Study on the Impacts of Capillary Number and Initial Water Saturation on the Residual Gas Distribution by NMR
Abstract
1. Introduction
2. Imbibition Experiments Theories and Methods
2.1. Material
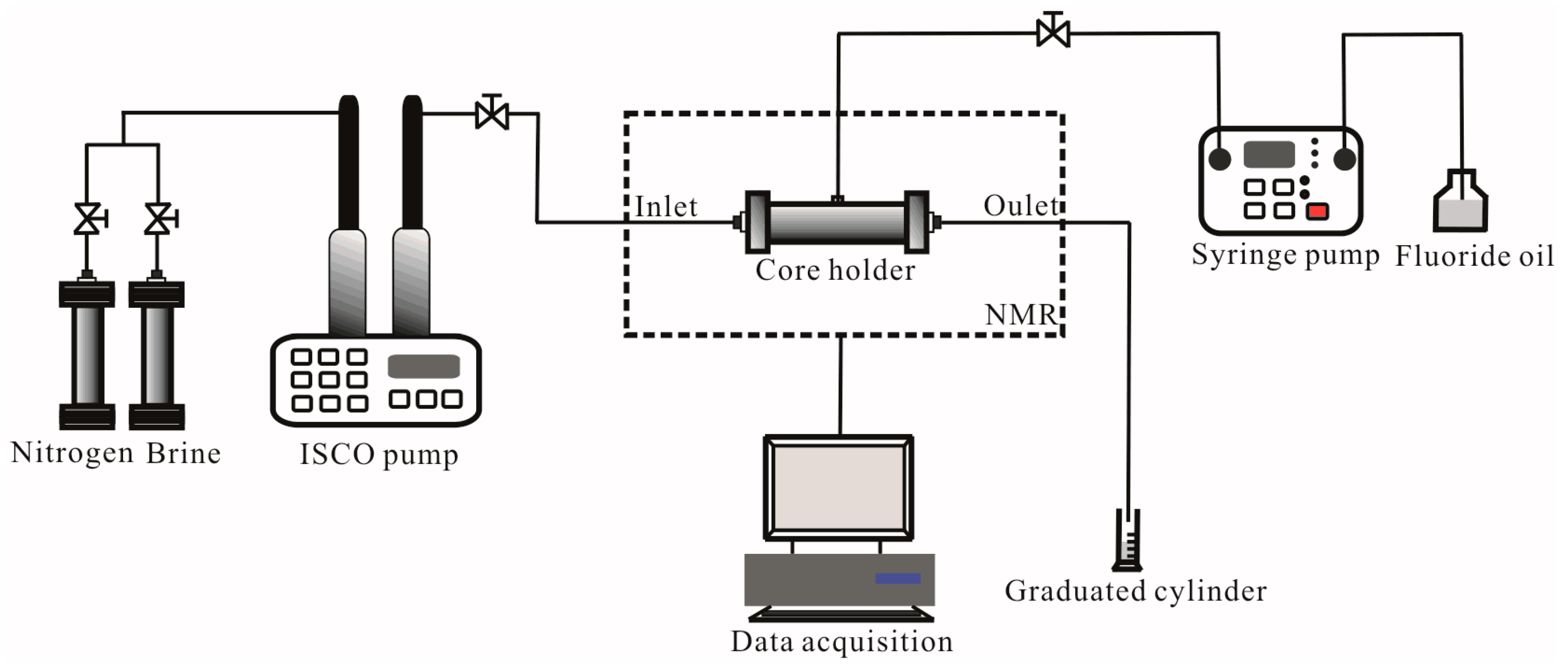
2.2. Setup
2.3. Procedures
2.4. Water/Gas Saturation Measurement Theories and Methods
3. Results and Discussion
3.1. The T2 Spectrum
3.2. Pore Radius and Pore Size Distribution
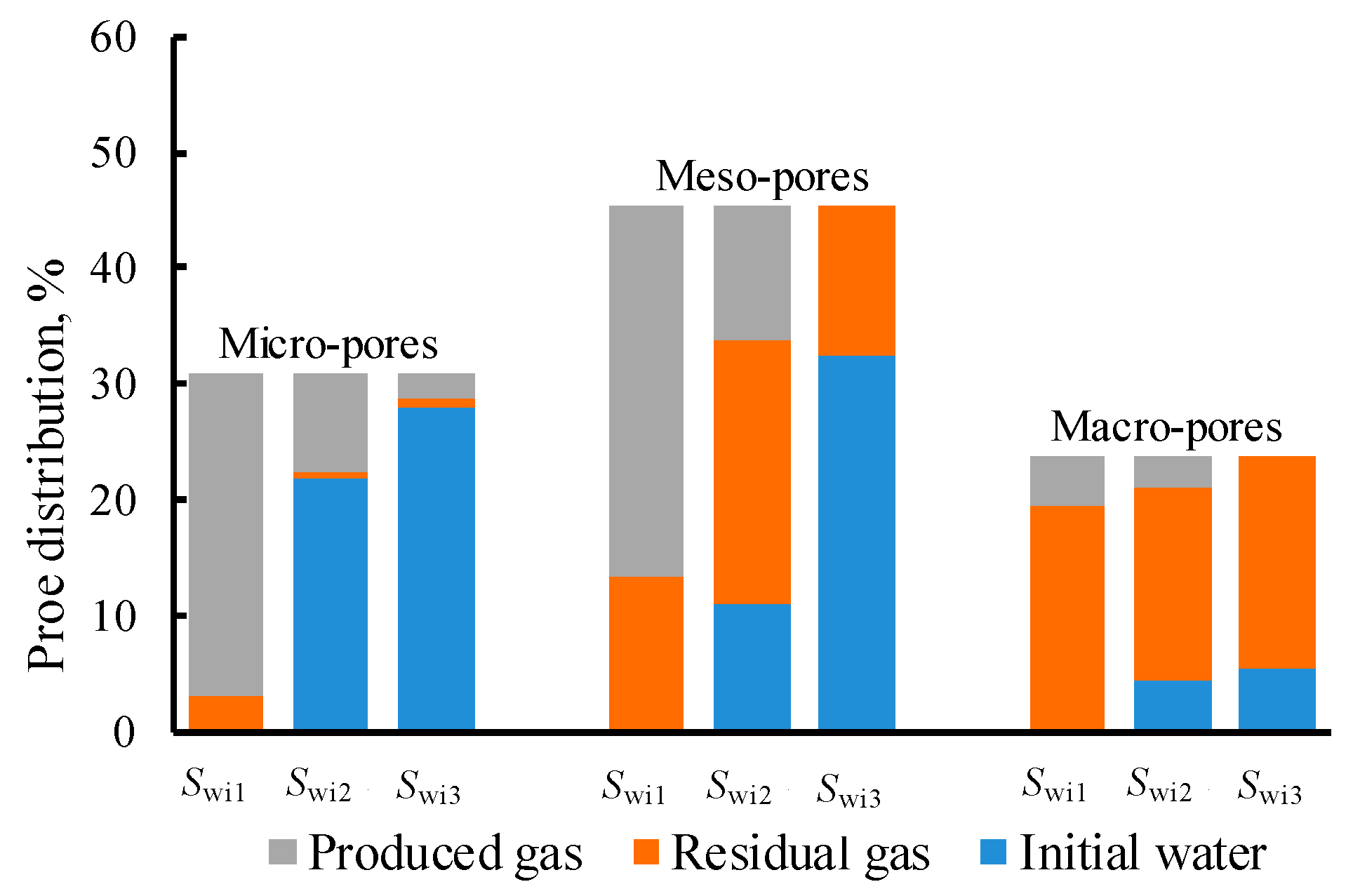
3.3. Initial Water, Produced Gas and Residual Gas Distribution
3.4. Impacts of Swi on Residual Gas Distribution
3.5. Impacts of Ca on Residual Gas Distribution
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lu, W.Y.; Huang, B.X. Numerical simulation of migration characteristics of the two-phase interface in water-gas displacement. Energy Explor. Exploit. 2018, 36, 246–264. [Google Scholar] [CrossRef]
- Geistlinger, H.; Mohammadian, S.; Schlueter, S. Quantification of capillary trapping of gas clusters using X-ray microtomogrephy. Water Resour. Res. 2014, 50, 4514–4529. [Google Scholar] [CrossRef]
- Meng, Q.B.; Liu, H.Q.; Wang, J. Entrapment of non-wetting phase during co-current spontaneous imbibition. Energy Fuels 2015, 29, 686–694. [Google Scholar] [CrossRef]
- Qian, K.; Yang, S.L.; Dou, H.G.; Wang, Q.; Wang, L.; Huang, Y. Experimental investigation on microscopic residual oil distribution during CO2 Huff-and-Puff process in tight oil reservoirs. Energies 2018, 11, 2843. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.L.; Peng, X.; Deng, H.; Meng, Z.; Qian, K.; Wang, Z.L.; Lei, H. An improved visual investigation on gas–water flow characteristics and trapped gas formation mechanism of fracture–cavity carbonate gas reservoir. J. Nat. Gas Sci. Eng. 2018, 49, 213–226. [Google Scholar] [CrossRef]
- Hamon, G.; Suzanne, K.; Billiotte, J.; Trocme, V. Field-wide variations of trapped gas saturation in heterogeneous sandstone reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–3 October 2001. [Google Scholar]
- Wang, S.; Li, Y.; Sun, L.; Wei, Y.; Wei, X.; Zhang, F. Measurements of residual gas saturation by unidirectional spontaneous imbibitions. Pet. Sci. Technol. 2014, 32, 2815–2821. [Google Scholar] [CrossRef]
- Hekmatzadeh, M.; Dadvar, M.; Emadi, M.A. Visual investigation of residual gas saturation in porous media. Int. J. Oil Gas Coal Technol. 2015, 10, 161–178. [Google Scholar] [CrossRef]
- Ding, M.; Kantzas, A. Capillary number correlations for gas-liquid systems. J. Can. Pet. Technol. 2007, 46, 27–32. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, S.C.; Wang, F.; Pan, Z.Q.; Mou, J.Y.; Zhou, T.; Ren, Z.X. Experimental investigation on imbibition-front progression in shale based on nuclear magnetic resonance. Energy Fuels 2016, 30, 9097–9105. [Google Scholar]
- Suzanne, K.; Hamon, G.; Billiotte, J.; Trocmé, V. Experimental relationships between residual gas saturation and initial gas saturation in heterogeneous sandstone reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5–8 October 2003. [Google Scholar]
- Batycky, J.; Irwin, D.; Fish, R. Trapped gas saturations in leduc-age reservoirs. J. Can. Pet. Technol. 1998, 37, 32–39. [Google Scholar] [CrossRef]
- Rezaee, M.; Rostami, B.; Pourafshary, P. Heterogeneity effect on non-wetting phase trapping in strong water drive gas reservoirs. J. Nat. Gas Sci. Eng. 2013, 14, 185–191. [Google Scholar] [CrossRef]
- Kumar, M.; Knackstedt, M.A.; Senden, T.J. Visualizing and quantifying the residual phase distribution in core material. Petrophysics 2010, 51, 323–332. [Google Scholar]
- Suekane, T.; Zhou, N.; Hosokawa, T. Direct observation of trapped gas bubbles by capillarity in sandy porous media. Transp. Porous Media 2010, 82, 111–122. [Google Scholar] [CrossRef]
- Iglauer, S.; Paluszny, A.; Blunt, M.J. Simultaneous oil recovery and residual gas storage: A pore-level analysis using in situ X-ray micro-tomography. Fuel 2013, 103, 905–914. [Google Scholar] [CrossRef]
- Pentland, C.H.; Itsekiri, E.; Al Mansoori, S.K.; Iglauer, S.; Branko, B.; Blunt, M.J. Measurement of nonwetting-phase trapping in sandpacks. SPE J. 2010, 15, 274–281. [Google Scholar] [CrossRef]
- Geistlinger, H.; Mohammadian, S. Capillary trapping mechanism in strongly water wet systems: Comparison between experiment and percolation theory. Adv. Water Resour. 2015, 79, 35–50. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E.C. Petrophysics, 2nd ed.; Gulf Professional Publishing: Houston, TX, USA, 2004; p. 352. [Google Scholar]
- Guo, H.; Dou, M.; Wang, H.Q.; Wang, F.Y.; Gu, Y.Y.; Yu, Z.Y.; Wang, Y.S.; Li, Y.Q. Proper Use of Capillary Number in Chemical Flooding. J. Chem. 2017, 4307368, 1–11. [Google Scholar] [CrossRef]
- Zendehboudi, S.; Chatzis, I. Experimental Study of Controlled Gravity Drainage in Fractured Porous Media. J. Can. Pet. Technol. 2011, 50. [Google Scholar] [CrossRef]
- Shen, Y.H.; Ge, H.K.; Meng, M.M.; Jiang, Z.X.; Yang, X.Y. Effect of water imbibition on shale permeability and its influence on gas production. Energy Fuels 2017, 31, 4873–4980. [Google Scholar] [CrossRef]
- Mohammadian, S.; Geistlinger, H.; Vogel, H.J. Quantification of Gas-Phase Trapping within the Capillary Fringe Using Computed Microtomography. Vadose Zone J. 2015, 14. [Google Scholar] [CrossRef]
- Buchgraber, M.; Kovscek, A.R.; Castanier, L.M. A study of microscale gas trapping using etched silicon micromodels. Transp. Porous Med. 2012, 95, 647–668. [Google Scholar] [CrossRef]
- Geistlinger, H.; Ataei-Dadavi, I.; Vogel, H.J. Impact of surface roughness on capillary trapping using 2D-micromodel visualization experiments. Transp. Porous Med. 2016, 112, 207–227. [Google Scholar] [CrossRef]
- Herring, A.L.; Andersson, L.; Schlüter, S.; Sheppard, A.; Wildenschild, D. Efficiently engineering pore-scale processes: The role of force dominance and topology during nonwetting phase trapping in porous media. Adv. Water Resour. 2015, 79, 91–102. [Google Scholar] [CrossRef]
- Yuan, Y.; Rezaee, R.; Verrall, M.; Hu, S.Y.; Zou, J.; Testmanti, N. Pore characterization and clay bound water assessment in shale with a combination of NMR and low-pressure nitrogen gas adsorption. Int. J. Coal Geol. 2018, 194, 11–21. [Google Scholar] [CrossRef]
- Slijkerman, W.F.J.; Hofman, J.P. Determination of surface relaxivity from NMR diffusion measurements. Magn. Reson. Imaging 1998, 16, 541–544. [Google Scholar] [CrossRef]
- Yang, P.; Guo, H.K.; Yang, D.Y. Determination of residual oil distribution during waterflooding in tight oil formations with NMR relaxometry measurements. Energy Fuels 2013, 27, 5750–5756. [Google Scholar] [CrossRef]
- Testamanti, M.N.; Rezaee, R. Determination of NMR T2 cut-off for clay bound water in shales: A case study of Carynginia Formation, Perth Basin, Western Australia. J. Pet. Sci. Eng. 2017, 149, 497–503. [Google Scholar] [CrossRef]
- Xu, H.J.; Fan, Y.R.; Hu, F.L.; Li, C.X.; Yu, J.; Liu, Z.C.; Wang, F.Y. Characterization of pore throat size distribution in tight sandstones with nuclear magnetic resonance and high-pressure mercury intrusion. Energies 2019, 12, 1528. [Google Scholar] [CrossRef]
- Lyu, C.H.; Wang, Q.; Ning, Z.F.; Chen, M.Q.; Li, M.Q.; Chen, Z.L.; Xia, Y.X. Investigation on the application of NMR to spontaneous imbibition recovery of tight sandstones: An experimental study. Energies 2018, 11, 2359. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Z.M.; Luo, Y.T.; Lin, W.; Xu, J.X.; Ding, Y.H.; Niu, J.L. Evaluation of displacement effects of different injection media in tight oil sandstone by online nuclear magnetic resonance. Energies 2018, 11, 2836. [Google Scholar] [CrossRef]
- Blümich, B.; Casanova, F.; Appelt, S. NMR at low magnetic fields. Chem. Phys. Lett. 2009, 477, 231–240. [Google Scholar] [CrossRef]
- Andersen, P.Ø.; Standnes, D.C.; Skjæveland, S.M. Waterflooding oil-saturated core samples-Analytical solutions for steady-state capillary end effects and correction of residual saturation. J. Pet. Sci. Eng. 2017, 157, 364–379. [Google Scholar] [CrossRef]
- Kibbey, T.C.G. The configuration of water on rough natural surfaces: Implications for understanding air-water interfacial area, film thickness, and imaging resolution. Water Resour. Res. 2013, 49, 4765–4774. [Google Scholar] [CrossRef]






| Sample | Mineral Component | Diameter (cm) | Length (cm) | Porosity (%) | Permeability (mD) |
|---|---|---|---|---|---|
| #1-1F #1-1M | 55% quartz + 35% feldspar + 10% cement | 2.440 | 4.534 2.500 | 24.01 | 82.26 |
| #1-2F #1-2M | 58% quartz + 34% feldspar + 8% cement | 2.451 | 4.580 2.500 | 22.72 | 92.22 |
| Composition | Concentration (mg/L) | Room Temperature and Brine Properties | |
|---|---|---|---|
| Sodium Chlorine | 7863.25 17,717.50 | Room temperature (°C) | 20.0 ± 1.0 |
| Density (g/mL) | 1.03 | ||
| Potassium Calcium | 2617.45 1801.80 | Viscosity (mPa·s) | 1.0 |
| Interfacial tension (mN/m) | 72.9 | ||
| Swi(%) | Spontaneous Imbibition | Forced Imbibition | ||||
|---|---|---|---|---|---|---|
| Sample | Sample | Q (mL/min) | v (m/d) | Ca | logCa | |
| 0 39.34 ± 1.57 62.98 ± 2.22 | #1-1F | #1-1F #1-2F | 0.001 | 0.0129 | 2.09 × 10−9 | −8.68 |
| 0.01 | 0.129 | 2.09 × 10−8 | −7.68 | |||
| 0.1 | 1.29 | 2.09 × 10−7 | −6.68 | |||
| 1 | 12.9 | 2.09 × 10−6 | −5.68 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wang, Y.; Li, M.; Ji, J.; Chang, L.; Wang, Z. Study on the Impacts of Capillary Number and Initial Water Saturation on the Residual Gas Distribution by NMR. Energies 2019, 12, 2714. https://doi.org/10.3390/en12142714
Li T, Wang Y, Li M, Ji J, Chang L, Wang Z. Study on the Impacts of Capillary Number and Initial Water Saturation on the Residual Gas Distribution by NMR. Energies. 2019; 12(14):2714. https://doi.org/10.3390/en12142714
Chicago/Turabian StyleLi, Tao, Ying Wang, Min Li, Jiahao Ji, Lin Chang, and Zheming Wang. 2019. "Study on the Impacts of Capillary Number and Initial Water Saturation on the Residual Gas Distribution by NMR" Energies 12, no. 14: 2714. https://doi.org/10.3390/en12142714
APA StyleLi, T., Wang, Y., Li, M., Ji, J., Chang, L., & Wang, Z. (2019). Study on the Impacts of Capillary Number and Initial Water Saturation on the Residual Gas Distribution by NMR. Energies, 12(14), 2714. https://doi.org/10.3390/en12142714




