Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Sources: Animal Manure and Inoculum
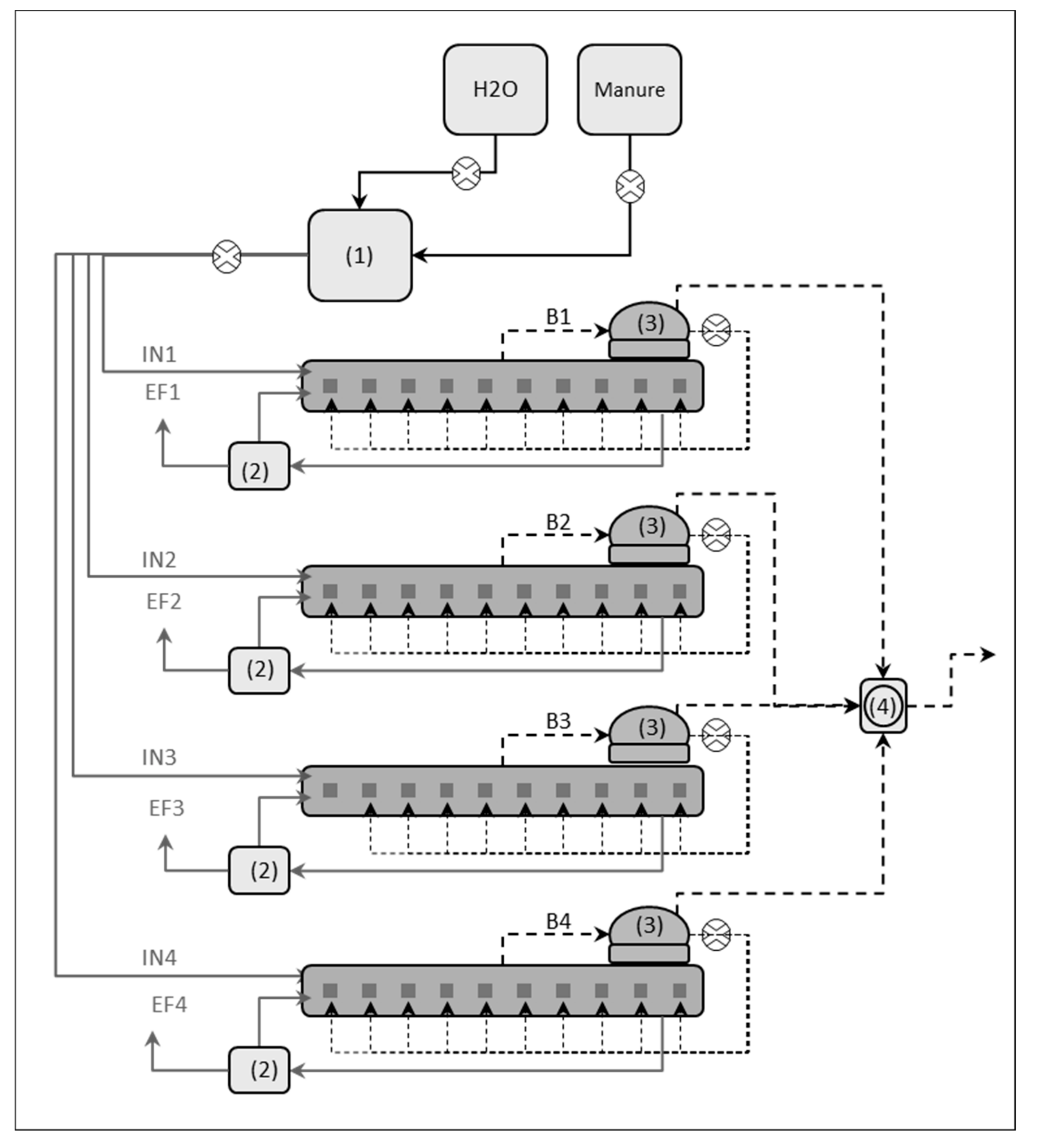
2.2. Pilot-Scale Biogas Plant
2.3. Experimental Conditions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Substrate Characterization
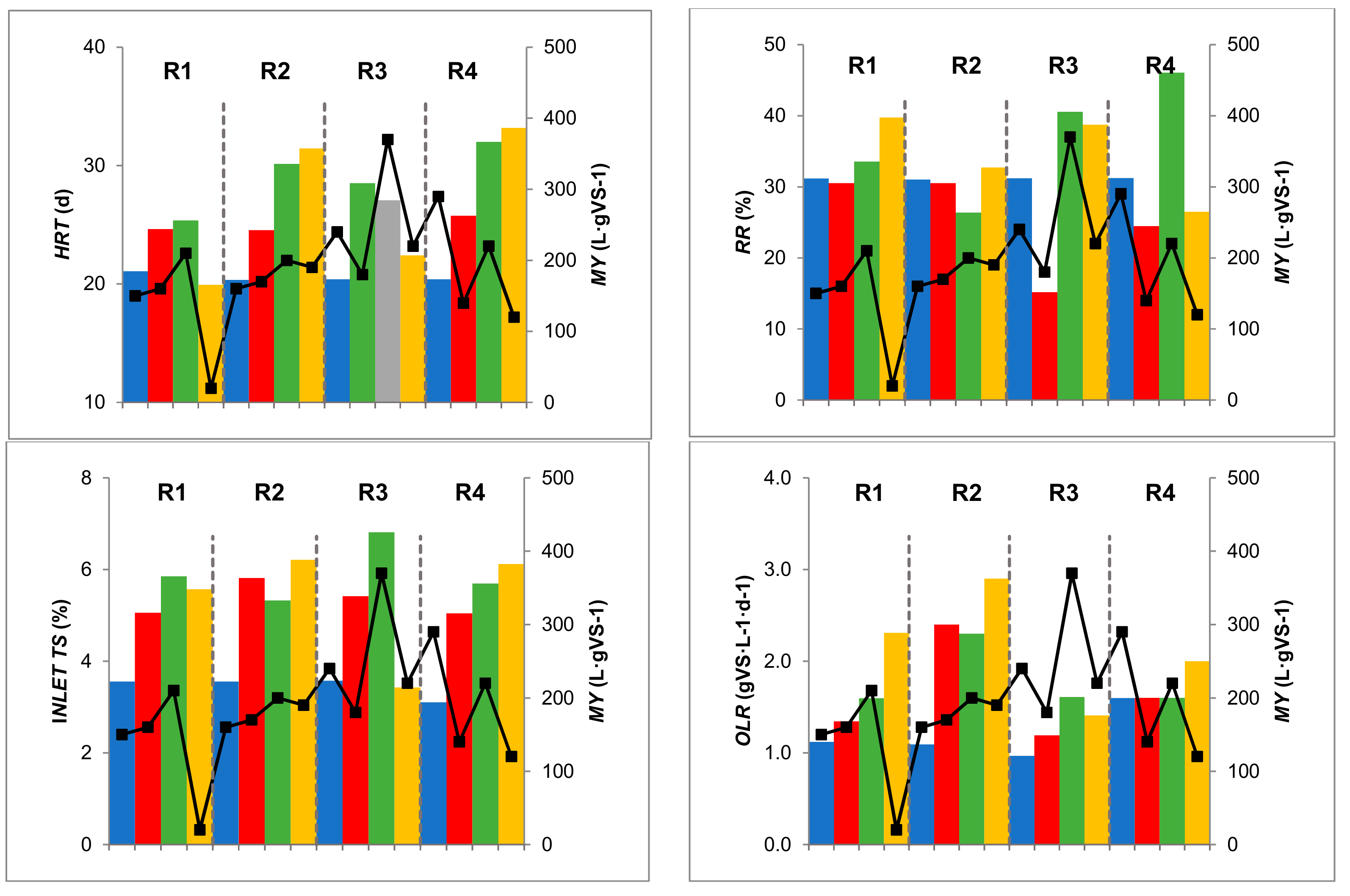
3.2. General Plant Performance
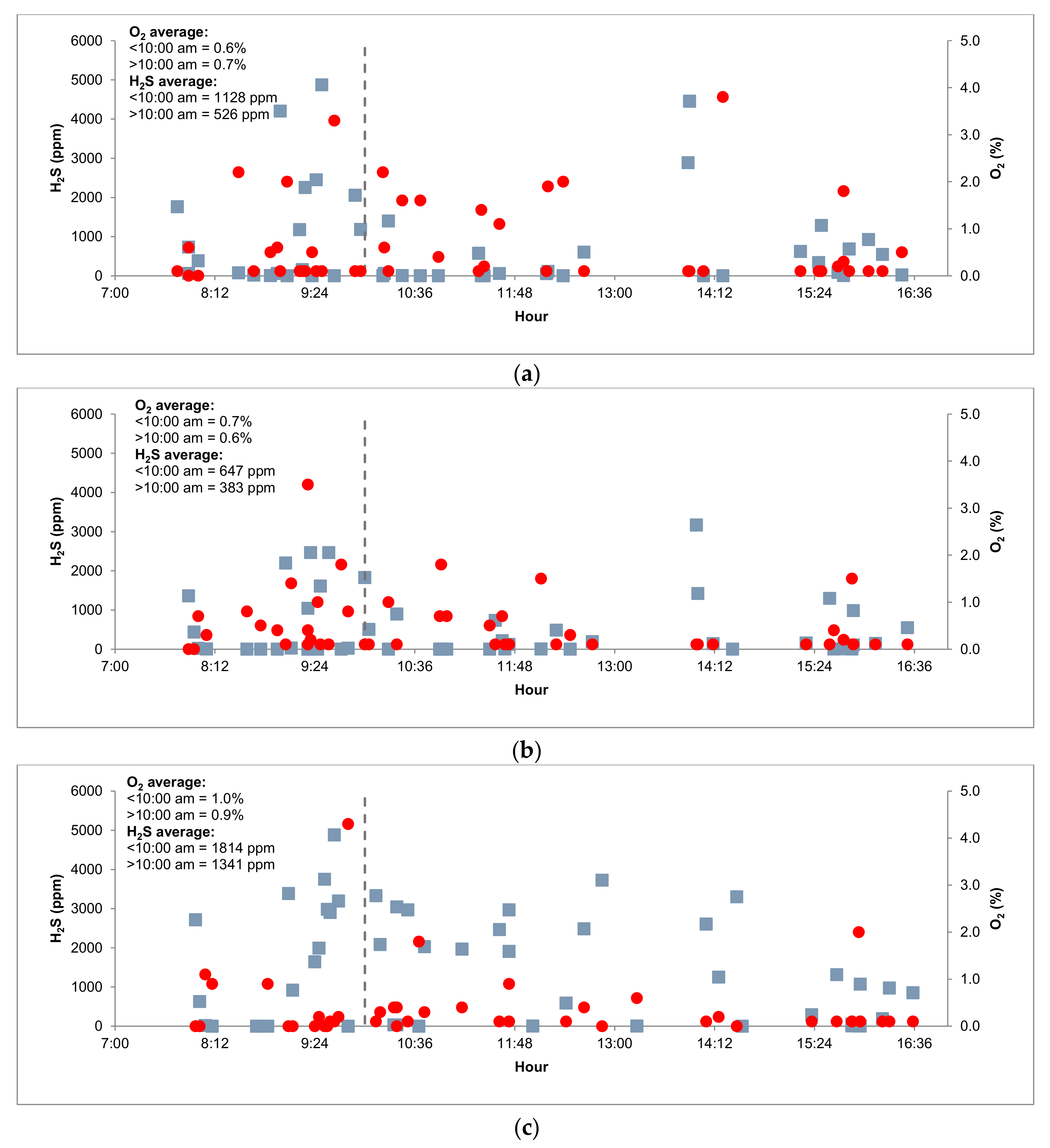
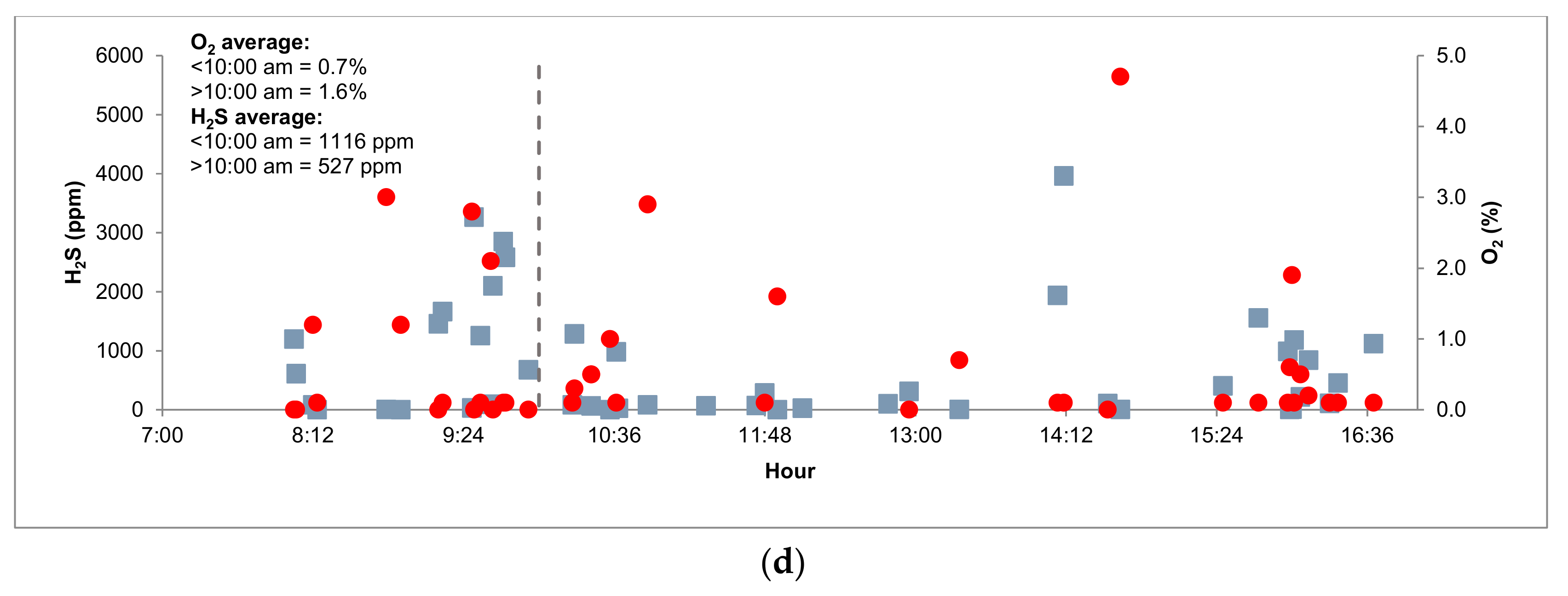
3.3. Pilot Plant Monitoring
3.3.1. Biogas Composition and Organic Matter Removal
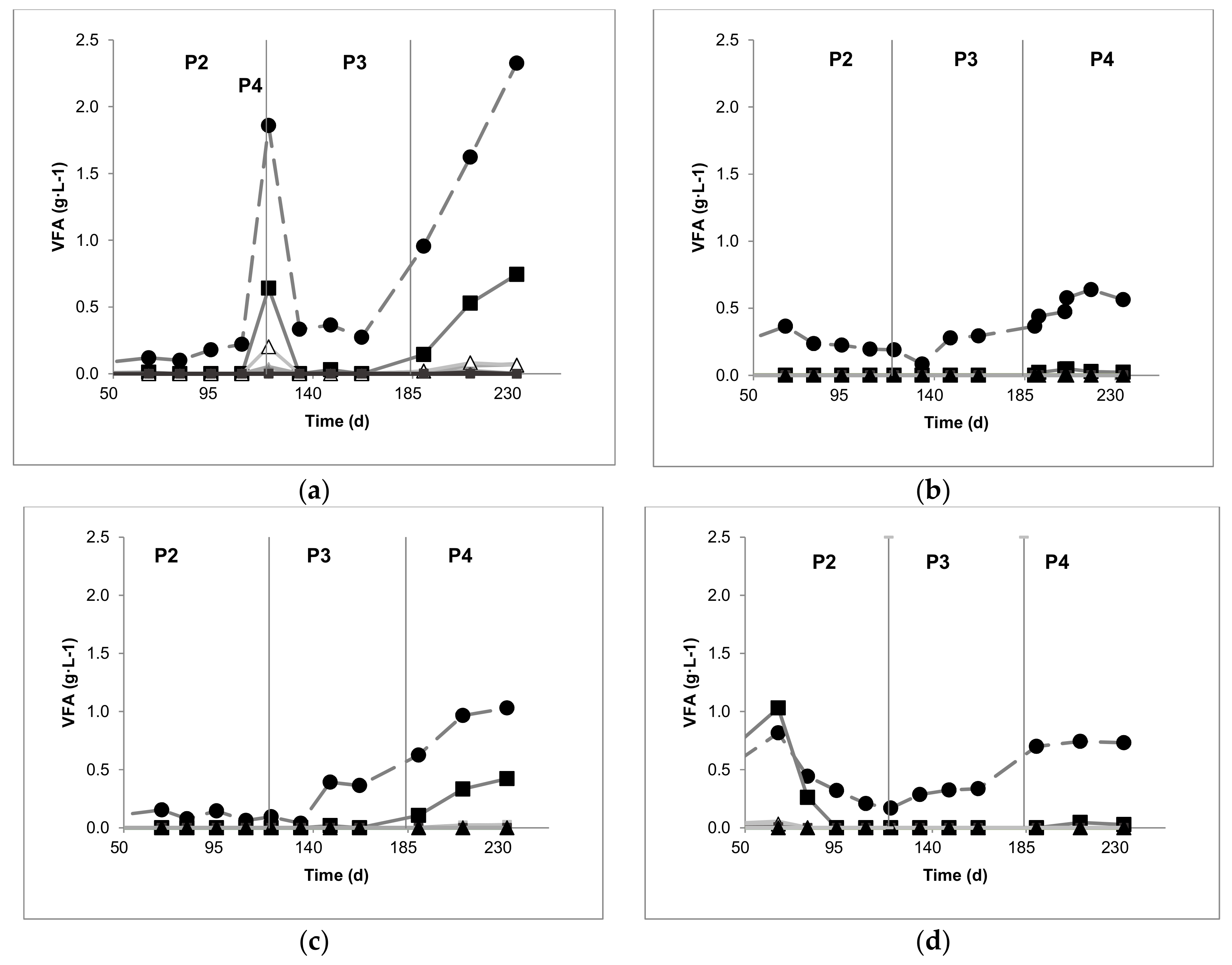
3.3.2. VFA, pH, and Alkalinity Profiles
3.3.3. Nitrogen (TKN, TAN) Profile
3.3.4. Effect of Recirculation
3.3.5. Effect of the Organic Loading Rate
3.3.6. Effect of Total Solid Content
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lukehurst, C.T.; Frost, P.; Al Seadi, T. Utilisation of digestate from biogas plants as biofertiliser. IEA Bioenergy 2010, 2010, 1–36. [Google Scholar]
- De Mes, T.Z.D.; Stams, A.J.M.; Reith, J.H.; Zeeman, G. Chapter 4: Methane production by anaerobic digestion of wastewater and solid wastes. In Bio-Methane Bio-Hydrogen: Status and Perspectives of Biological Methane and Hydrogen Production; Dutch Biological Hydrogen Foundation: The Hague, The Netherlands, 2003; pp. 58–102. [Google Scholar]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A.; Makaka, G.; Simon, M.; Manyi-Loh, C.E.; Mamphweli, S.N.; Meyer, E.L.; Okoh, A.I.; et al. Microbial Anaerobic Digestion (Bio-Digesters) as an Approach to the Decontamination of Animal Wastes in Pollution Control and the Generation of Renewable Energy. Int. J. Environ. Res. Public Health 2013, 10, 4390–4417. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Crittenden, J.C.; Montgomery, W.H. Water Treatment Principles and Design; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Wen, Z.; Frear, C.; Chen, S. Anaerobic digestion of liquid dairy manure using a sequential continuous-stirred tank reactor system. J. Chem. Technol. Biotechnol. 2007, 82, 758–766. [Google Scholar] [CrossRef]
- Burke, D.A. Dairy Waste Anaerobic Digestion Handbook; Environmental Energy Company: Olympia, WA, USA, 2001; Volume 6007, pp. 17–27. [Google Scholar]
- Krich, K.; Augenstein, D.; Batmale, J.P.; Benemann, J.; Rutledge, B.; Salour, D. Chapter 8 Financial Analysis of Biomethane Production. In Biomethane from Dairy Waste. A Sourcebook for the Production and Use of Renewable Natural Gas in California; Western United Dairymen: Modesto, CA, USA, 2005; pp. 147–162. [Google Scholar]
- Li, Y.-F.; Chen, P.-H.; Yu, Z. Spatial and temporal variations of microbial community in a mixed plug-flow loop reactor fed with dairy manure. Microb. Biotechnol. 2014, 7, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Azbar, N.; Ursillo, P.; Speece, R.E. Effect of process configuration and substrate complexity on the performance of anaerobic processes. Water Res. 2001, 35, 817–829. [Google Scholar] [CrossRef]
- Batstone, D.J.; Puyol, D.; Flores-Alsina, X.; Rodríguez, J. Mathematical modelling of anaerobic digestion processes: Applications and future needs. Rev. Environ. Sci. Bio/Technol. 2015, 14, 595–613. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Ducey, T.; Ro, K.S.; Hunt, P.G. Livestock waste-to-bioenergy generation opportunities. Bioresour. Technol. 2008, 99, 7941–7953. [Google Scholar] [CrossRef]
- Speece, R.E.; Duran, M.; Demirer, G.; Zhang, H.; DiStefano, T. The role of process configuration in the performance of anaerobic systems. Water Sci. Technol. 1997, 36, 539–547. [Google Scholar] [CrossRef]
- Monnet, F. An introduction to anaerobic digestion of organic wastes. Remade Scotl. 2003, 379, 1–48. [Google Scholar]
- Wilkie, A.C. Anaerobic digestion of dairy manure: Design and process considerations. Dairy Manure Manag. Treat. Handl. Community Relat. 2005, 301, 312. [Google Scholar]
- Dvorak, S.; Frear, C. Commercial Demonstration of Nutrient Recovery of Ammonium Sulfate and Phosphorous Rich Fines from AD Effluent. 2014. Available online: http://hdl.handle.net/1813/36526 (accessed on 7 July 2019).
- Lansing, S.; Martin, J.F.; Botero, R.B.; da Silva, T.N.; da Silva, E.D. Methane production in low-cost, unheated, plug-flow digesters treating swine manure and used cooking grease. Bioresour. Technol. 2010, 101, 4362–4370. [Google Scholar] [CrossRef] [PubMed]
- Lansing, S.; Botero, R.B.; Martin, J.F. Waste treatment and biogas quality in small-scale agricultural digesters. Bioresour. Technol. 2008, 99, 5881–5890. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H. An Evaluation of a Mesophilic, Modified Plug Flow Anaerobic Digester for Dairy Cattle Manure; EPA Contract No. GS 10F-0036K Work Assignment/Task Order 9; East. Res. Group, Inc.: Morrisville, NC, USA, 2005. [Google Scholar]
- Martin, J.H. Comparison of Dairy Cattle Manure Management with and without Anaerobic Digestion and Biogas Utilization; Eastern Research Group, Inc.: Boston MA for AgStar Program; U.S. Environmental Protection Agency: Washington, DC, USA, 2003. [Google Scholar]
- St-Pierre, B.; Wright, A.-D.G. Metagenomic analysis of methanogen populations in three full-scale mesophilic anaerobic manure digesters operated on dairy farms in Vermont, USA. Bioresour. Technol. 2013, 138, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Morrison, M.; Schanbacher, F.L. Production and utilization of methane biogas as renewable fuel. Biomass to biofuels Strateg. Glob. Ind. 2010, 403–433. [Google Scholar] [CrossRef]
- Beddoes, J.C.; Bracmort, K.S.; Burns, R.T.; Lazarus, W.F. An analysis of energy production costs from anaerobic digestion systems on US livestock production facilities. USDA NRCS Tech. Note 2007, 1, 7–10. [Google Scholar]
- Chynoweth, D.P.; Isaacson, R. Anaerobic Digestion of Biomass; Chynoweth, D.P., Isaacson, R., Eds.; Elsevier applied Science; Springer: Dordrecht, The Netherlands, 1987; ISBN 978-1-85166-069-8. [Google Scholar]
- Jagadish, K.; Chanakya, H.; Rajabapaiah, P.; Anand, V. Plug flow digestors for biogas generation from leaf biomass. Biomass Bioenergy 1998, 14, 415–423. [Google Scholar] [CrossRef]
- Karim, K.; Thomas Klasson, K.; Hoffmann, R.; Drescher, S.R.; DePaoli, D.W.; Al-Dahhan, M.H. Anaerobic digestion of animal waste: Effect of mixing. Bioresour. Technol. 2005, 96, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Meroney, R.N.; Colorado, P.E. CFD simulation of mechanical draft tube mixing in anaerobic digester tanks. Water Res. 2009, 43, 1040–1050. [Google Scholar] [CrossRef] [PubMed]
- Stalin, N.; Prabhu, H.J. Performance evaluation of partial mixing anaerobic digester. ARPN J. Eng. Appl. Sci. 2007, 2, 1–6. [Google Scholar]
- Vesvikar, M. Understanding the Hydrodynamics and Performance of Anaerobic Digesters, Chemical Reaction Engineering Laboratory; Washingt. Univ.: St. Louis, MO, USA, 2007. [Google Scholar]
- Kaparaju, P.; Buendia, I.; Ellegaard, L.; Angelidakia, I. Effects of mixing on methane production during thermophilic anaerobic digestion of manure: Lab-scale and pilot-scale studies. Bioresour. Technol. 2008, 99, 4919–4928. [Google Scholar] [CrossRef] [PubMed]
- Agency, E.P. EPA Design Information Report–anaerobic digester mixing systems. J. Water Pollut. Control Fed. 1987, 59, 162–170. [Google Scholar]
- Wu, B. Large eddy simulation of mechanical mixing in anaerobic digesters. Biotechnol. Bioeng. 2012, 109, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Couper, R.; Penney, W.R.; Fair, J.R.; Walas, S.M. Chemical Process Equipment, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2005; ISBN 978-0-7506-7510-9. [Google Scholar]
- Rodríguez-Abalde, Á.; Fernández García, B.; Flotats Ripoll, X. Effect of thermal pre-treatments on biogas production potential of solid slaughterhouse wastes. In Proceedings of the 17th European Biomass Conference & Exhibition—From Research to Industry and Markets, Hamburg, Germany, 29 June–3 July 2009; pp. 252–256. [Google Scholar]
- Soto, M.; Méndez, R.; Lema, J.M. Methanogenic and non-methanogenic activity tests. Theoretical basis and experimental set up. Water Res. 1993, 27, 1361–1376. [Google Scholar] [CrossRef]
- Li, P.; Li, W.; Sun, M.; Xu, X.; Zhang, B.; Sun, Y. Evaluation of Biochemical Methane Potential and Kinetics on the Anaerobic Digestion of Vegetable Crop Residues. Energies 2019, 12, 26. [Google Scholar] [CrossRef]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Association, A.P.H.; Association, A.W.W.; Federation, W.P.C.; Federation, W.E. Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Noguerol-Arias, J.; Rodríguez-Abalde, A.; Romero-Merino, E.; Flotats, X. Determination of Chemical Oxygen Demand in Heterogeneous Solid or Semisolid Samples Using a Novel Method Combining Solid Dilutions as a Preparation Step Followed by Optimized Closed Reflux and Colorimetric Measurement. Anal. Chem. 2012, 84, 5548–5555. [Google Scholar] [CrossRef]
- Kinyua, M.N.; Rowse, L.E.; Ergas, S.J. Review of small-scale tubular anaerobic digesters treating livestock waste in the developing world. Renew. Sustain. Energy Rev. 2016, 58, 896–910. [Google Scholar] [CrossRef]
- Agency, E.P. Anaerobic Digester Conservation Practice Standard Number 366; USDA Natural Resources Conservation Service: New York, NY, USA, 2009. [Google Scholar]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Methane productivity of manure, straw and solid fractions of manure. Biomass Bioenergy 2004, 26, 485–495. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Flotats, X.; Bonmatí, A.; Fernández, B.; Magrí, A. Manure treatment technologies: On-farm versus centralized strategies. NE Spain as case study. Bioresour. Technol. 2009, 100, 5519–5526. [Google Scholar] [CrossRef]
- Hreiz, R.; Adouani, N.; Fünfschilling, D.; Marchal, P.; Pons, M.-N. Rheological characterization of raw and anaerobically digested cow slurry. Chem. Eng. Res. Des. 2017, 119, 47–57. [Google Scholar] [CrossRef]
- Nagy, G.; Wopera, A. Biogas production from pig slurry-feasibility and challenges. Mat. Sci. Eng. 2012, 37, 65–75. [Google Scholar]
- Muzondiwa, R.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Sunada, N.S.; Amorim, A.C.; Previdelli, M.A.; Miranda, F.; Garófallo, R.; Mendes, A.R. Potential of biogas and methane production from anaerobic of poultry slaughterhouse effluent. R. Bras. Zootec. 2012, 41. [Google Scholar] [CrossRef]
- Donoso-Bravo, A.; Sadino-Riquelme, C.; Gómez, D.; Segura, C.; Valdebenito, E.; Hansen, F. Modelling of an anaerobic plug-flow reactor. Process analysis and evaluation approaches with non-ideal mixing coniderations. Bioresour. Technol. 2018, 260, 95–104. [Google Scholar] [CrossRef]
- El-Mashad, H.M.; Zhang, R.; Arteaga, V.; Rumsey, T.; Mitloehner, F.M. Volatile Fatty acids and Alcohols Production during Anaerobic Storage of Dairy Manure. Trans. ASABE 2011, 54, 599–607. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Saleart, T. Effects of sludge recirculation rate and mixing time on performance of a prototype single-stage anaerobic digester for conversion of food wastes to biogas and energy recovery. Environ. Sci. Pollut. Res. 2016, 23, 7092–7098. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Boe, K.; Ellegaard, L. Effect of operating conditions and reactor configuration on efficiency of full-scale biogas plants. Water Sci. Technol. 2005, 52, 189–194. [Google Scholar] [CrossRef]
- Michele, P.; Giuliana, D.; Carlo, M.; Sergio, S.; Fabrizio, A. Optimization of solid state anaerobic digestion of the OFMSW by digestate recirculation: A new approach. Waste Manag. 2015, 35, 111–118. [Google Scholar] [CrossRef]
- Page, L.H.; Ni, J.Q.; Heber, A.J.; Mosier, N.S.; Liu, X.; Joo, H.S.; Ndegwa, P.M.; Harrison, J.H. Characteristics of volatile fatty acids in stored dairy manure before and after anaerobic digestion. Biosyst. Eng. 2014, 118, 16–28. [Google Scholar] [CrossRef]
| Fresh Manure (FM) | FM1 | FM2 | FM3 | FM4 | FM5 | Averages |
|---|---|---|---|---|---|---|
| COD (gO2 kg−1) | 120.5 | 96.1 | 90.4 | 83.1 | 113.5 | 100.7 ± 14.1 |
| TS (g kg−1) | 105.1 | 105.6 | 99.3 | 104.4 | 113.0 | 105.5 ± 4.4 |
| VS (g kg−1) | 75.2 | 84.3 | 79.3 | 75.7 | 80.0 | 78.9 ± 3.3 |
| TKN (gN kg−1) | 6.0 | 6.1 | 6.0 | 6.8 | 6.3 | 6.2 ± 0.3 |
| TAN (gN kg−1) | 3.7 | 3.8 | 4.0 | 4.5 | 4.2 | 4.0 ± 0.3 |
| Biodegrability (%COD) | 33% | nd | 31% | 47% | nd | 37 ± 7.0 |
| CH4 Yield (m3CH4 kgVS−1) | 0.184 | nd | 0.125 | 0.181 | nd | 0.163 ± 0.027 |
| R (m3CH4 kgCOD−1 d−1) | 5.8 | - | 6.9 | 14.0 | - | 8.9 ± 4.1 |
| λ (d) | 1.9 | - | 14.0 | - | - |
| Parameters | P1 | P2 | P3 | P4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Interval (Days) | 1–56 | 57–120 | 121–186 | 187–255 | ||||||||
| Digester R1 | Av. | Error | Av. | Error | Av. | Error | Av. | Error | ||||
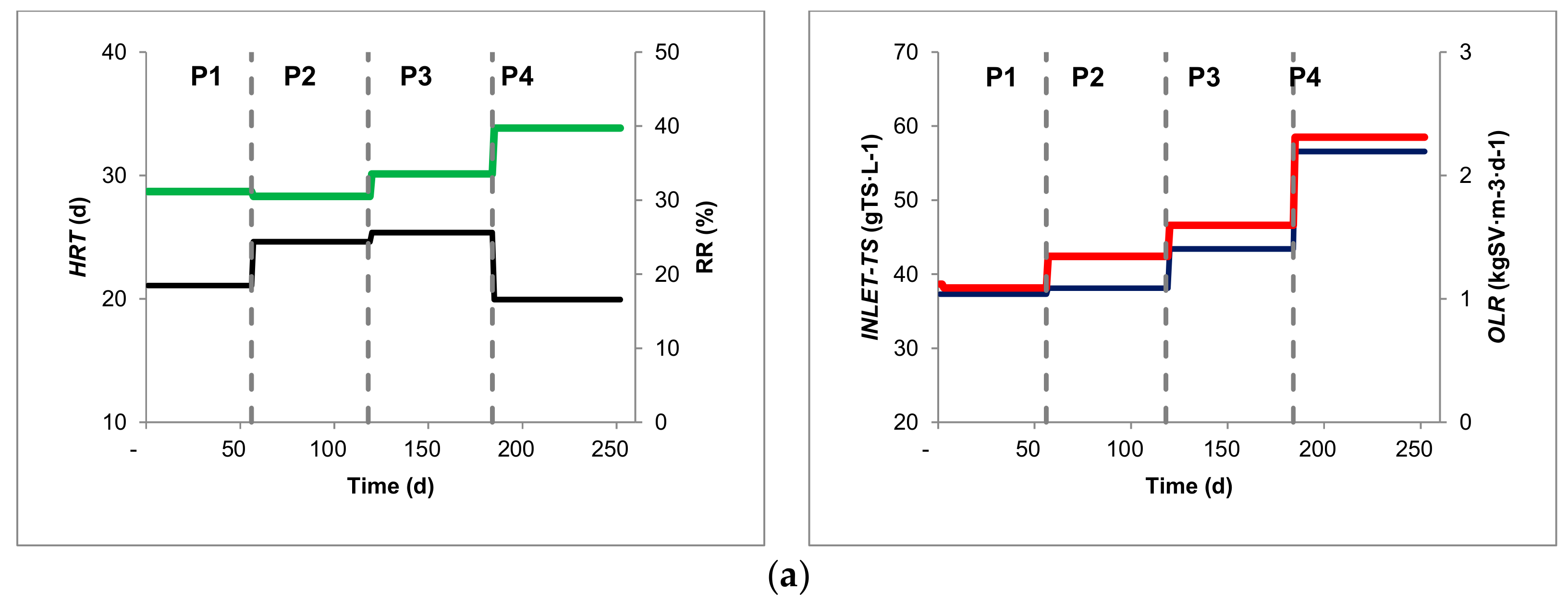
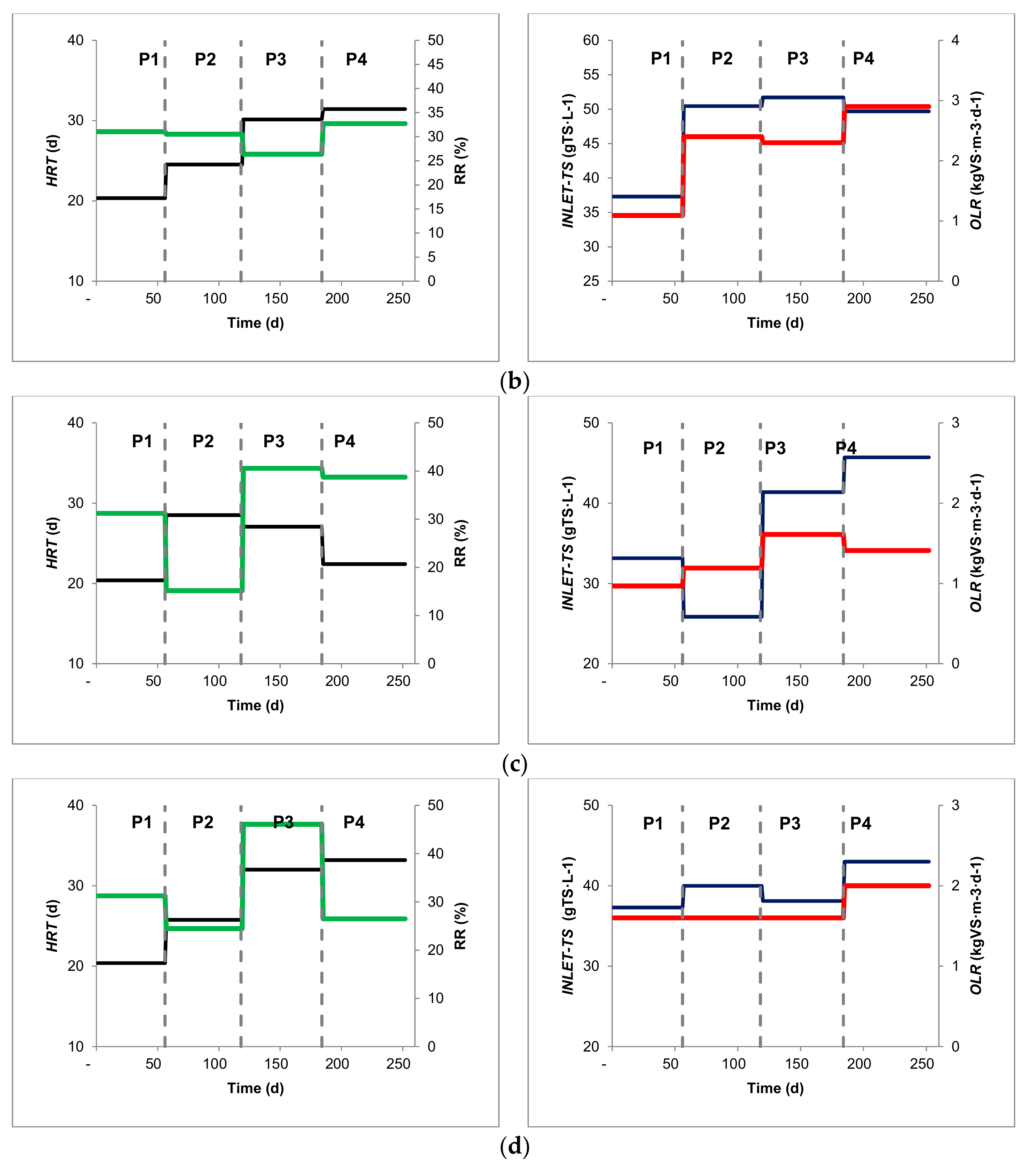
| Inlet-TS (%) | 3.58 | ± | 0.01 | 5.30 | ± | 0.54 | 5.85 | ± | 0.68 | 5.57 | ± | 1.07 |
| HRT (d) | 21.08 | ± | 4.26 | 24.64 | ± | 2.84 | 25.37 | ± | 3.60 | 19.94 | ± | 2.68 |
| RR (%effluent) | 31.17 | ± | 6.23 | 30.52 | ± | 4.15 | 33.55 | ± | 5.51 | 39.74 | ± | 4.59 |
| OLR (kgVS m−3 d−1) | 1.12 | ± | 0.22 | 1.39 | ± | 0.15 | 1.50 | ± | 0.24 | 1.92 | ± | 0.29 |
| Digester R2 | ||||||||||||
| Inlet TS (%) | 3.68 | ± | 0.05 | 5.64 | ± | 0.57 | 5.45 | ± | 0.99 | 6.21 | ± | 0.33 |
| HRT (d) | 20.35 | ± | 4.08 | 24.54 | ± | 3.00 | 30.15 | ± | 3.82 | 31.44 | ± | 2.54 |
| RR (%effluent) | 31.03 | ± | 6.21 | 30.53 | ± | 3.94 | 26.37 | ± | 4.38 | 32.71 | ± | 4.93 |
| OLR (kgVS m−3 d−1) | 1.14 | ± | 0.23 | 2.40 | ± | 0.13 | 2.30 | ± | 0.60 | 2.90 | ± | 0.50 |
| Digester R3 | ||||||||||||
| Inlet TS (%) | 3.57 | ± | 0.00 | 5.60 | ± | 0.86 | 6.81 | ± | 1.35 | 3.43 | ± | 0.18 |
| HRT (d) | 20.39 | ± | 4.09 | 28.51 | ± | 4.41 | 27.06 | ± | 4.37 | 22.41 | ± | 2.24 |
| RR (%effluent) | 31.21 | ± | 6.24 | 15.17 | ± | 16.15 | 40.57 | ± | 7.99 | 38.73 | ± | 4.89 |
| OLR (kgVS m−3 d−1) | 1.11 | ± | 0.22 | 1.21 | ± | 0.18 | 1.61 | ± | 0.33 | 1.41 | ± | 0.17 |
| Digester R4 | ||||||||||||
| Inlet TS (%) | 3.10 | ± | 0.00 | 5.10 | ± | 0.40 | 5.70 | ± | 0.69 | 6.12 | ± | 0.31 |
| HRT (d) | 20.39 | ± | 4.09 | 25.76 | ± | 3.57 | 32.01 | ± | 4.07 | 33.19 | ± | 3.07 |
| RR (%effluent) | 31.23 | ± | 6.25 | 24.46 | ± | 4.17 | 46.08 | ± | 9.81 | 26.50 | ± | 4.78 |
| OLR (kgVS m−3 d−1) | 1.60 | ± | 0.20 | 1.60 | ± | 0.30 | 1.60 | ± | 0.10 | 2.00 | ± | 0.20 |
| Parameters | P1 | P2 | P3 | P4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time Interval (Days) | 1–56 | 57–120 | 121–186 | 187–255 | ||||||||
| Digester R1 | Av. | Error | Av. | Error | Av. | Error | Av. | Error | ||||
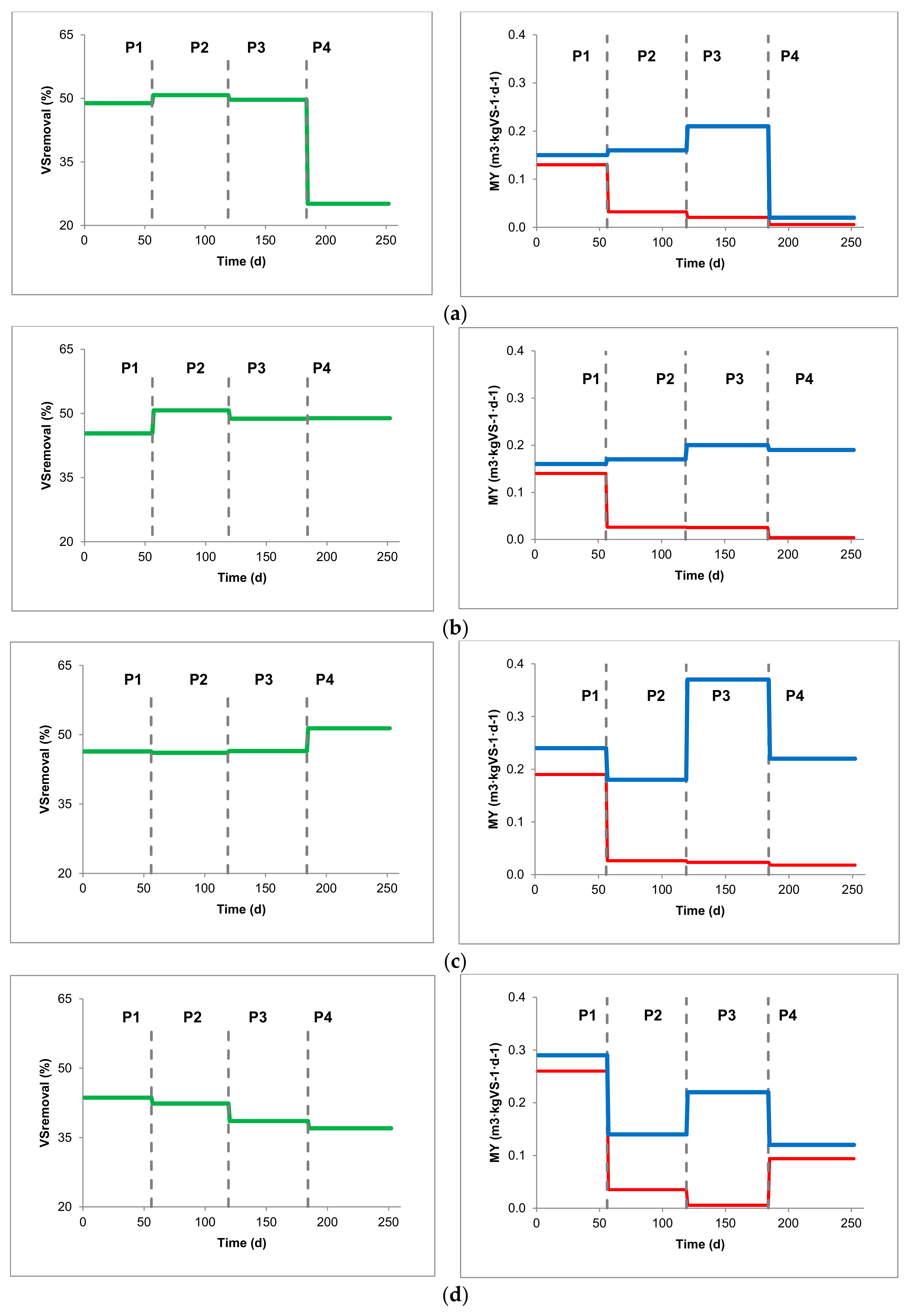
| MY (m3CH4 kgVS−1) | 0.15 | ± | 0.02 | 0.16 | ± | 0.10 | 0.21 | ± | 0.10 | 0.02 | ± | 0.02 |
| VMY (m3CH4 m−3 d−1) | 0.19 | ± | 0.01 | 0.21 | ± | 0.12 | 0.30 | ± | 0.14 | 0.04 | ± | 0.04 |
| CH4 (%) | 63.01 | ± | 1.52 | 62.97 | ± | 3.34 | 61.52 | ± | 1.62 | 57.22 | ± | 2.02 |
| O2 (%) | 0.60 | ± | 1.12 | 0.26 | ± | 0.32 | 0.71 | ± | 0.71 | 1.14 | ± | 1.15 |
| VSremoval (%VS inlet) | 48.86 | ± | 16.53 | 50.77 | ± | 18.08 | 49.68 | ± | 16.70 | 25.12 | ± | 30.01 |
| Digester R2 | ||||||||||||
| MY (m3CH4 kgVS−1) | 0.16 | ± | 0.02 | 0.17 | ± | 0.04 | 0.20 | ± | 0.06 | 0.19 | ± | 0.04 |
| VMY (m3CH4 m−3 d−1) | 0.20 | ± | 0.01 | 0.22 | ± | 0.06 | 0.25 | ± | 0.10 | 0.23 | ± | 0.06 |
| CH4 (%) | 61.32 | ± | 2.10 | 62.06 | ± | 3.75 | 62.90 | ± | 1.49 | 58.81 | ± | 3.32 |
| O2 (%) | 0.80 | ± | 0.54 | 0.31 | ± | 0.37 | 0.43 | ± | 0.42 | 1.04 | ± | 1.40 |
| VSremoval (%VS inlet) | 45.34 | ± | 9.44 | 50.72 | ± | 10.05 | 48.78 | ± | 13.83 | 48.91 | ± | 14.08 |
| Digester R3 | ||||||||||||
| MY (m3CH4 kgVS−1) | 0.24 | ± | 0.03 | 0.18 | ± | 0.12 | 0.37 | ± | 0.05 | 0.22 | ± | 0.11 |
| VMY (m3CH4 m−3 d−1) | 0.30 | ± | 0.02 | 0.21 | ± | 0.13 | 0.57 | ± | 0.04 | 0.42 | ± | 0.13 |
| CH4 (%) | 60.12 | ± | 2.21 | 59.26 | ± | 3.29 | 62.51 | ± | 2.33 | 59.38 | ± | 4.89 |
| O2 (%) | 0.41 | ± | 0.76 | 0.45 | ± | 0.57 | 0.23 | ± | 0.32 | 1.06 | ± | 2.50 |
| VSremoval (%VS inlet) | 46.38 | ± | 20.11 | 46.08 | ± | 29.33 | 46.47 | ± | 20.03 | 51.38 | ± | 26.01 |
| Digester R4 | ||||||||||||
| MY (m3CH4 kgVS−1) | 0.29 | ± | 0.03 | 0.14 | ± | 0.11 | 0.22 | ± | 0.04 | 0.12 | ± | 0.04 |
| VMY (m3CH4 m−3 d−1) | 0.23 | ± | 0.01 | 0.16 | ± | 0.13 | 0.26 | ± | 0.05 | 0.16 | ± | 0.05 |
| CH4 (%) | 56.49 | ± | 1.76 | 57.54 | ± | 1.95 | 55.01 | ± | 5.01 | 57.23 | ± | 4.34 |
| O2 (%) | 1.52 | ± | 0.81 | 0.43 | ± | 0.44 | 3.13 | ± | 3.34 | 0.63 | ± | 1.24 |
| VSremoval (%VS inlet) | 43.62 | ± | 20.75 | 42.36 | ± | 32.29 | 38.57 | ± | 14.66 | 37.01 | ± | 18.53 |
| Parameter | Period | OLR (1) | HRT (d) | VMY (2) | MY (3) | RR (%) | NTK (4) | TAN (4) | pH | IA (5) | VFA (6) | VS-Rem (%) | CH4 (%) | CO2 (%) | H2S (ppm) | COD-Rem (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | 1 | 0.411 ** | −0.086 | −0.115 | −0.168 | 0.370 ** | 0.306 * | 0.079 | −0.301* | 0.461 ** | 0.412 ** | −0.213 * | −0.356 * | 0.344 * | 0.535** | −0.218 * |
| OLR (1) | 1 | −0.800 ** | −0.150 | −0.279 | 0.438 ** | 0.636 ** | 0.139 | 0.044 | 0.392 ** | 0.684 ** | −0.331 * | 0.062 | −0.073 | 0.467 ** | −0.339 ** | |
| HRT (d) | 1 | 0.190 | 0.311 * | −0.323 * | −0.567 ** | −0.175 | −0.100 | −0.308 * | −0.582** | 0.331 * | −0.201 | 0.205 | −0.142 | 0.279 | ||
| VMY (2) | 1 | 0.972 ** | 0.051 | −0.448 ** | −0.243 | −0.240 | −0.191 | −0.275 | 0.596 ** | −0.001 | −0.005 | 0.271 | 0.812 ** | |||
| MY (3) | 1 | −0.053 | −0.502 ** | −0.299 * | −0.247 | −0.233 | −0.329 * | 0.658 ** | −0.073 | 0.070 | 0.168 | 0.866 ** | ||||
| RR (%) | 1 | 0.236 | −0.004 | 0.021 | 0.159 | 0.217 | −0.156 | 0.030 | −0.038 | 0.364 * | −0.136 | |||||
| NTK (4) | 1 | −0.204 | 0.028 | 0.259 | 0.515 ** | −0.277 | 0.201 | −0.200 | −0.062 | −0.423 ** | ||||||
| TAN (4) | 1 | 0.000 | 0.378 * | 0.251 | −0.623 ** | −0.134 | 0.129 | 0.220 | −0.474 ** | |||||||
| pH | 1 | −0.277 | 0.018 | −0.122 | 0.008 | −0.002 | −0.251 | −0.343 * | ||||||||
| IA (5) | 1 | 0.682 ** | −0.367 * | −0.283 | 0.277 | 0.219 | −0.266 | |||||||||
| VFA (6) | 1 | −0.465 ** | −0.217 | 0.212 | 0.217 | −0.362 * | ||||||||||
| VS-Rem (%) | 1 | 0.112 | −0.113 | 0.037 | 0.818 ** | |||||||||||
| CH4 (%) | 1 | −1.000 ** | −0.045 | −0.019 | ||||||||||||
| CO2 (%) | 1 | 0.023 | 0.018 | |||||||||||||
| H2S (ppm) | 1 | 0.034 | ||||||||||||||
| COD-Rem (%) | 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, D.; Ramos-Suárez, J.L.; Fernández, B.; Muñoz, E.; Tey, L.; Romero-Güiza, M.; Hansen, F. Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures. Energies 2019, 12, 2628. https://doi.org/10.3390/en12132628
Gómez D, Ramos-Suárez JL, Fernández B, Muñoz E, Tey L, Romero-Güiza M, Hansen F. Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures. Energies. 2019; 12(13):2628. https://doi.org/10.3390/en12132628
Chicago/Turabian StyleGómez, Daniel, Juan Luis Ramos-Suárez, Belén Fernández, Eduard Muñoz, Laura Tey, Maycoll Romero-Güiza, and Felipe Hansen. 2019. "Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures" Energies 12, no. 13: 2628. https://doi.org/10.3390/en12132628
APA StyleGómez, D., Ramos-Suárez, J. L., Fernández, B., Muñoz, E., Tey, L., Romero-Güiza, M., & Hansen, F. (2019). Development of a Modified Plug-Flow Anaerobic Digester for Biogas Production from Animal Manures. Energies, 12(13), 2628. https://doi.org/10.3390/en12132628







