Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent †
Abstract
1. Introduction
2. Material and Methods
2.1. Seed Sludge and Substrate
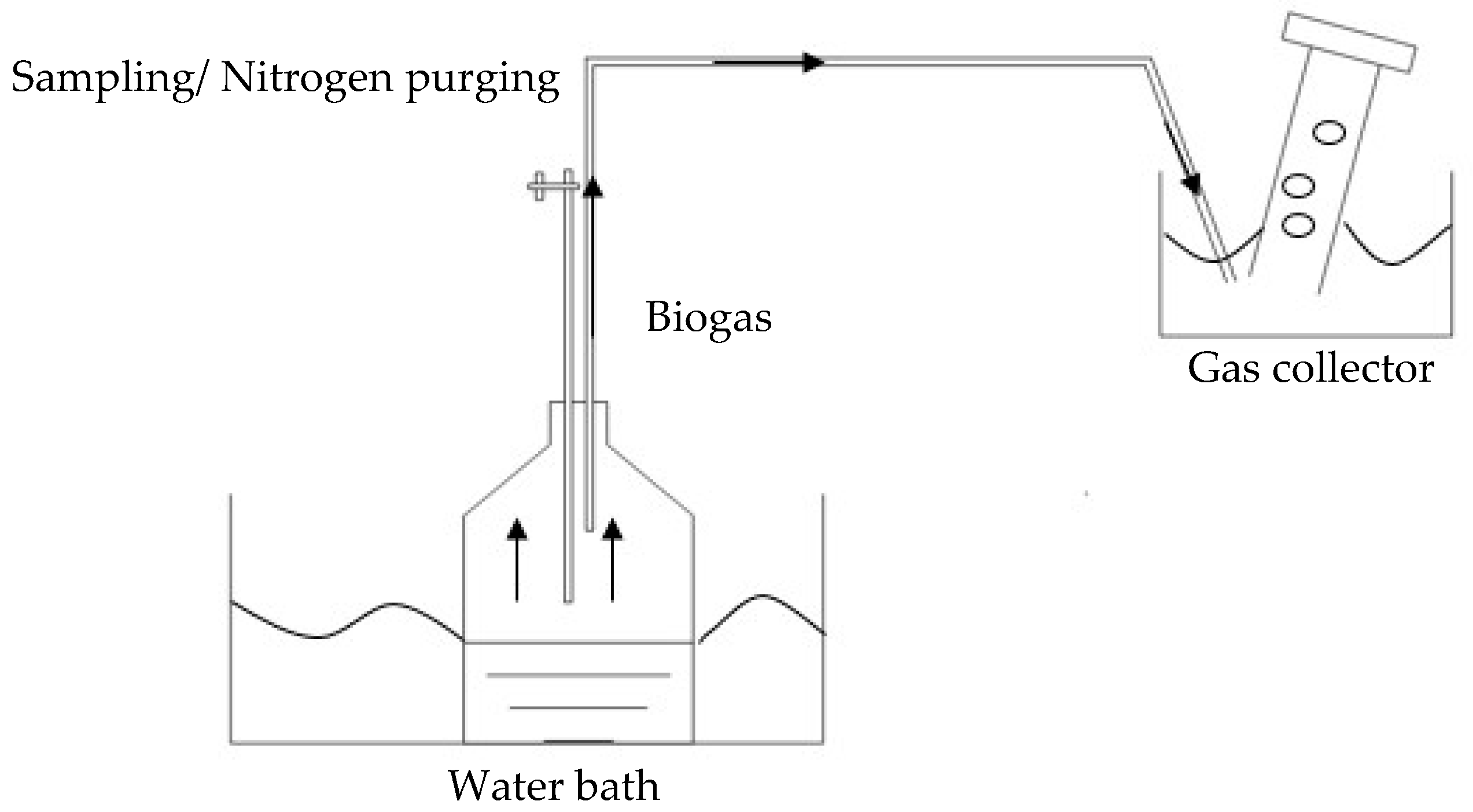
2.2. Experimental Set-Up and Digester Operation
2.3. Analytical Method
3. Results and Discussion
3.1. Start-Up Experiments
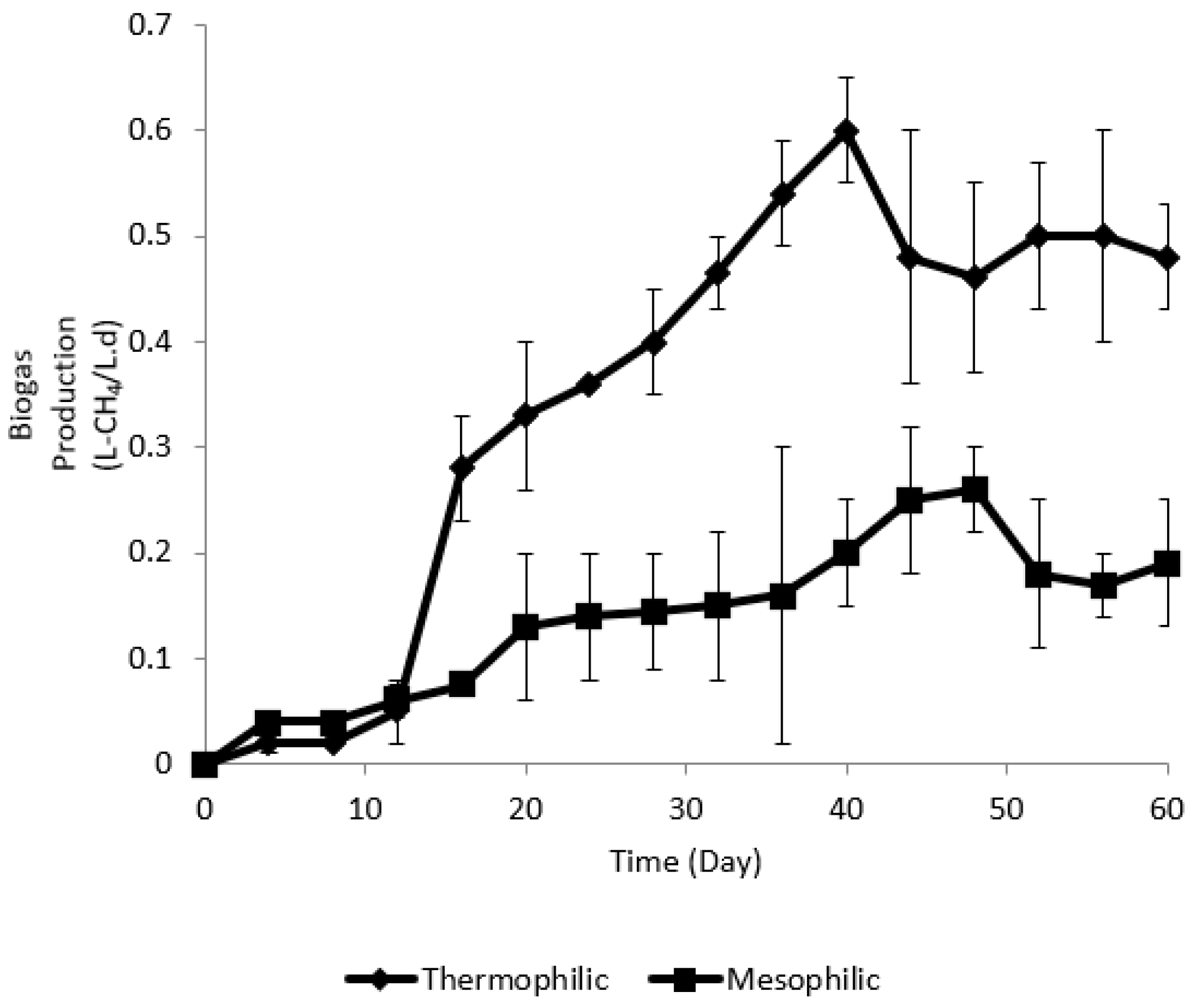
3.2. Biogas Productivity
3.3. Substrate Removal
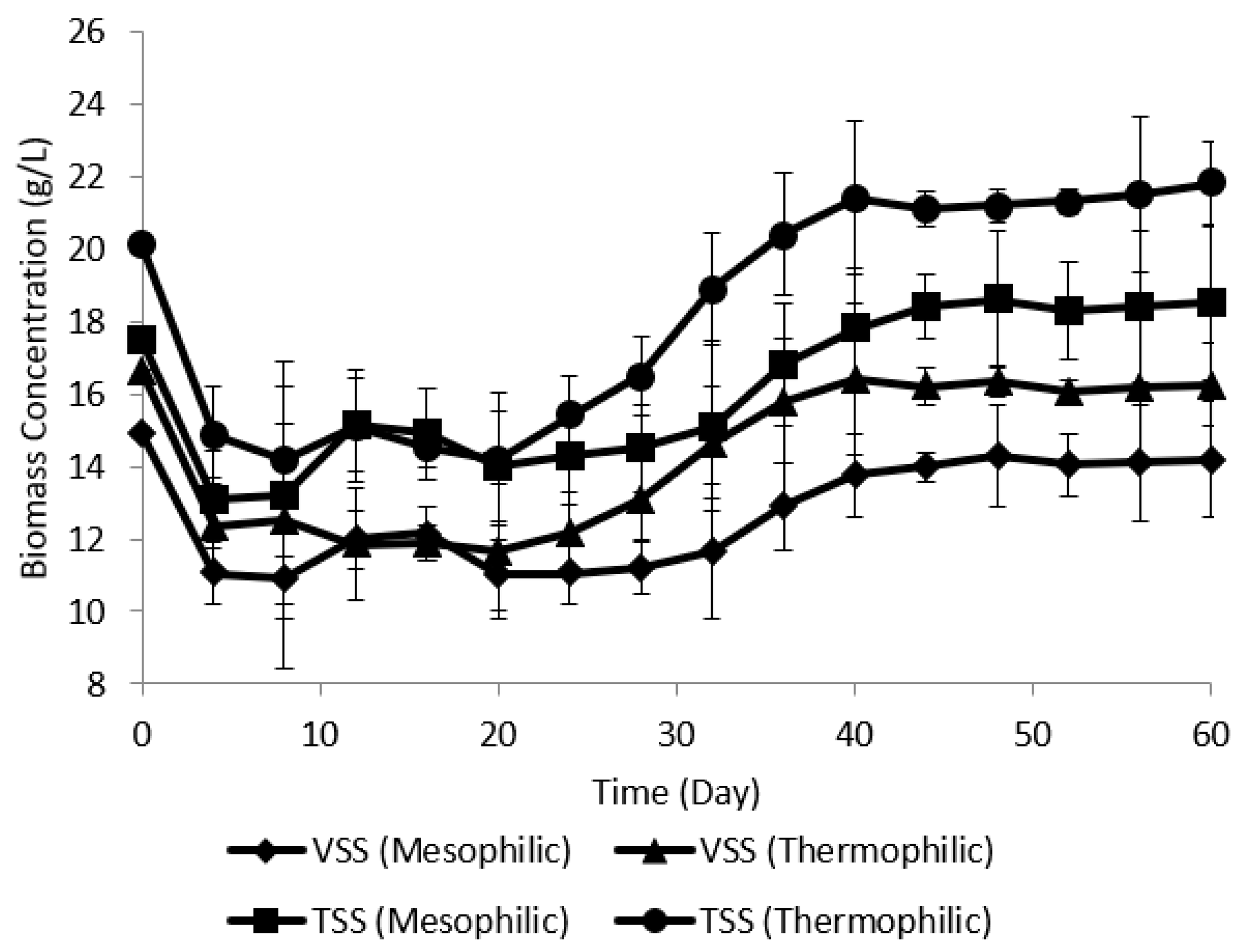
3.4. Variation of Volatile Suspended Solid and Total Suspended Solid
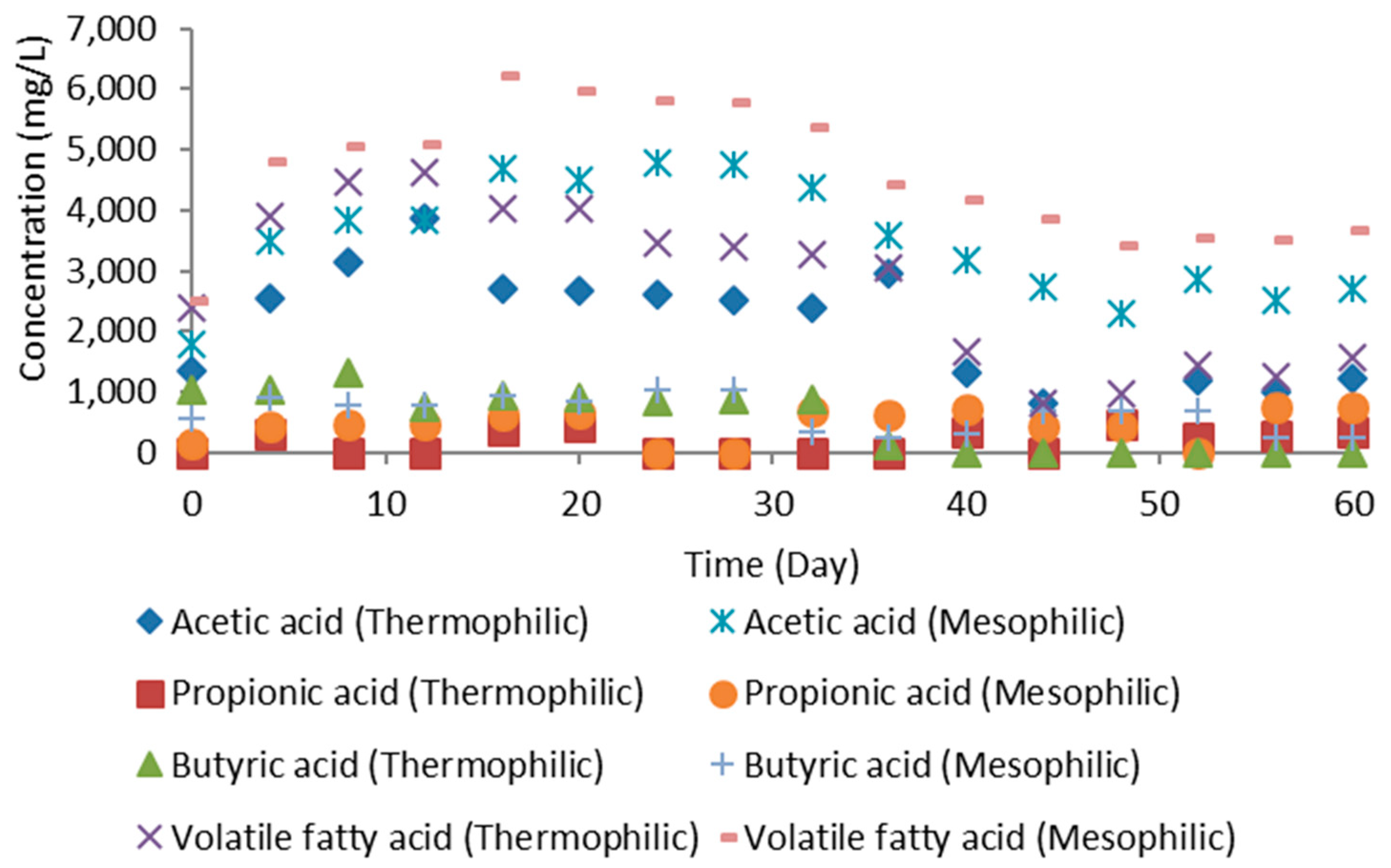
3.5. Volatile Fatty Acids Production
3.6. Stability of The Digesters
3.7. Total Alkalinity
3.8. Overall Performance
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Production of Crude Palm Oil for the Month of January–December 2016 & 2017. 2018. Available online: http://bepi.mpob.gov.my/index.php/en/statistics/production/177-production-2017/792-production-of-crude-oil-palm-2017.html (accessed on 30 October 2018).
- Maaroff, R.M.; Md Jahim, J.; Azahar, A.M.; Abdul, P.M.; Masdar, M.S.; Nordin, D.; Abd Nasir, M.A. Biohydrogen production from palm oil mill effluent (POME) by two stage anaerobic sequencing batch reactor (ASBR) system for better utilization of carbon sources in POME. Int. J. Hydrogen Energy 2018, 44, 3395–3406. [Google Scholar] [CrossRef]
- Hanum, F.; Yuan, L.C.; Kamahara, H.; Aziz, H.A.; Atsuta, Y.; Yamada, T.; Daimon, H. Treatment of sewage sludge using anaerobic digestion in Malaysia: Current state and challenges. Front. Energy Res. 2019, 7. [Google Scholar] [CrossRef]
- Chin, M.J.; Poh, P.E.; Tey, B.T.; Chan, E.S.; Chin, K.L. Biogas from palm oil mill effluent (POME): Opportunities and challenges from Malaysia’s perspective. Renew. Sustain. Energy Rev. 2013, 26, 717–726. [Google Scholar] [CrossRef]
- Wu, T.Y.; Mohammad, A.W.; Jahim, J.M.; Anuar, N. Pollution control technologies for the treatment of palm oil mill effluent (POME) through end-of-pipe processes. J. Environ. Manag. 2010, 91, 1467–1490. [Google Scholar] [CrossRef] [PubMed]
- Gebreeyessus, G.; Jenicek, P. Thermophilic versus mesophilic anaerobic digestion of sewage sludge: A comparative review. Bioengineering 2016, 3, 15. [Google Scholar] [CrossRef]
- İnce, E.; İnce, M.; Önkal Engin, G. Comparison of thermophilic and mesophilic anaerobic treatment for potato processing wastewater using a contact reactor. Glob. NEST J. 2017, 19, 318–326. [Google Scholar]
- Jeong, J.-Y.; Son, S.-M.; Pyon, J.-H.; Park, J.-Y. Performance comparison between mesophilic and thermophilic anaerobic reactors for treatment of palm oil mill effluent. Bioresour. Technol. 2014, 165, 122–128. [Google Scholar] [CrossRef]
- Sulaiman, A.; Tabatabaei, M.; Yusoff, M.Z.M.; Ibrahim, M.F.; Hassan, M.A.; Shirai, Y. Accelerated start-up of a semi-commercial digester tank treating palm oil mill effluent with sludge seeding for methane production. World Appl. Sci. J. 2010, 8, 247–258. [Google Scholar]
- Burak, D.; Orhan, Y. Changes in microbial ecology in an anaerobic reactor. Bioresour. Technol. 2006, 97, 1201–1208. [Google Scholar] [CrossRef]
- Hansen, T.L.; Schmidt, J.E.; Angelidaki, I.; Marca, E.; Jansen, J.C.; Mosbæk, H.; Christensen, T.H. Measurement of methane potentials of solid organic waste. Waste Manag. 2004, 24, 393–400. [Google Scholar] [CrossRef]
- Massé, D.I.; Croteau, F.; Masse, L.; Danesh, S. The effect of scale-up on the digestion of swine manure slurry in psychrophilic anaerobic sequencing batch reactors. Trans. ASAE 2004, 47, 1367–1373. [Google Scholar] [CrossRef]
- Mir, M.A.; Hussain, A.; Verma, C. Design considerations and operational performance of anaerobic digester: A review. Cogent Eng. 2016, 3. [Google Scholar] [CrossRef]
- Alrawi, R.A.; Ahmad, A.; Norli, I.; Mohd Omar, A.K. Methane production during start-up phase of mesophilic semi-continues suspended growth anaerobic digester. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Sidik, U.; Razali, F.; Alwi, S.; Maigari, F. Biogas production through co-digestion of palm oil mill effluent with cow manure. Niger. J. Basic Appl. Sci. 2013, 21, 79–84. [Google Scholar] [CrossRef]
- Ergüder, T.; Tezel, U.; Güven, E.; Demirer, G. Anaerobic biotransformation and methane generation potential of cheese whey in batch and UASB reactors. Waste Manag. 2001, 21, 643–650. [Google Scholar] [CrossRef]
- Badiei, M.; Jahim, J.M.; Anuar, N.; Sheikh Abdullah, S.R. Effect of hydraulic retention time on biohydrogen production from palm oil mill effluent in anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2011, 36, 5912–5919. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA; New York, NY, USA, 2005. [Google Scholar]
- Logan, B.E.; Oh, S.-E.; Kim, I.S.; Van Ginkel, S. Biological hydrogen production measured in batch anaerobic respirometers. Environ. Sci. Technol. 2002, 36, 2530–2535. [Google Scholar] [CrossRef]
- Abd Nasir, M.A.; Jahim, J.M.; Abdul, P.M.; Silvamany, H.; Maaroff, R.M.; Mohammed Yunus, M.F. The use of acidified palm oil mill effluent for thermophilic biomethane production by changing the hydraulic retention time in anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Seadi, T.A.; Rutz, D.; Prassl, H.; Kottner, M.; Finsterwalder, T.; Volk, S.; Janssen, R. Biogas Handbook; University of Southern Denmark: Esbjerg, Denmark, 2008. [Google Scholar]
- Meegoda, J.; Li, B.; Patel, K.; Wang, L. A review of the processes, parameters, and optimization of anaerobic digestion. Int. J. Environ. Res. Public Health 2018, 15, 2224. [Google Scholar] [CrossRef]
- Richards, M.A.; Lie, T.J.; Zhang, J.; Ragsdale, S.W.; Leigh, J.A.; Price, N.D. Exploring hydrogenotrophic. methanogenesis: A genome scale metabolic reconstruction of Methanococcus maripaludis. J. Bacteriol. 2016, 198, 3379–3390. [Google Scholar] [CrossRef]
- Wong, Y.S.; Ong, S.A.; Lim, K.K.; Lee, H.C. Acclimatization and performance study of acidogenesis anaerobic degradation process for palm oil mill effluent. In Proceedings of the 2011 International Conference on Environment and Industrial Innovation (IPCBEE), Singapore, 26–28 February 2011. [Google Scholar]
- Yacob, S.; Shirai, Y.; Hassan, M.A.; Wakisaka, M.; Subash, S. Start-up operation of semi-commercial closed anaerobic digester for palm oil mill effluent treatment. Process. Biochem. 2006, 41, 962–964. [Google Scholar] [CrossRef]
- Enzmann, F.; Mayer, F.; Rother, M.; Holtmann, D. Methanogens: Biochemical background and biotechnological applications. AMB Express 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-Q.; Fang, H.H.; Gu, G.-W. Comparative performance of mesophilic and thermophilic acidogenic upflow reactors. Process. Biochem. 2002, 38, 447–454. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Substrate removal kinetics in an upflow anaerobic sludge blanket reactor decolorising simulated textile wastewater. Process. Biochem. 2005, 40, 1189–1198. [Google Scholar] [CrossRef]
- Echiegu, E.A. Kinetic Models for Anaerobic Fermentation Processes—A Review. Am. J. Biochem. Biotechnol. 2015, 11, 132–148. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.T.; Wong, Y.-S.; Ong, S.-A.; Norhashimah, M.; Rafatullah, M. Start-up operation of anaerobic degradation process for palm oil mill effluent in anaerobic bench scale reactor (ABSR). Procedia Environ. Sci. 2013, 18, 442–450. [Google Scholar] [CrossRef]
- Switzenbaum, M.S.; Jewell, W.J. Anaerobic attached film expanded bed reactor treatment. J. Water Poll. Control. Fed. 1980, 52, 1953–1965. [Google Scholar]
- Idris, N.; Lutpi, N.A.; Wong, Y.S.; Izhar, T.N.T. Acclimatization study for biohydrogen production from palm oil mill effluent (pome) in continuous-flow system. E3S Web Conf. 2018, 34, 2054. [Google Scholar] [CrossRef]
- Sperling, M.V. Activated Sludge and Aerobic Biofilm Reactors; IWA Publishing: London, UK, 2007. [Google Scholar]
- Trisakti, B.; Manalu, V.; Taslim, I.; Turmuzi, M. Acidogenesis of palm oil mill effluent to produce biogas: Effect of hydraulic retention time and pH. Procedia Soc. Behav. Sci. 2015, 195, 2466–2474. [Google Scholar] [CrossRef]
- Song, Y.-C.; Kwon, S.-J.; Woo, J.-H. Mesophilic and thermophilic temperature co-phase anaerobic digestion compared with single-stage mesophilic- and thermophilic digestion of sewage sludge. Water Res. 2004, 38, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food waste to energy: An overview of sustainable approaches for food waste management and nutrient recycling. Biomed. Res. Int. 2017, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Diamantis, V.; Aivasidis, A. Performance of an ECSB reactor for high-rate anaerobic treatment of cheese industry wastewater: Effect of pre-acidification on process efficiency and calcium precipitation. Water Sci. Technol. 2018, 78, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Bonk, F.; Popp, D.; Weinrich, S.; Sträuber, H.; Kleinsteuber, S.; Harms, H.; Centler, F. Ammonia inhibition of anaerobic volatile fatty acid degrading microbial communities. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Yenigün, O. Two-phase anaerobic digestion processes: A review. J. Chem. Technol. Biotechnol. 2002, 77, 743–755. [Google Scholar] [CrossRef]
- Siegert, I.; Banks, C. The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process. Biochem. 2005, 40, 3412–3418. [Google Scholar] [CrossRef]
- Kumar, G.; Sivagurunathan, P.; Park, J.-H.; Kim, S.-H. Anaerobic digestion of food waste to methane at various organic loading rates (OLRs) and hydraulic retention times (HRTs): Thermophilic vs. mesophilic regimes. J. Environ. Eng. 2016, 21, 69–73. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Conventional mesophilic vs. thermophilic anaerobic digestion: A trade-off between performance and stability? Water Res. 2014, 53, 249–258. [Google Scholar] [CrossRef]
- Kardos, L.; Juhász, A.; Palkó, G.Y.; Oláh, J.; Barkács, J.; Záray, G.Y. Comparing of mesophilic and thermophilic anaerobic fermented sewage sludge based on chemical and biochemical tests. Appl. Ecol. Environ. Res. 2011, 9, 293–302. [Google Scholar] [CrossRef]
- Raposo, F.; Borja, R.; Martín, M.A.; de la Rubia, M.A.; Rincón, B. Influence of inoculum–substrate ratio on the anaerobic digestion of sunflower oil cake in batch mode: Process stability and kinetic evaluation. Chem. Eng. J. 2009, 149, 70–77. [Google Scholar] [CrossRef]
- Labatut, R.A.; Gooch, C.A. Monitoring of anaerobic digestion process to optimize performance and prevent system failure. Proceeding Got Manure Enhancing Environment. Econ. Sustain. 2012, 14, 209–225. [Google Scholar]
- Kugelman, I.J.; Guida, V.G. Comparative Evaluation of Mesophilic and Thermophilic Anaerobic Digestion: Phase II—Steady State Studies; National Service Centre for Environmental Publication: Cincinnati, OH, USA, 1989.
- Girardi, G.; Berman, J.; Redecha, P.; Spruce, L.; Thurman, J.M.; Kraus, D.; Hollmann, T.J.; Casali, P.; Caroll, M.C.; Wetsel, R.A.; et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Investig. 2003, 112, 54. [Google Scholar] [CrossRef]
- Mamimin, C.; Prasertsan, P.; Kongjan, P.; O-Thong, S. Effects of volatile fatty acids in biohydrogen effluent on biohythane production from palm oil mill effluent under thermophilic condition. Electron. J. Biotechnol. 2017, 29, 78–85. [Google Scholar] [CrossRef]
- Hans, M.; Kumar, S. Biohythane production in two-stage anaerobic digestion system. Int. J. Hydrogen Energy 2018. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, L.; Sakinah, M.; Thakur, S.; Wahid, Z.A.; Sohaili, J. Effect of organic loading rate on hydrogen (H2) and methane (CH4) production in two-stage fermentation under thermophilic conditions using palm oil mill effluent (POME). Energy Sustain. Dev. 2016, 34, 130–138. [Google Scholar] [CrossRef]



| ID. | Parameter | Concentration Range | |
|---|---|---|---|
| Acidified POME | Raw POME [2] | ||
| 1 | pH | 5.14 ± 0.1 | 5.90 ± 0.2 |
| 2 | Chemical oxygen demand (g/L) | 44.3 ± 3.7 | 50.1 ± 1.4 |
| 3 | Total solid (g/L) | 31.2 ± 2.3 | 61.5 ± 1.5 |
| 4 | Total suspended solid (g/L) | 24.1 ± 4.4 | 42.3 ± 1.1 |
| 5 | Volatile suspended solid (g/L) | 20.4 ± 2.9 | 39.5 ± 1.3 |
| 6 | Volatile fatty acid (mg/L as ) | 6383.5 ± 1348.3 | 4600.0 ± 1300.0 |
| 7 | Total nitrogen (mg/L) | 254 ± 27 | N/D |
| ID | Parameter | [31] | [14] | Present Study | |
|---|---|---|---|---|---|
| 1 | Substrate | Palm oil mill effluent | Palm oil mill effluent | Acidified palm oil mill effluent | |
| 2 | Hydraulic retention time (days) & Organic loading rate (g COD/L·d ) | Hydraulic retention time = 40 Organic loading rate = 1.4 | Hydraulic retention time = 20 Organic loading rate = 3.4 | Hydraulic retention time = 30 Organic loading rate = 1.22 | |
| 3 | pH | Mesophilic (7.30) | Mesophilic (7.30) | Mesophilic (7.00) | Thermophilic (7.20) |
| 4 | Total alkalinity (mg/L as ) | Mesophilic (11,610) | N/D | Mesophilic (7792) | Thermophilic (9540) |
| 5 | Volatile fatty acid (mg/L as ) | N/D | N/D | Mesophilic (4360.3) | Thermophilic (2714.2) |
| 6 | Chemical oxygen demand effluent (g/L) | Mesophilic (14.4) | Mesophilic (47.5) | Mesophilic (15.2) | Thermophilic (14.3) |
| 7 | Production rate (L-/L·d) | N/D | N/D | Mesophilic (0.14) | Thermophilic (0.34) |
| Independent Samples Test | Levene’s Test for Equality of Variances | t-Test for Equality of Means | |||||
| F | Sig. | t | df | sig. (2-tailed) | Mean Difference | ||
| Productivity | Equal Variances Assumed | 13.920 | 0.001 | 3.745 | 30 | 0.001 | 0.206 |
| Equal variances not assumed | 3.745 | 19 | 0.001 | 0.206 | |||
| Group Statistics | |||||||
| Condition | N | Mean | Std Deviation | Std Error Mean | |||
| Productivity | Thermophilic | 16 | 0.34 | 0.21 | 0.05 | ||
| Mesophilic | 16 | 0.14 | 0.08 | 0.02 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamzah, M.A.F.; Jahim, J.M.; Abdul, P.M.; Asis, A.J. Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent. Energies 2019, 12, 2473. https://doi.org/10.3390/en12132473
Hamzah MAF, Jahim JM, Abdul PM, Asis AJ. Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent. Energies. 2019; 12(13):2473. https://doi.org/10.3390/en12132473
Chicago/Turabian StyleHamzah, Muhammad Arif Fikri, Jamaliah Md Jahim, Peer Mohamed Abdul, and Ahmad Jaril Asis. 2019. "Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent" Energies 12, no. 13: 2473. https://doi.org/10.3390/en12132473
APA StyleHamzah, M. A. F., Jahim, J. M., Abdul, P. M., & Asis, A. J. (2019). Investigation of Temperature Effect on Start-Up Operation from Anaerobic Digestion of Acidified Palm Oil Mill Effluent. Energies, 12(13), 2473. https://doi.org/10.3390/en12132473






