Experimental Investigation of the Effects of Drilling Fluid Activity on the Hydration Behavior of Shale Reservoirs in Northwestern Hunan, China
Abstract
:1. Introduction
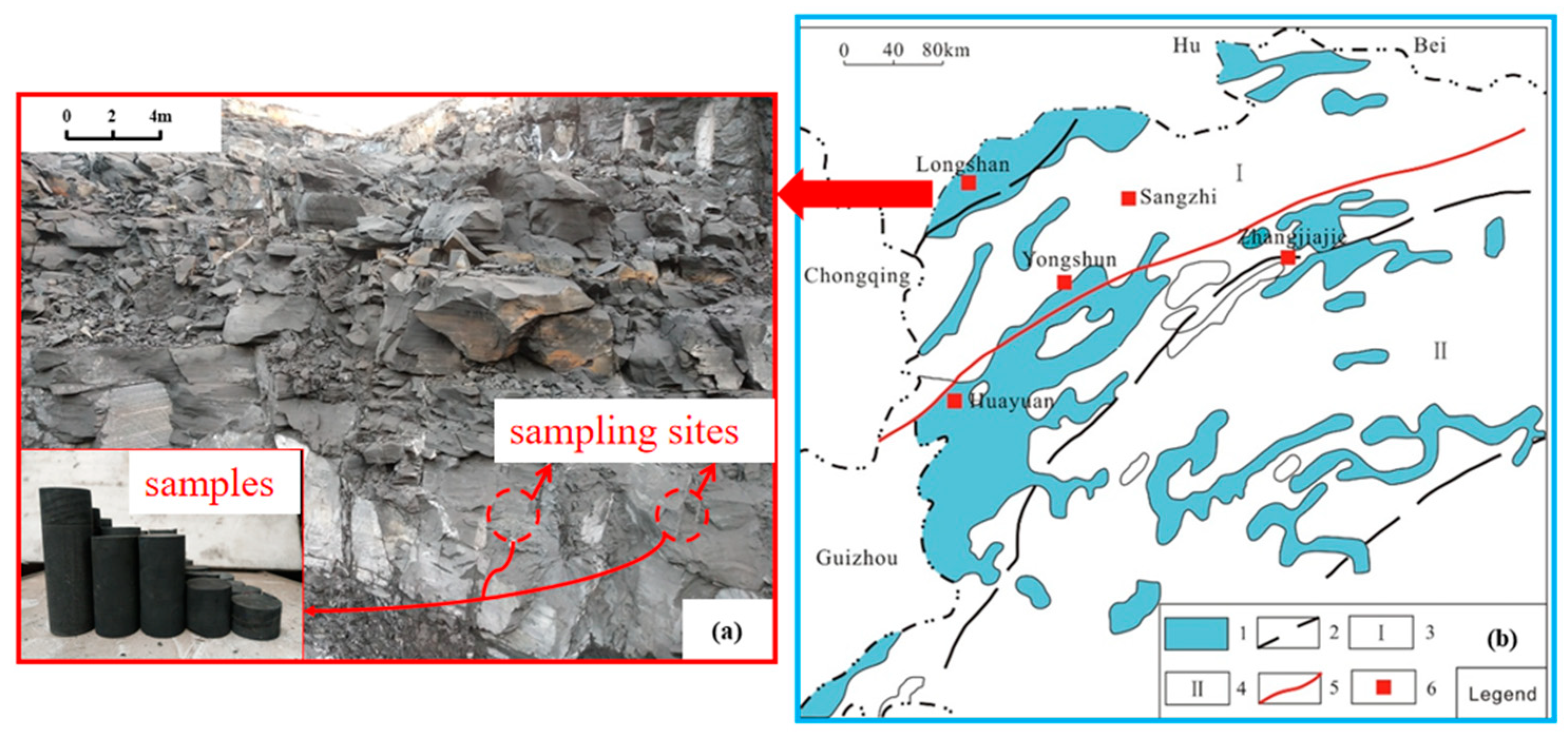
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Fluid Activity Tests
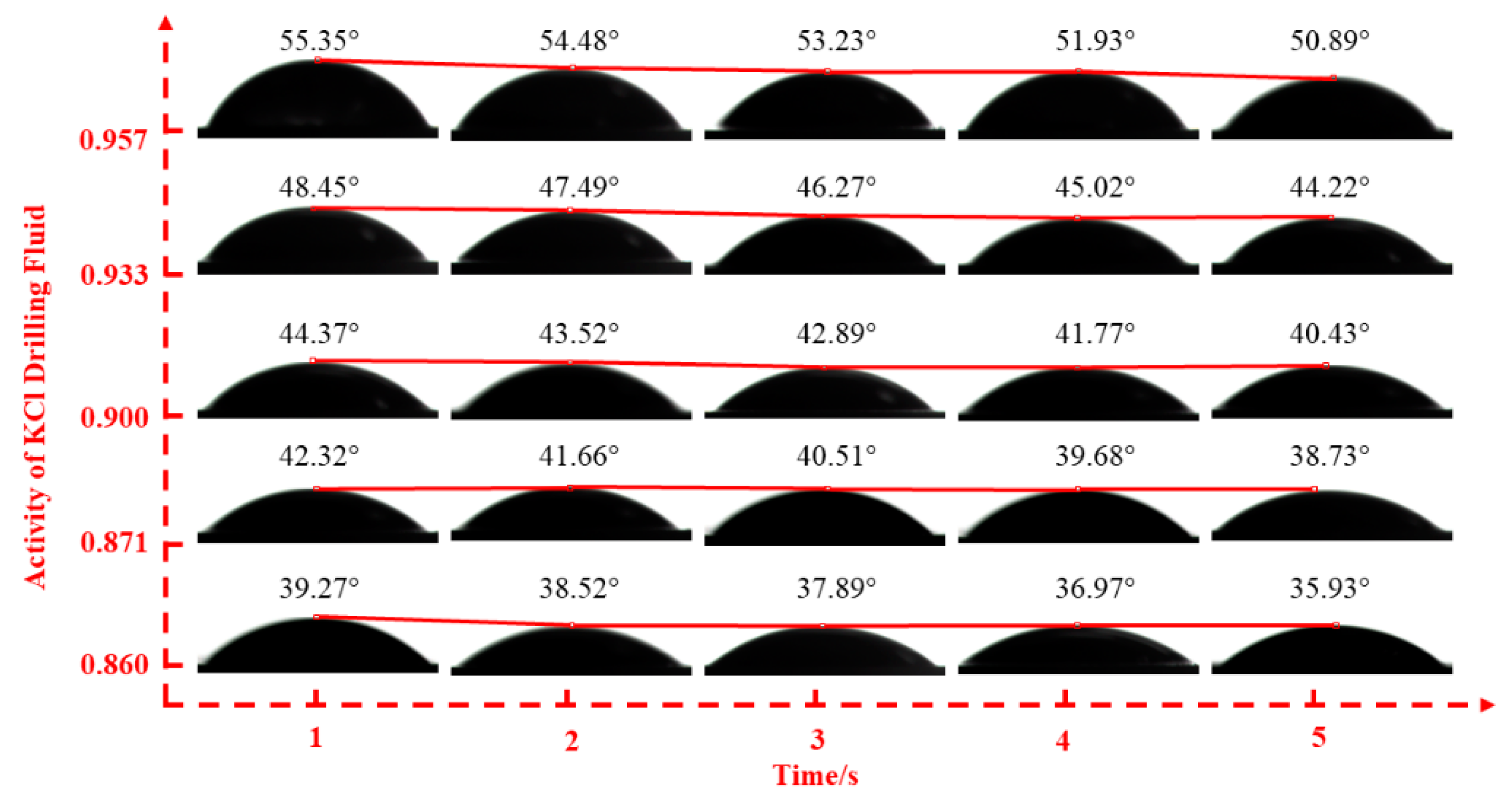
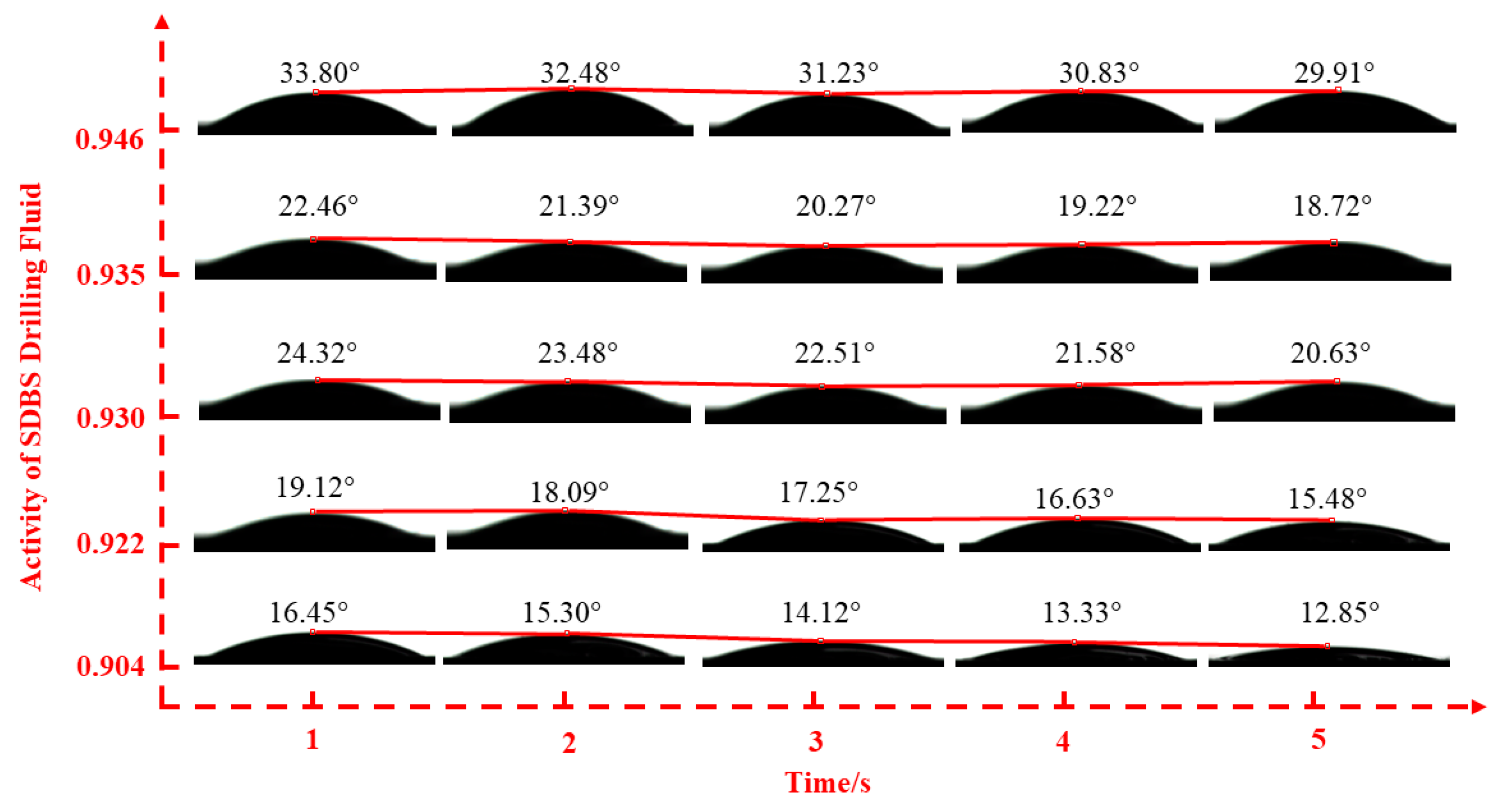
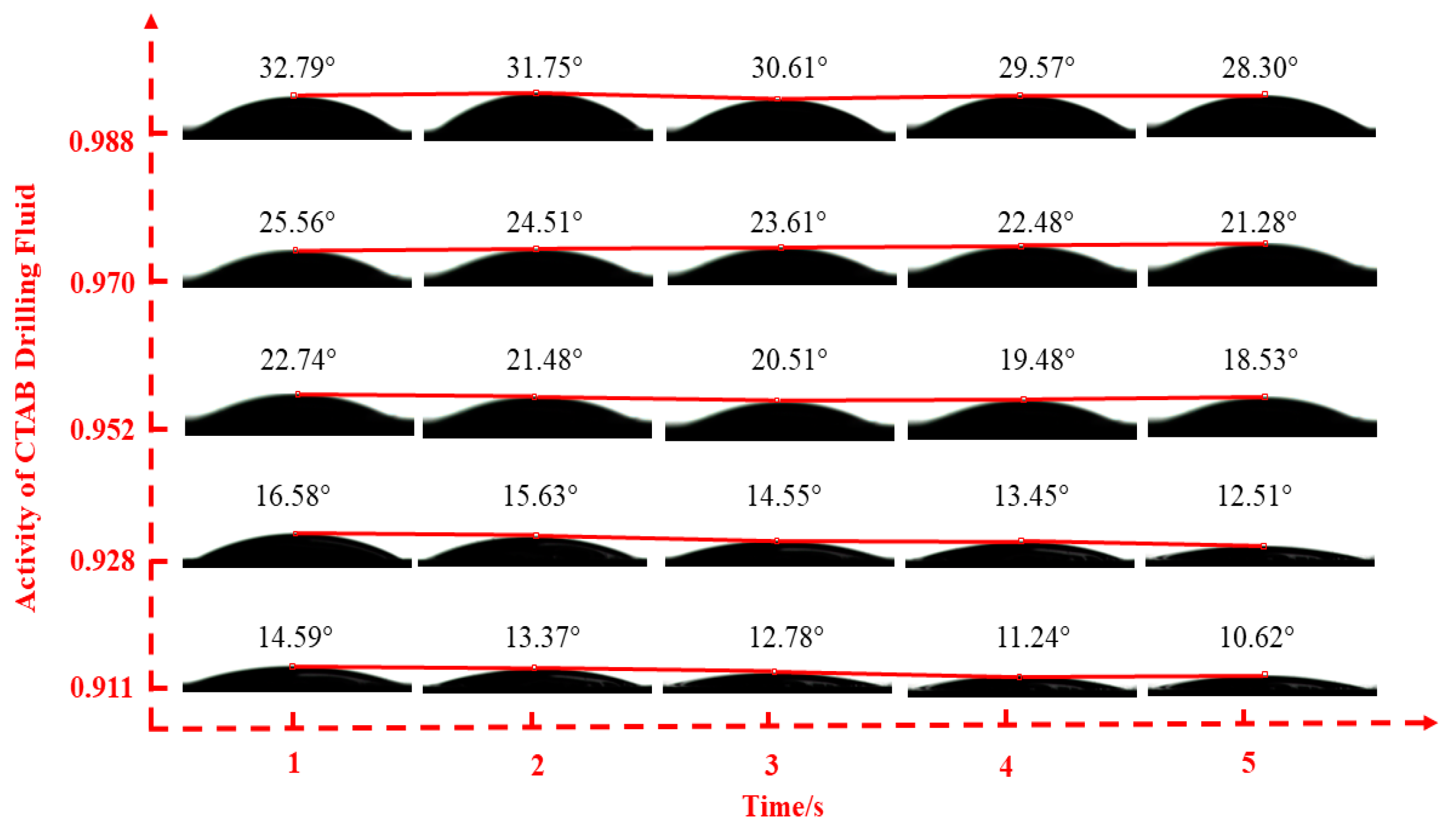
2.2.2. Fluid–Shale Contact Angle Tests
2.2.3. Shale Swelling Ratio Tests
3. Results and Discussion
3.1. Effect of Inhibitor Concentration on Drilling Fluid Activity
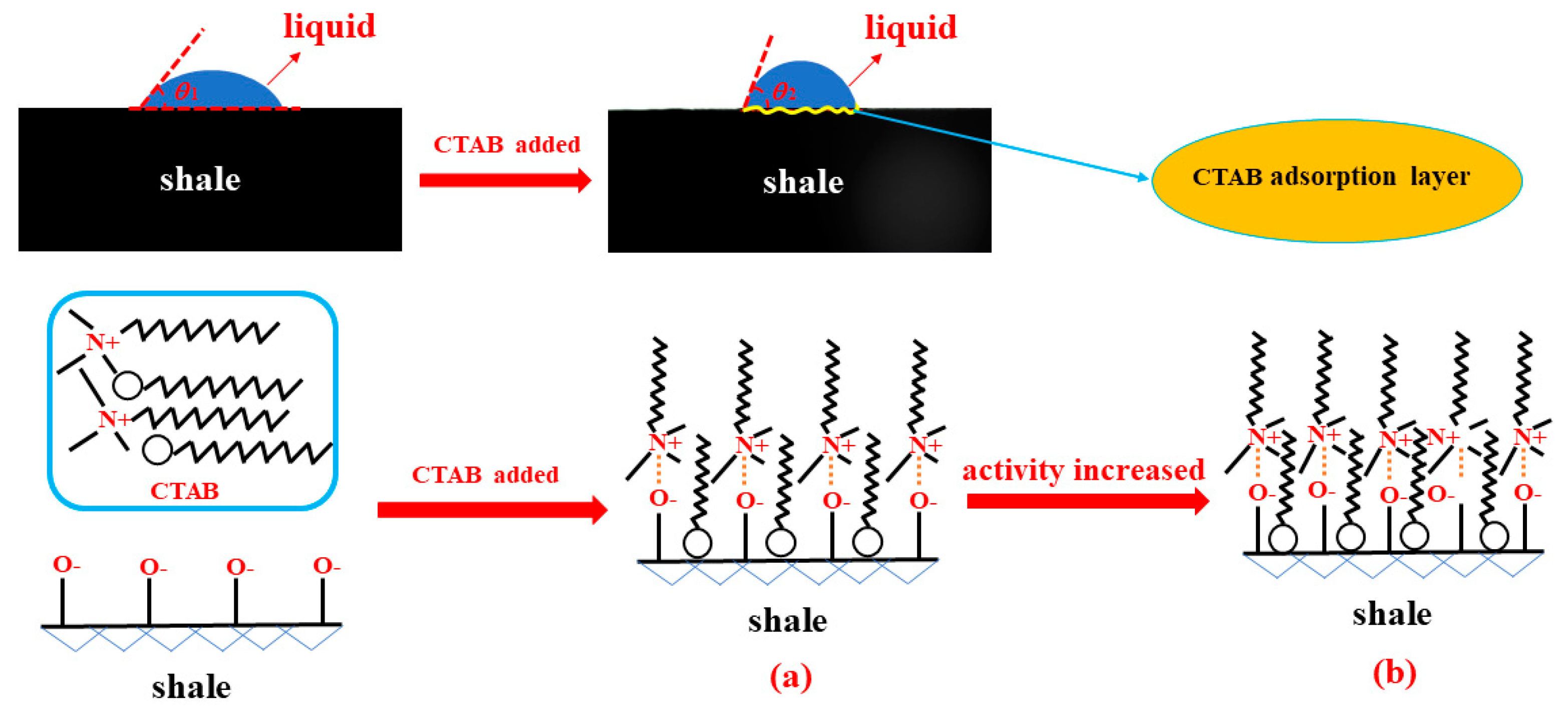
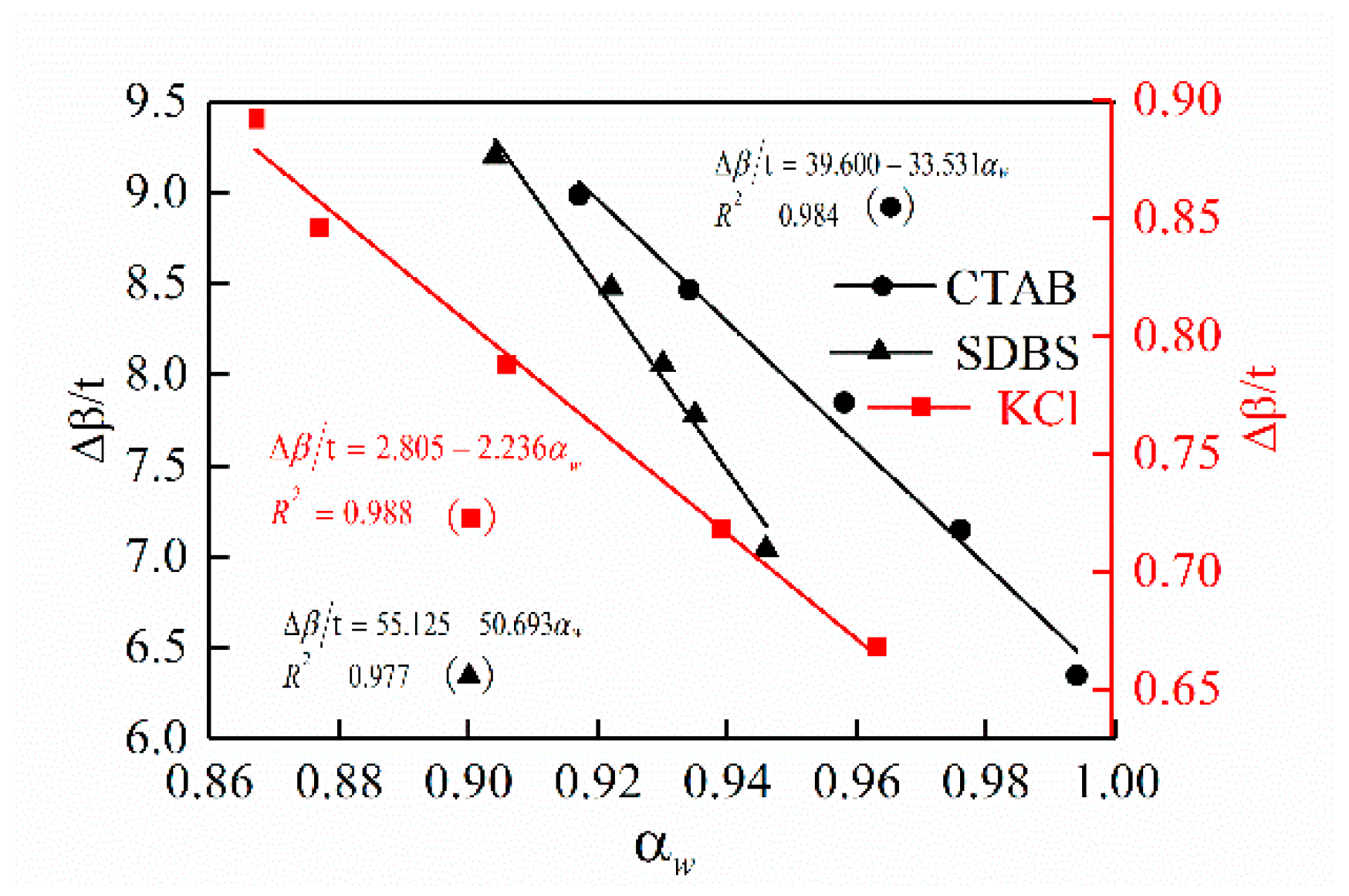
3.2. Effect of Drilling Fluid Activity on Surface Hydration of Shale
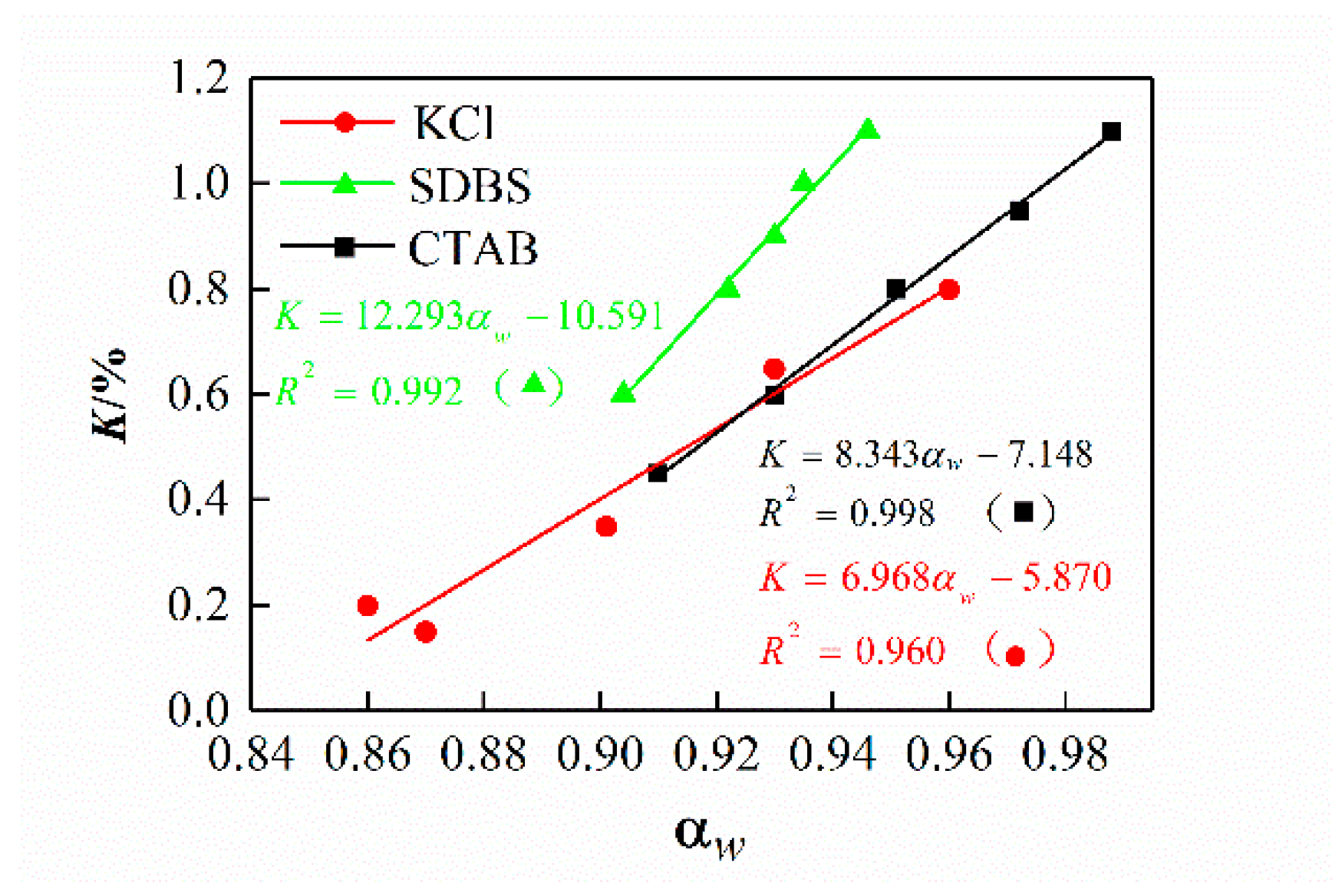
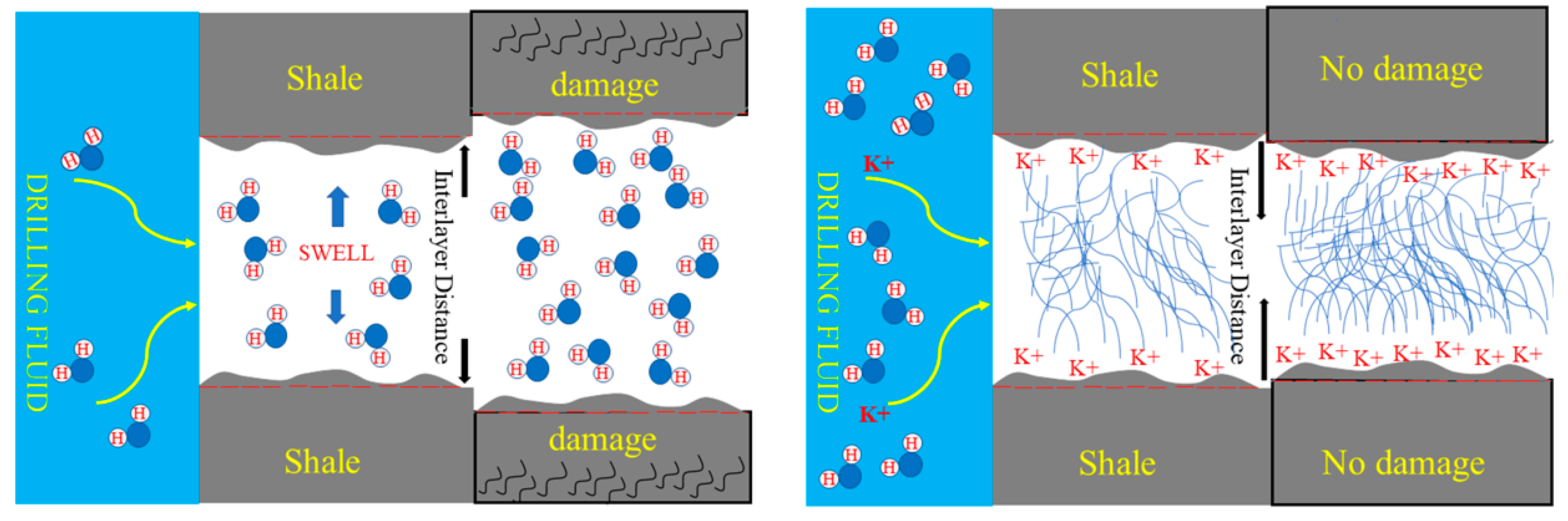
3.3. Effect of Drilling Fluid Activity on the Osmotic Hydration of Shale
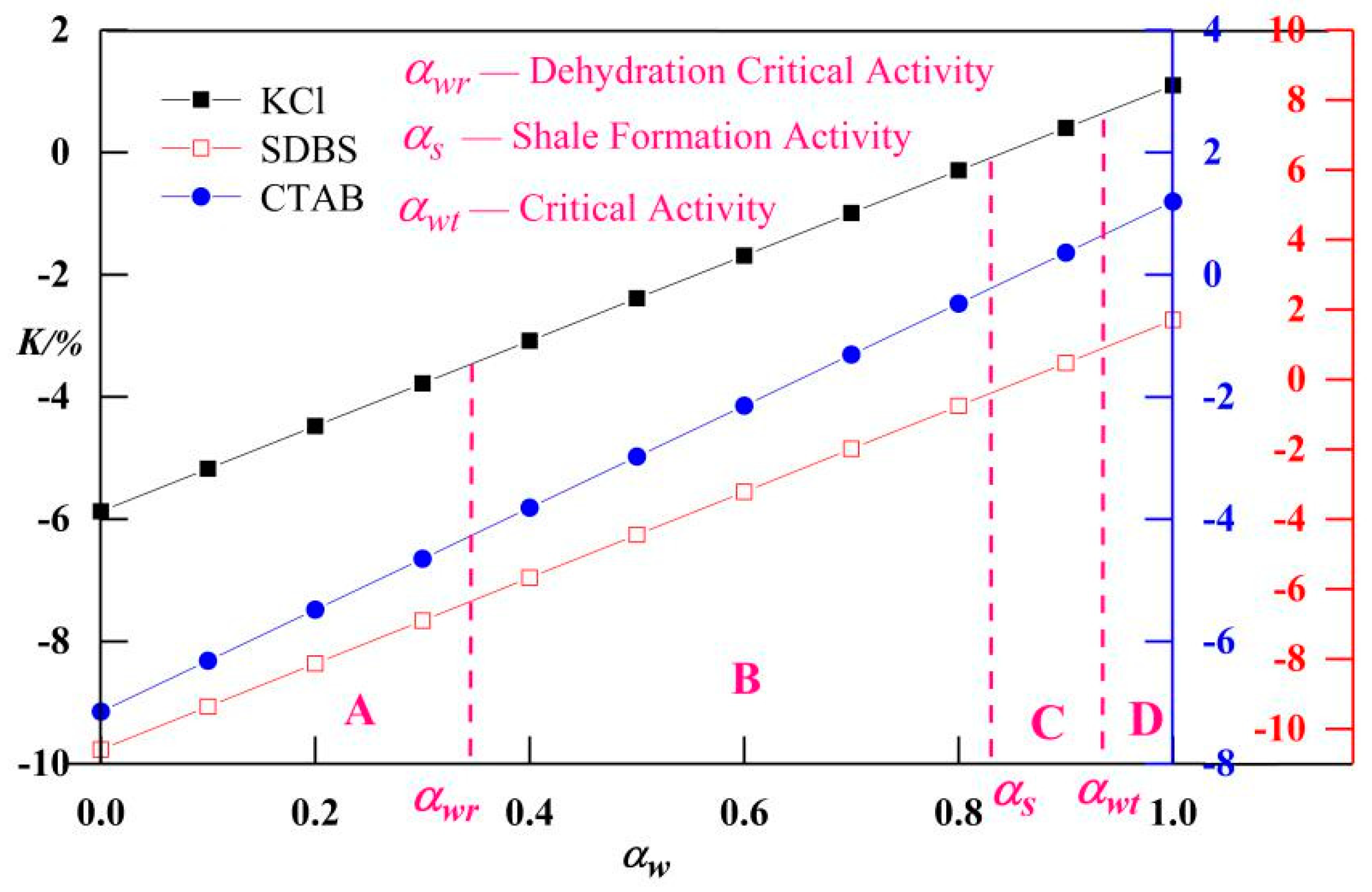
3.4. Classification of Safety Levels of Drilling Fluid Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| KCl | potassium chloride |
| SDBS | sodium dodecylbenzene sulfonate |
| CTAB | cetyltrimethylammonium bromide |
| b | negative extreme swelling ratio |
| αw | drilling fluid activity |
| ∆β/t | change ratio of the fluid-shale contact angle |
| K | shale swelling ratio |
| a | the ratio of the shale swelling ratio to the drilling fluid activity |
| αwo | critical drilling fluid activity |
| αs | shale formation activity |
| αwt | drilling fluid critical activity |
| αwr | dehydration critical activity |
References
- Moslemizadeh, A.; Saeed Khezerloo-ye, A.; Shahbazi, K.; Zendehboudi, S. A triterpenoid saponin as an environmental friendly and biodegradable clay swelling inhibitor. J. Mol. Liq. 2017, 247, 269–280. [Google Scholar] [CrossRef]
- Zeynali, M.E. Mechanical and physical-chemical aspects of wellbore stability during drilling operations. J. Pet. Sci. Eng. 2012, 82, 120–124. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Moslemizadeh, A.; Dezaki, A.S. A novel nonionic surfactant for inhibiting shale hydration. Appl. Clay Sci. 2015, 118, 74–86. [Google Scholar] [CrossRef]
- Steiger, R.P.; Leung, P.K. Quantitative Determination of the Mechanical Properties of Shales. SPE Drill. Eng. 1992, 7, 181–185. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, S.; Wang, Z.; Yang, X.; Peng, Y.; Cai, J.; Nasr-El-Din, H.A. Improving wellbore stability of shale by adjusting its wettability. J. Pet. Sci. Eng. 2017, 161, 692–702. [Google Scholar] [CrossRef]
- Diaz-Perez, A.; Cortes-Monroy, I.; Roegiers, J. The role of water/clay interaction in the shale characterization. J. Pet. Sci. Eng. 2007, 58, 83–98. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, L.; Yu, P.; Chen, Z. Experimental study on the application of an ionic liquid as a shale inhibitor and inhibitive mechanism. Appl. Clay Sci. 2017, 150, 267–274. [Google Scholar] [CrossRef]
- Liang, L.; Xiong, J.; Liu, X. Effects of hydration swelling and wettability on propagation mechanism of shale formation crack. Pet. Geol. Exp. 2014, 36, 780–786. [Google Scholar]
- Wang, G. Hydration Characteristics of Hard Brittle Shale and Its Influence on Collapse Pressure. Master Thesis, Southwest Petroleum University, Chengdu, China, 2014. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10615-1018021314.htm (accessed on 15 August 2019).
- Chenevert, E.; Shale, M. Alteration by Water Adsorption. J. Pet. Technol. 1970, 22, 1141–1148. [Google Scholar] [CrossRef]
- Talal, M.A.; Kuwait, U.; Zhang, J.; Baker, A.; Martin, E.C.; Mukul, M. Factors Controlling the Membrane Efficiency of Shales When Interacting with Water-Based and Oil-Based Muds. In Proceedings of the SPE International Oil & Gas Conference and Exhibition, Beijing, China, 5–7 December 2006; Available online: https://www.onepetro.org/conference-paper/SPE-100735-MS (accessed on 15 August 2019).
- Van Oort, E. On the physical and chemical stability of shales. J. Pet. Sci. Eng. 2003, 38, 213–235. [Google Scholar] [CrossRef]
- Tang, W. Study on Effects of Shale Hydration on Wellbore Stability. Ph.D. Thesis, China University of Petroleum (East China), Qingdao, China, 2011. Available online: http://cdmd.cnki.com.cn/Article/CDMD-10425-1011287063.htm (accessed on 15 August 2019).
- Ding, R.; Li, J. The Effect of Water Activity and Semipermeable Membrane on Shale Hydration. Drill. Fluid Complet. Fluid 1994, 23–28. Available online: http://www.cnki.com.cn/Article/CJFDTotal-ZJYW199403004.htm (accessed on 15 August 2019).
- Al-Bazali, T.; Rakf, M.A. The Role of Chemical Potential and Molecular Diffusion on the geo-Mechanical Stability of Shale. In Proceedings of the 2018 World Congress on Advances in Civil, Environmental, & Materials Research (ACEM18), Incheon, Korea, 27 31 August 2018; Available online: http://www.i-asem.org/publication_conf/acem18/2.ICGE18/W3B.4.GE1160_5034F1.pdf (accessed on 15 August 2019).
- Chenevert, E.; Shale, M. Control with Balanced-Activity Oil-Continuous Muds. J. Pet. Technol. 1970, 22, 1309–1316. [Google Scholar] [CrossRef]
- Wen, H.; Chen, M.; Jin, Y.; Wen, Z.; Ye, X.; Yang, S. Experimental research on brittle shale failure caused by drilling fluid activity. Oil Drill. Prod. Technol. 2014, 57–60. [Google Scholar] [CrossRef]
- Yew, H.C.; Wang, L.C.; Chenevert, E.M.A. Theory on Water Activity Between Drill-Fluid and Shale. In Proceedings of the Rock Mechanics, Tilerson & Wawersik, Santa Fe, NM, USA, 3–5 June 1992; Available online: https://www.onepetro.org/conference-paper/ARMA-92-0717 (accessed on 15 August 2019).
- Huang, W.; Li, X.; Qiu, Z.; Jia, J.; Wang, Y.; Li, X. Inhibiting the surface hydration of shale formation using preferred surfactant compound of polyamine and twelve alkyl two hydroxyethyl amine oxide for drilling. J. Pet. Sci. Eng. 2017, 159, 791–798. [Google Scholar] [CrossRef]
- Du, D.; Fan, S.; Chenevert, M.E. Study on the osmotic hydration of shale in water based fluid. Drill. Fluid Complet. Fluid 1996, 5–10. Available online: http://www.cnki.com.cn/Article/CJFDTOTAL-ZJYW199603001.htm (accessed on 15 August 2019).
- Liu, J.; Sun, J. Effects of Drilling Fluid Activity on Hydration and Dispersion of Formation Rocks in Shale Gas Drilling in Chuan-Dian Area. Drill. Fluid Compl. Fluid 2016, 33, 31–35. [Google Scholar] [CrossRef]
- Cai, J.; Yue, Y.; Cao, J.; Yang, X.; Wu, X. Experimental study on the effect of drilling fluid wettability on shale wellbore stability. J. China Coal Soc. 2016, 41, 228–233. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Shadizadeh, S.R. Minimizing water invasion into kazhdumi shale using nanoparticles. Iran. J. Oil Gas Sci. Technol. 2015, 4, 15–32. Available online: http://ijogst.put.ac.ir/article_12475_a66037b9fcaa4c236494db533a218905.pdf (accessed on 15 August 2019).
- Liu, X.; Xiong, J.; Liang, L.; Luo, C.; Zhang, A. Analysis of the wettability of Longmaxi Formation shale in the south region of Sichuan Basin and its influence. Nat. Gas Geosci. 2017, 161, 692–702. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Guo, J.; Su, Q.; Zhuo, X. Wettability, oil recovery, and interfacial tension with an SDBS–dodecane–kaolin system. J. Colloid Interface Sci. 1999, 214, 368–372. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Guo, J.; Su, Q.; Zhuo, X. Effects of inhibitor KCl on shale expansibility and mechanical properties. Petroleum 2018. [Google Scholar] [CrossRef]
- Moslemizadeh, A.; Aghdam, S.K.; Shahbazi, K.; Aghdam, H.K.; Alboghobeish, F. Assessment of swelling inhibitive effect of CTAB adsorption on montmorillonite in aqueous phase. Appl. Clay Sci. 2016, 127, 111–122. [Google Scholar] [CrossRef]
- Cao, H.; Wang, T.; Bao, T.; Sun, P.; Zhang, Z.; Wu, J. Effective Exploitation Potential of Shale Gas from Lower Cambrian Niutitang Formation, Northwestern Hunan, China. Energies 2018, 11. [Google Scholar] [CrossRef]
- Liu, X.; Liu, K.; Gou, S.; Liang, L.; Cheng, L.; Guo, Q. Water-Soluble Acrylamide Sulfonate Copolymer for Inhibiting Shale Hydration. Ind. Eng. Chem. Res. 2014, 53, 2903–2910. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Wang, T.; Sun, D. Effect of poly(oxypropylene)diamine adsorption on hydration and dispersion of montmorillonite particles in aqueous solution. Colloids Surf. Physicochem. Eng. Asp. 2011, 381, 41–47. [Google Scholar] [CrossRef]
| Based Fluid Formula | Inhibitor Concentration |
|---|---|
| 0.35% CMC + 0.08% PH | 0.5%~2.5% KCl |
| 0.5%~2.5% SDBS | |
| 0.5%~2.5% CTAB |
| Quartz (%) | Feldspar (%) | Clay Mineral (%) | Anatase (%) | MICA (%) | Amorphous (%) |
|---|---|---|---|---|---|
| 21~30 | 10~15 | 20~27 | 5~8 | 4~9 | 21~29 |
| Elastic Modulus (GPa) | Poisson’s Ratio | Brittleness Index | Porosity (%) | Cohesion (MPa) | Internal Friction Angle (°) |
|---|---|---|---|---|---|
| 1.25~3.86 | 0.19~0.24 | 0.20~0.37 | 2.24~4.47 | 0.57~0.98 | 33.82~41.70 |
| Inhibitor Concentration | 0.5% | 1.0% | 1.5% | 2.0% | 2.5% | |
|---|---|---|---|---|---|---|
| KCl | drilling fluid activities | 0.860 | 0.871 | 0.900 | 0.933 | 0.957 |
| SDBS | 0.904 | 0.922 | 0.930 | 0.935 | 0.946 | |
| CTAB | 0.911 | 0.928 | 0.952 | 0.970 | 0.988 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, H.; Zhang, Z.; Bao, T.; Sun, P.; Wang, T.; Gao, Q. Experimental Investigation of the Effects of Drilling Fluid Activity on the Hydration Behavior of Shale Reservoirs in Northwestern Hunan, China. Energies 2019, 12, 3151. https://doi.org/10.3390/en12163151
Cao H, Zhang Z, Bao T, Sun P, Wang T, Gao Q. Experimental Investigation of the Effects of Drilling Fluid Activity on the Hydration Behavior of Shale Reservoirs in Northwestern Hunan, China. Energies. 2019; 12(16):3151. https://doi.org/10.3390/en12163151
Chicago/Turabian StyleCao, Han, Zheng Zhang, Ting Bao, Pinghe Sun, Tianyi Wang, and Qiang Gao. 2019. "Experimental Investigation of the Effects of Drilling Fluid Activity on the Hydration Behavior of Shale Reservoirs in Northwestern Hunan, China" Energies 12, no. 16: 3151. https://doi.org/10.3390/en12163151
APA StyleCao, H., Zhang, Z., Bao, T., Sun, P., Wang, T., & Gao, Q. (2019). Experimental Investigation of the Effects of Drilling Fluid Activity on the Hydration Behavior of Shale Reservoirs in Northwestern Hunan, China. Energies, 12(16), 3151. https://doi.org/10.3390/en12163151





