Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrates
2.2. Experimental Design
2.3. Analytical Methods
2.4. Kinetic Study
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Substrates and Inoculum
3.2. Effect of ISRs on the Mono-Digestion PPW
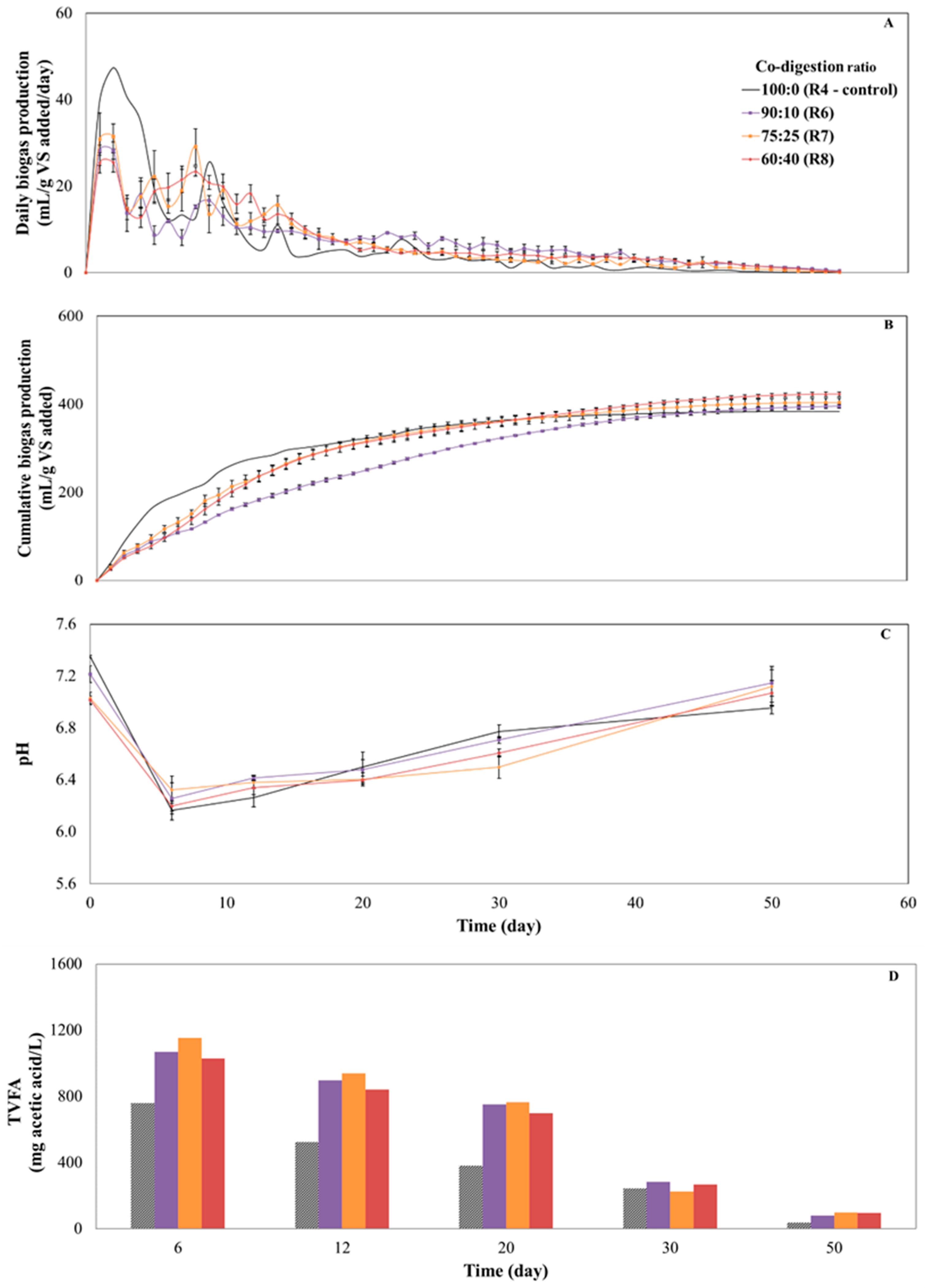
3.3. Co-Digestion of Potato Peel Waste with Cow Manure: Influence on Process Performance
3.4. Effect of Pretreatment on Biogas Production in Co-Digestion of Potato Peels and Cow Manure
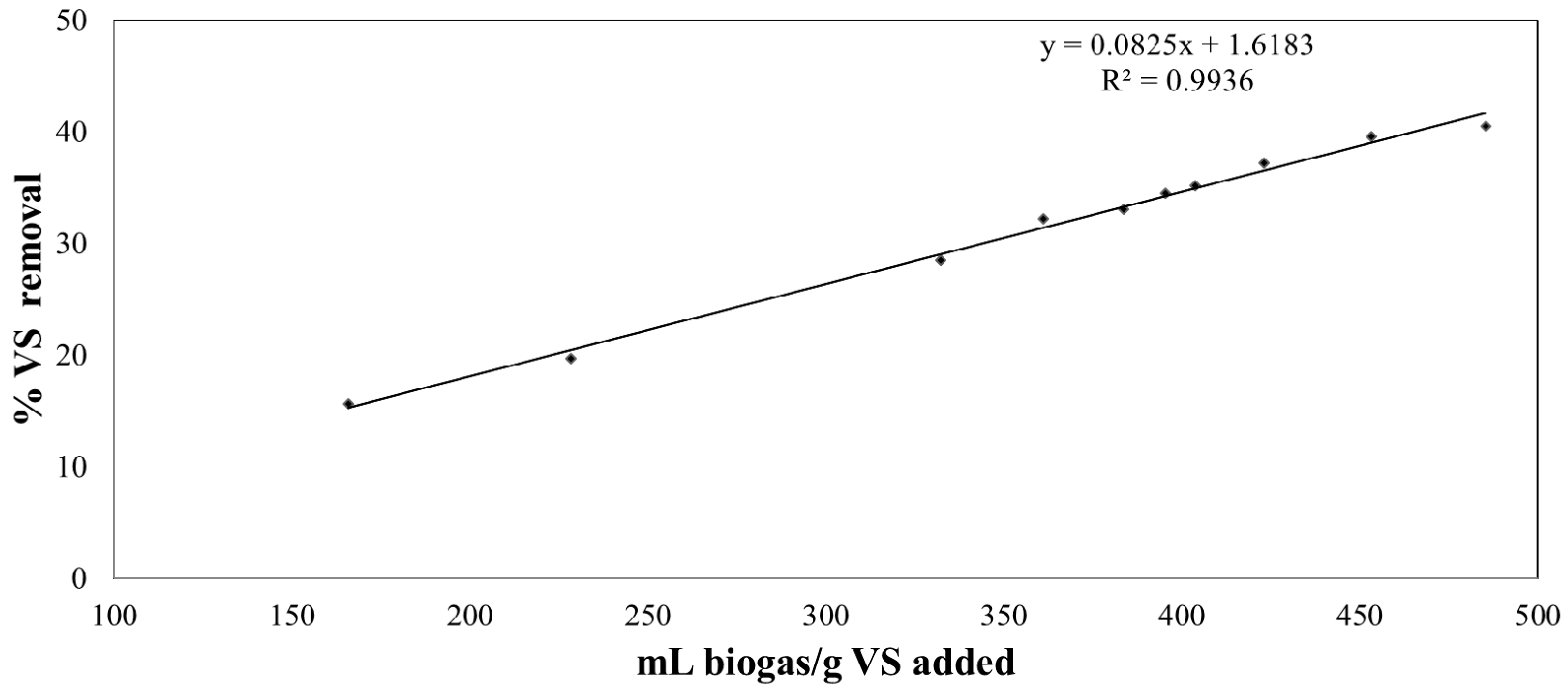
3.5. VS Removal
3.6. Kinetics Results and Correlation with Process Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. FAOSTAT Agricultural Data. Food and Agricultural Commodities Production. Available online: http://faostat.fao.org/site/339/default.aspx (accessed on 12 June 2014).
- VAVI. Association for the Potato Processing Industry. Available online: http://www.vavi.nl/index.shtml (accessed on 26 November 2018).
- Chang, K.C. Polyphenol antioxidants from potato peels: Extraction, optimization and application to stabilizing lipid oxidation in foods. In Proceedings of the National Conference on Undergraduate Research (NCUR), Ithaca College, NY, USA, 31 March–2 April 2011. [Google Scholar]
- Wu, D. Recycle Technology for Potato Peel Waste Processing: A Review. Procedia Environ. Sci. 2016, 31, 103–107. [Google Scholar] [CrossRef]
- Nelson, M.L. Utilization and application of wet potato processing coproducts for fishing cattle. J. Anim. Sci. 2010, 88, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mader, J.; Rawel, H.; Kroh, L. Composition of phenolic compounds and glycoalkaloids a-solanine and a-chaconine during commercial potato processing. J. Agric. Food Chem. 2009, 57, 6292–6297. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—Recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Camire, M.E.; Violette, D.; Dougherty, M.P.; McLaughlin, M.A. Potato peel dietary fiber composition: Effects of peeling and extrusion cooking processes. J. Agric. Food Chem. 1997, 45, 1404–1408. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Chemical and thermal characterization of potato peel waste and its fermentation residue as potential resources for biofuel and bioproducts production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Han, Y.; Wei, L.; McDonald, A.G. Production and characterization of bio-oil and bio-char from pyrolysis of potato peel wastes. Biomass Convers. Biorefin. 2015, 5, 237–246. [Google Scholar] [CrossRef]
- Arapoglou, D.; Varzakas, T.; Vlyssides, A.; Israilides, C. Ethanol production from potato peel waste (PPW). Waste Manag. 2010, 30, 1898–1902. [Google Scholar] [CrossRef]
- Peschel, W.; Sanchez-Rabaneda, F.; Diekmann, W.; Plescher, A.; Gartzia, I.; Jimenez, D.; Lamuela-Raventos, R.; Buxaderas, S.; Codina, C. An industrial approach in the search of natural antioxidants from vegetable and fruit wastes. Food Chem. 2006, 97, 137–150. [Google Scholar] [CrossRef]
- Liang, S.; Gliniewicz, K.; Mendes-Soares, H.; Settles, M.L.; Forney, L.J.; Coats, E.R.; McDonald, A.G. Comparative analysis of microbial community of novel lactic acid fermentation inoculated with different under fined mixed cultures. Bioresour. Technol. 2015, 179, 268–274. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G.; Coats, E.R. Lactic acid production from potato peel waste by anaerobic sequencing batch fermentation using undefined mixed culture. Waste Manag. 2015, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A.; Boscaro, D.; da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from fresh spring and summer grass: Effect of the harvesting period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V.; Euverink, G.J.W.M. A technological overview of biogas production from biowaste. Engineering 2017, 3, 299–307. [Google Scholar] [CrossRef]
- Li, W.W.; Yu, H.Q. Advances in energy-producing anaerobic biotechnologies for municipal wastewater treatment. Engineering 2016, 2, 438–446. [Google Scholar] [CrossRef]
- Chen, J.F. Green chemical engineering. Engineering 2017, 3, 283–284. [Google Scholar] [CrossRef]
- Sahajwalla, V. Green Processes: Transforming Waste into Valuable Resources. Engineering 2018, 4, 309–310. [Google Scholar] [CrossRef]
- Matsakas, L.; Gao, Q.; Jansson, S.; Rova, U.; Christakopoulos, P. Green conversion of municipal solid wastes into fuels and chemicals. Electron. J. Biotechnol. 2017, 26, 69–83. [Google Scholar] [CrossRef]
- RedCorn, R.; Fatemi, S.; Engelberth, A.S. Comparing end-use potential for industrial food-waste sources. Engineering 2018, 4, 371–380. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Chacón-Pérez, Y.; Cardona-Alzate, C.A. Evaluation of biogas and syngas as energy vectors for heat and power generation using lignocellulosic biomass as raw material. Electron. J. Biotechnol. 2018, 33, 52–62. [Google Scholar] [CrossRef]
- Achinas, S.; Achinas, V. Biogas combustion: An introductory briefing. In Biogas: Production, Applications and Global Developments; Vico, A., Artemio, N., Eds.; Nova Science Publishers, Inc.: New York, NY, USA, 2017; pp. 179–193. [Google Scholar]
- Chen, P.; Anderson, E.; Addy, M.; Zhang, R.; Cheng, Y.; Peng, P.; Ma, Y.; Fan, L.; Zhang, Y.; Lu, Q.; et al. Breakthrough technologies for the biorefining of organic solid and liquid wastes. Engineering 2018, 4, 574–580. [Google Scholar] [CrossRef]
- EBA. Biogas Report 2014—Biogas Production in Europe; European Biogas Association: Bruxelles, Belgium, 2015. [Google Scholar]
- Fabbri, A.; Serranti, S.; Bonifazi, G. Biochemical methane potential (BMP) of artichoke waste: The inocolum effect. Waste Manag. Res. 2014, 32, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Valenti, F.; Porto, S.M.C.; Cascone, G.; Arcidiacono, C. Potential biogas production from agricultural by-products in Sicily. A case study of citrus pulp and olive pomace. J. Agric. Eng. 2017, 48, 196–202. [Google Scholar] [CrossRef]
- Scaglione, S.; Caffaz, S.; Ficara, E.; Malpei, F.; Lubello, C. A simple method to evaluate the short-term biogas yield in anaerobic codigestion of WAS and organic wastes. Water Sci. Technol. 2008, 58, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Dinuccio, E.; Gioelli, F.; Cuk, D.; Rollè, L.; Balsari, P. The use of co-digested solid fraction as feedstock for biogas plants. J. Agric. Eng. 2013, 44, 153–159. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Feasibility study of biogas production from hardly degradable material in co-inoculated bioreactor. Energies 2019, 12, 1040. [Google Scholar] [CrossRef]
- Gomez, X.; Cuetos, M.J.; Cara, J.; Moran, A.; Garcia, A.I. Anaerobic co-digestion of primary sludge and the fruit and vegetable fraction of the municipal solid wastes: Conditions for mixing and evaluation of the organic loading rate. Renew. Energy 2006, 31, 2017–2024. [Google Scholar]
- Bolzonella, D.; Innocenti, L.; Cecchi, F. BNR wastewater treatments and sewage sludge anaerobic mesophilic digestion performances. Water Sci. Technol. 2002, 46, 199–208. [Google Scholar] [CrossRef]
- Perazzolo, F.; Mattachini, G.; Tambone, F.; Calcante, A.; Provolo, G. Nutrient losses from cattle co-digestate slurry during storage. J. Agric. Eng. 2016, 47, 94–99. [Google Scholar] [CrossRef][Green Version]
- Pontoni, L.; Panico, A.; Salzano, E.; Frunzo, L.; Iodice, P.; Cavinato, F. Innovative parameters to control the efficiency of anaerobic digestion process. Chem. Eng. Trans. 2015, 43, 2089–2094. [Google Scholar]
- Cavinato, C.; Fatone, F.; Bolzonella, D.; Pavan, P. Thermophilic anaerobic co-digestion of cattle manure with agro-wastes and energy crops: Comparison of pilot and full-scale experiences. Bioresour. Technol. 2010, 101, 545–550. [Google Scholar] [CrossRef]
- Naik, L.; Gebreegziabher, Z.; Tumwesige, V.; Balana, B.B.; Mwirigi, J.; Austin, G. Factors determining the stability and productivity of small-scale anaerobic digesters. Biomass Bioenergy 2014, 70, 51–57. [Google Scholar] [CrossRef]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Solvent pretreatments of lignocellulosic materials to enhance biogas production: A review. Energy Fuels 2016, 30, 1892–1903. [Google Scholar] [CrossRef]
- Nelson, M.J.; Nakhla, G.; Zhu, J. Fluidized-bed bioreactor applications for biological wastewater treatment: A review of research and developments. Engineering 2017, 3, 330–342. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Mesophilic anaerobic digestion of waste activated sludge: Influence of the solid retention time in the wastewater treatment process. Process Biochem. 2005, 40, 1453–1460. [Google Scholar] [CrossRef]
- Fabbri, A.; Bonifazi, G.; Serranti, S. Micro-scale energy valorization of grape MARCS in winery production plants. Waste Manag. 2015, 36, 156–165. [Google Scholar] [CrossRef] [PubMed]
- APHA-AWWA-WEF. Standard Methods for the Examination of Water and Wastewater; APHA-AWWA-WEF: Washington, DC, USA, 2005. [Google Scholar]
- Lossie, U.; Pütz, P. Targeted Control of Biogas Plants with the Help of FOS/TAC. Practice Report Hach-Lange. 2008. Available online: https://www.semanticscholar.org/paper/Targeted-control-of-biogas-plants-with-the-help-of-Lossie-Putz/b71fa7e5578e02348b65d22f7be11844e646c7c8 (accessed on 16 December 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Wickham, R.; Galway, B.; Bustamante, H.; Nghiem, L.D. Biomethane potential evaluation of co-digestion of sewage sludge and organic wastes. Int. Biodeterior. Biodegrad. 2016, 13, 3–8. [Google Scholar] [CrossRef]
- Morosini, C.; Conti, F.; Torretta, V.; Rada, E.C.; Passamani, G.; Schiavon, M.; Cioca, L.I.; Ragazzi, M. Biochemical methane potential assays to test the biogas production from the anaerobic digestion of sewage sludge and other organic matrices. WIT Trans. Ecol. Environ. 2016, 205, 235–244. [Google Scholar]
- WRC. Equipment for Measurement of Gas Production at Low Rates of Flow; Technical Memorandum TM104—Water Research Centre: Swindon, UK, 1975. [Google Scholar]
- Dinuccio, E.; Balsari, P.; Gioelli, F.; Menardo, S. Evaluation of the biogas productivity potential of some Italian agro-industrial biomasses. Bioresour. Technol. 2010, 101, 3780–3783. [Google Scholar] [CrossRef]
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 102, 2255–2264. [Google Scholar] [CrossRef]
- Luna-del Risco, M.; Normak, A.; Orupõld, K. Biochemical methane potential of different organic wastes and energy crops from Estonia. Agron. Res. 2011, 9, 331–342. [Google Scholar]
- Stone, R.J. Improved statistical procedure for the evaluation of solar radiation estimation models. Sol. Energy 1993, 51, 289–291. [Google Scholar] [CrossRef]
- Bhattarai, S.; Oh, J.H.; Euh, S.H.; Kafle, G.K.; Kim, D.H. Simulation and model validation of sheet and tube type photovoltaic thermal solar system and conventional solar collecting system in transient states. Sol. Energy Mater. Sol. Cells 2012, 103, 184–193. [Google Scholar] [CrossRef]
- Parawira, W.; Murto, M.; Read, J.S.; Mattiasson, B. A study of two-stage anaerobic digestion of solid potato waste using reactors under mesophilic and thermophilic conditions. Environ. Technol. 2007, 28, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Parawira, W.; Murto, M.; Zvauya, R.; Mattiasson, B. Anaerobic batch digestion of solid potato waste alone and in combination with sugar beet leaves. Renew. Energy 2004, 29, 1811–1823. [Google Scholar]
- Muhondwa, J.P.; Martienssen, M.; Burkhardt, M. Feasibility of anaerobic digestion of potato peels for biogas as mitigation of greenhouse gases emission potential. Int. J. Environ. Res. 2015, 9, 481–488. [Google Scholar]
- Cater, M.; Zorec, M.; Logar, R.M. Methods for improving anaerobic lignocellulosic substrates degradation for enhanced biogas production. Springer Sci. Rev. 2014, 2, 51–61. [Google Scholar] [CrossRef]
- Franchi, O.; Rosenkranz, F.; Chamy, R. Key microbial populations involved in anaerobic degradation of phenol and p-cresol using different inocula. Electron. J. Biotechnol. 2018, 35, 33–38. [Google Scholar] [CrossRef]
- Świątek, M.; Lewicki, A.; Szymanowska, D.; Kubiak, P. The effect of introduction of chicken manure on the biodiversity and performance of an anaerobic digester. Electron. J. Biotechnol. 2019, 37, 25–33. [Google Scholar] [CrossRef]
- Liotta, F.; Esposito, G.; Fabbricino, M.; van Hullebusch, E.D.; Lens, P.N.L.; Pirozzi, F.; Pontoni, L. Methane and VFA production in anaerobic digestion of rice straw under dry, semi-dry and wet conditions during start-up phase. Environ. Technol. 2016, 37, 505–512. [Google Scholar] [CrossRef]
- Franke-Whittle, I.H.; Walter, A.; Ebner, C.; Insam, H. Investigation into the effect of high concentrations of volatile fatty acids in anaerobic digestion on methanogenic communities. Waste Manag. 2014, 34, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Pellera, F.M.; Gidarakos, E. Effect of substrate to inoculum ratio and inoculum type on the biochemical methane potential of solid agro-industrial waste. J. Environ. Chem. Eng. 2016, 4, 3217–4229. [Google Scholar] [CrossRef]
- Cestonaro, T.; de Mendonça Costa, M.S.S.; de Mendonça Costa, L.A.; Rozatti, M.A.T.; Pereira, D.C.; Lorin, H.E.F.; Carneiro, L.J. The anaerobic co-digestion of sheep bedding and >50% cattle manure increases biogas production and improves biofertilizer quality. Waste Manag. 2015, 46, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Liden, G. Low temperature anaerobic digestion of mixtures of llama, cow and sheep manure for improved methane production. Biomass Bioenergy 2009, 33, 527–533. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobu, M.; Ogawa, H.I.; Kato, Y. Degradation of volatile fatty acids in highly efficient anaerobic digestion. Biomass Bioenergy 1999, 16, 407–416. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Consolidated briefing of biochemical ethanol production form lignocellulosic biomass. Electron. J. Biotechnol. 2016, 23, 44–53. [Google Scholar] [CrossRef]
- Johnson, D.K.; Elander, R.E. Treatment for enhanced digestability of feedstocks. In Biomass Recalcitrance: Deconstructing the Plant Cell Wall for Bioenergy; Himmel, M.E., Ed.; Blackwell Pub.: Oxford, UK, 2008; pp. 436–453. [Google Scholar]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]


| Particular | Experimental Design | |||
|---|---|---|---|---|
| ISR (based on VS) | Co-Digestion Ratio (based on VS) | Organic Load (g VS added per L) | Pretreatment | |
| Experiment 1 | ||||
| R1 | 0.25 | 100:0 | 10 | - |
| R2 | 0.5 | 100:0 | 10 | - |
| R3 | 1 | 100:0 | 10 | - |
| R4 | 2 | 100:0 | 10 | - |
| R5 | 4 | 100:0 | 10 | - |
| Experiment 2 | ||||
| R6 | 2 | 90:10 | 10 | - |
| R7 | 2 | 75:25 | 10 | - |
| R8 | 2 | 60:40 | 10 | - |
| Experiment 3 | ||||
| R9 | 2 | 60:40 | 10 | Grinding |
| R10 | 2 | 60:40 | 10 | Acid hydrolysis |
| Parameter | Inoculum | Potato Peels Waste (PPW) | Cow Manure (CM) |
|---|---|---|---|
| TS (g/kg) | 50.9 (0.2) | 144.0 (4.3) | 144.6 (1.5) |
| VS (g/kg) | 29.1 (1.2) | 139.4 (4.8) | 119.4 (1.1) |
| VS/TS | 0.60 | 0.97 | 0.78 |
| COD (g/kg) | 47.2 (1.0) | 123.8 (4.2) | 138.4 (1.9) |
| pH | 7.36 | NA | NA |
| Insoluble lignin (%TS) | NA | 19.1 (0.7) | 24.6 (2.5) |
| Soluble lignin (%TS) | NA | 1.59 (0.1) | 1.2 (0.4) |
| Ash (%) | NA | 0.58 (0.1) | 5.5 (1.1) |
| Parameter | ISR | ||||
|---|---|---|---|---|---|
| 0.25 (R1) | 0.5 (R2) | 1 (R3) | 2 (R4) | 4 (R5) | |
| Biogas yield (mL/g VSadded) | 165.8 ± 7.9 | 228.1 ± 6.8 | 332.2 ± 7.6 | 383.7 ± 10.7 | 361.2 ± 14.4 |
| Methane content (%) | 50.2 ± 0.5 | 51.1 ± 1.7 | 52.4 ± 0.5 | 56.8 ± 0.3 | 53.8 ± 0.3 |
| Methane yield (mL/g VSadded) | 83.3 ± 0.9 | 116.6 ± 3.9 | 177.4 ± 2.3 | 217.7 ± 1.2 | 190.7 ± 2.9 |
| VSadded degradation (%) | 15.6 ± 1.2 | 19.7 ± 3.0 | 28.5 ± 2.1 | 33.1 ± 2.7 | 32.1 ± 3.4 |
| Technical digestion time (d) | 22 | 22 | 26 | 17 | 24 |
| Parameter | Co-Digestion Ratio | ||
|---|---|---|---|
| 90:10 (R6) | 75:25 (R7) | 60:40(R8) | |
| Biogas yield (mL/g VSadded) | 395.4 ± 4.3 | 403.7 ± 9.8 | 423.1 ± 5.2 |
| CH4 content (%) | 52.3 ± 1.6 | 53.4 ± 2.3 | 56.1 ± 0.8 |
| CH4 yield (mL/g VSadded) | 206.7 ± 3.6 | 215.5 ± 7.6 | 237.4 ± 3.1 |
| Weighted methane yield (mL/g VSadded) | 211.6 | 202.5 | 193.3 |
| Differential (EMY-weighted MY) (mL/g VSadded) | −4.9 | 13.1 | 44.2 |
| Synergistic effect | No | Yes | Yes |
| VSadded degradation (%) | 34.5 ± 2.8 | 35.2 ± 4.3 | 37.2 ± 4.7 |
| Technical digestion time (day) | 29 | 21 | 25 |
| Parameter | Pretreatment | |
|---|---|---|
| Grinding/Homogenizing (R9) | Acid Hydrolysis (R10) | |
| Biogas yield (mL/g VSadded) | 453.2 ± 11.0 | 485.4 ± 14.6 |
| Methane content (%) | 57.5 ± 1.4 | 58.3 ± 0.8 |
| Methane yield (mL/g VSadded) | 260.3 ± 3.2 | 283.4 ± 2.7 |
| VSadded degradation (%) | 39.5 ± 2.5 | 40.5 ± 3.2 |
| Technical digestion time (day) | 23 | 19 |
| Reactor | 1-Order | ||||
|---|---|---|---|---|---|
| K (1/day) | R2 | RMSE | Measured (mL/g VSadded) | Predicted (mL/g VSadded) | |
| R1 | 0.0741 | 0.9934 | 4.05 | 165.8 | 163.3 |
| R2 | 0.0733 | 0.9977 | 6.46 | 228.1 | 224.6 |
| R3 | 0.067 | 0.9786 | 16.57 | 332.2 | 324.9 |
| R4 | 0.0911 | 0.0323 | 15.49 | 383.7 | 381.6 |
| R5 | 0.0667 | 0.0323 | 15.30 | 361.2 | 353.1 |
| R6 | 0.0537 | 0.9924 | 14.15 | 395.4 | 376.9 |
| R7 | 0.0767 | 0.999 | 5.49 | 403.7 | 398.6 |
| R8 | 0.066 | 0.9943 | 8.89 | 423.1 | 413.3 |
| R9 | 0.0702 | 0.9982 | 10.47 | 453.2 | 444.9 |
| R10 | 0.0921 | 0.9909 | 19.38 | 485.4 | 482.9 |
| Reactor | Cone | |||||
|---|---|---|---|---|---|---|
| K (1/day) | n | R2 | RMSE | Measured (mL/g VSadded) | Predicted (mL/g VSadded) | |
| R1 | 0.1108 | 1.69 | 0.9657 | 8.273 | 165.8 | 158.4 |
| R2 | 0.1258 | 1.59 | 0.9762 | 9.187 | 228.1 | 217.9 |
| R3 | 0.0817 | 2.03 | 0.9724 | 16.51 | 332.2 | 317.3 |
| R4 | 0.176 | 1.51 | 0.9818 | 12.54 | 383.7 | 371.6 |
| R5 | 0.1155 | 1.55 | 0.9481 | 21.59 | 361.2 | 341.7 |
| R6 | 0.0798 | 1.79 | 0.965 | 21.17 | 395.4 | 368.9 |
| R7 | 0.1098 | 1.78 | 0.9871 | 12.61 | 403.7 | 387.8 |
| R8 | 0.0958 | 1.80 | 0.9886 | 12.77 | 423.1 | 402.7 |
| R9 | 0.1129 | 1.68 | 0.9779 | 18.13 | 453.2 | 432.9 |
| R10 | 0.1111 | 1.98 | 0.9838 | 17.56 | 485.4 | 472.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies 2019, 12, 2311. https://doi.org/10.3390/en12122311
Achinas S, Li Y, Achinas V, Euverink GJW. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies. 2019; 12(12):2311. https://doi.org/10.3390/en12122311
Chicago/Turabian StyleAchinas, Spyridon, Yu Li, Vasileios Achinas, and Gerrit Jan Willem Euverink. 2019. "Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation" Energies 12, no. 12: 2311. https://doi.org/10.3390/en12122311
APA StyleAchinas, S., Li, Y., Achinas, V., & Euverink, G. J. W. (2019). Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies, 12(12), 2311. https://doi.org/10.3390/en12122311




