Tailoring Ni and Sr2Mg0.25Ni0.75MoO6−δ Cermet Compositions for Designing the Fuel Electrodes of Solid Oxide Electrochemical Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Materials Characterization
3. Results and Discussion
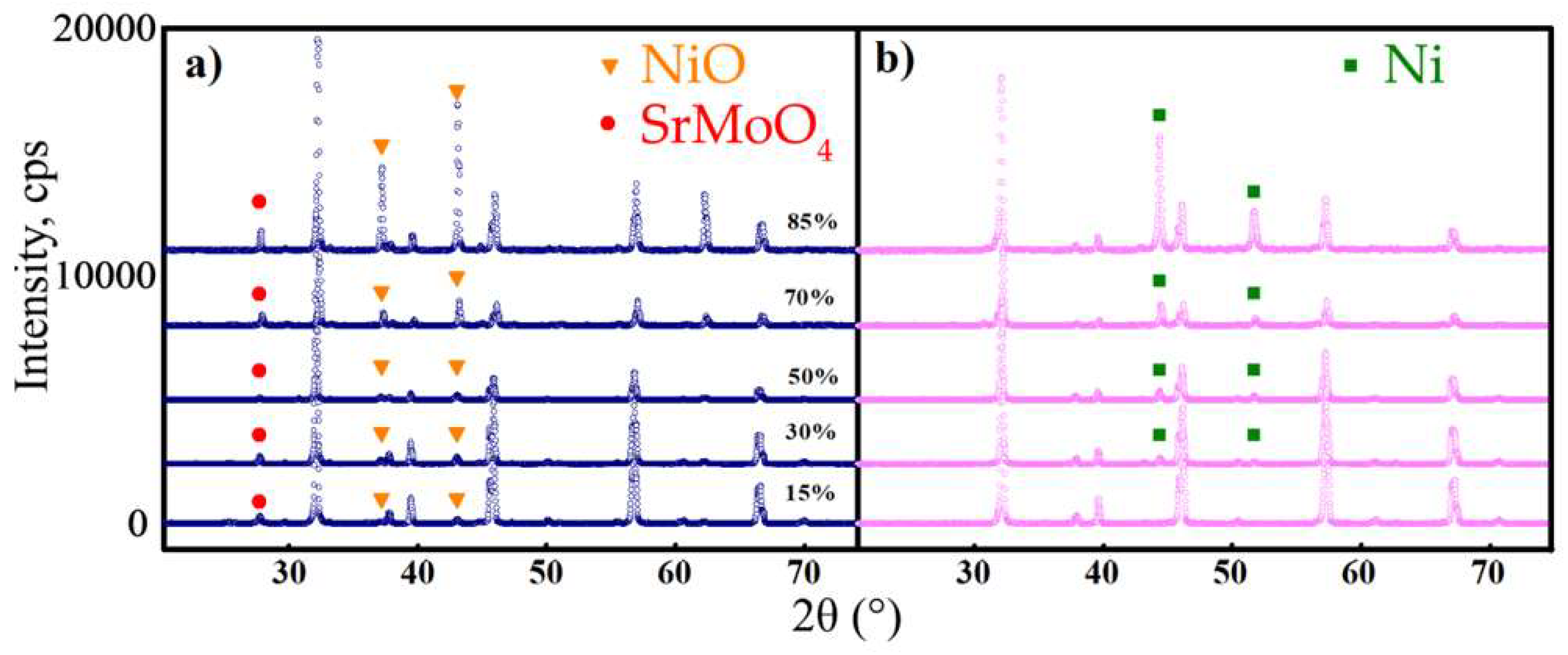
3.1. Phase Relation
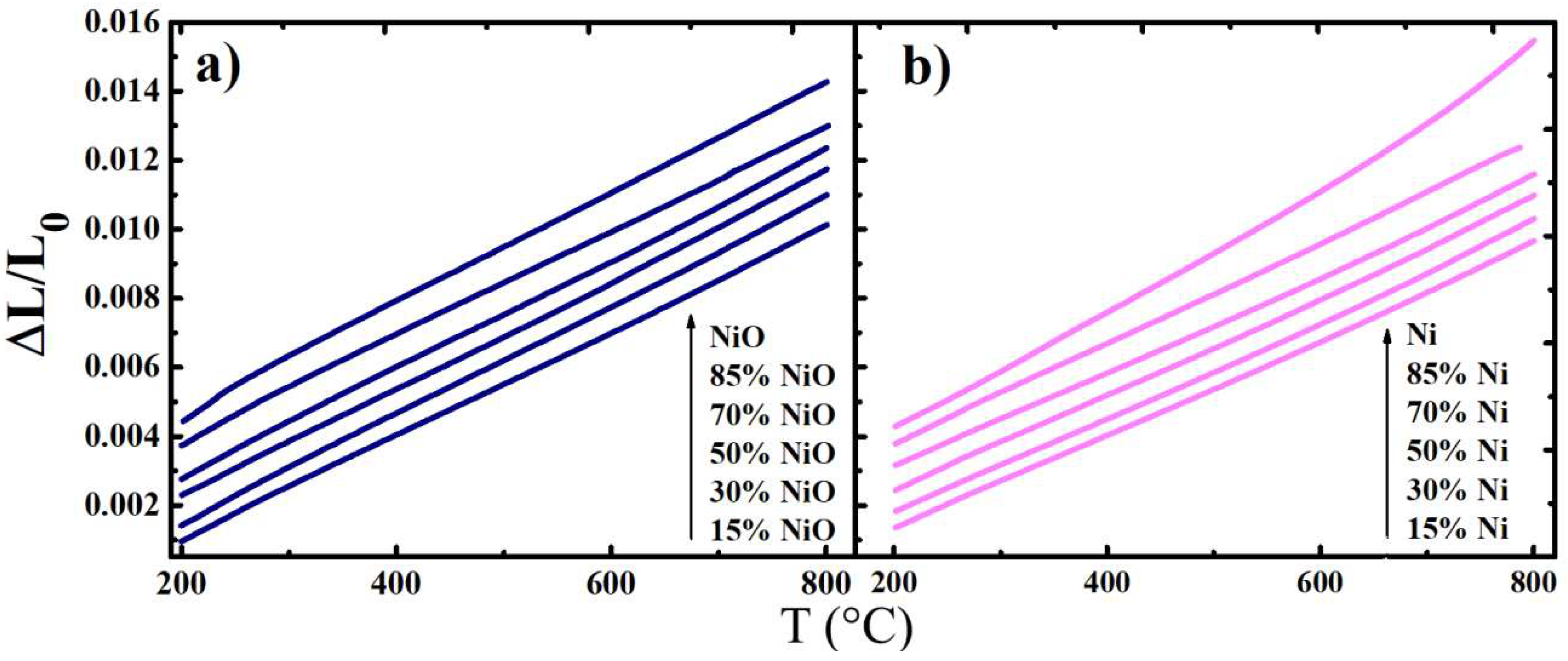
3.2. Thermal Behaviour
- The molybdenum ions reduction, Mo6+ → Mo5+ (Equation (2)), results in a slight increase in the average ionic radii of elements occupied B-position of the A2BB’O6 structure, since r(Mo6+) = 0.59 Å and r(Mo5+) = 0.61 Å [45].
- Together with a minor strain in cationic sublattice, the dimension change (contraction) in the anionic sublattice is estimated to be more pronounced due to oxygen desorption ( = 1.40 Å, = 1.18 Å [46,47]) occurring as a compensation of the Mo-ions reduction process. Here, the ionic radii values are provided using the Shannon’s system [48].
- NiO undergoes a complete reduction in a hydrogen atmosphere until the formation of a Ni metallic phase. The volume changes during this reduction amount ~40% [49].
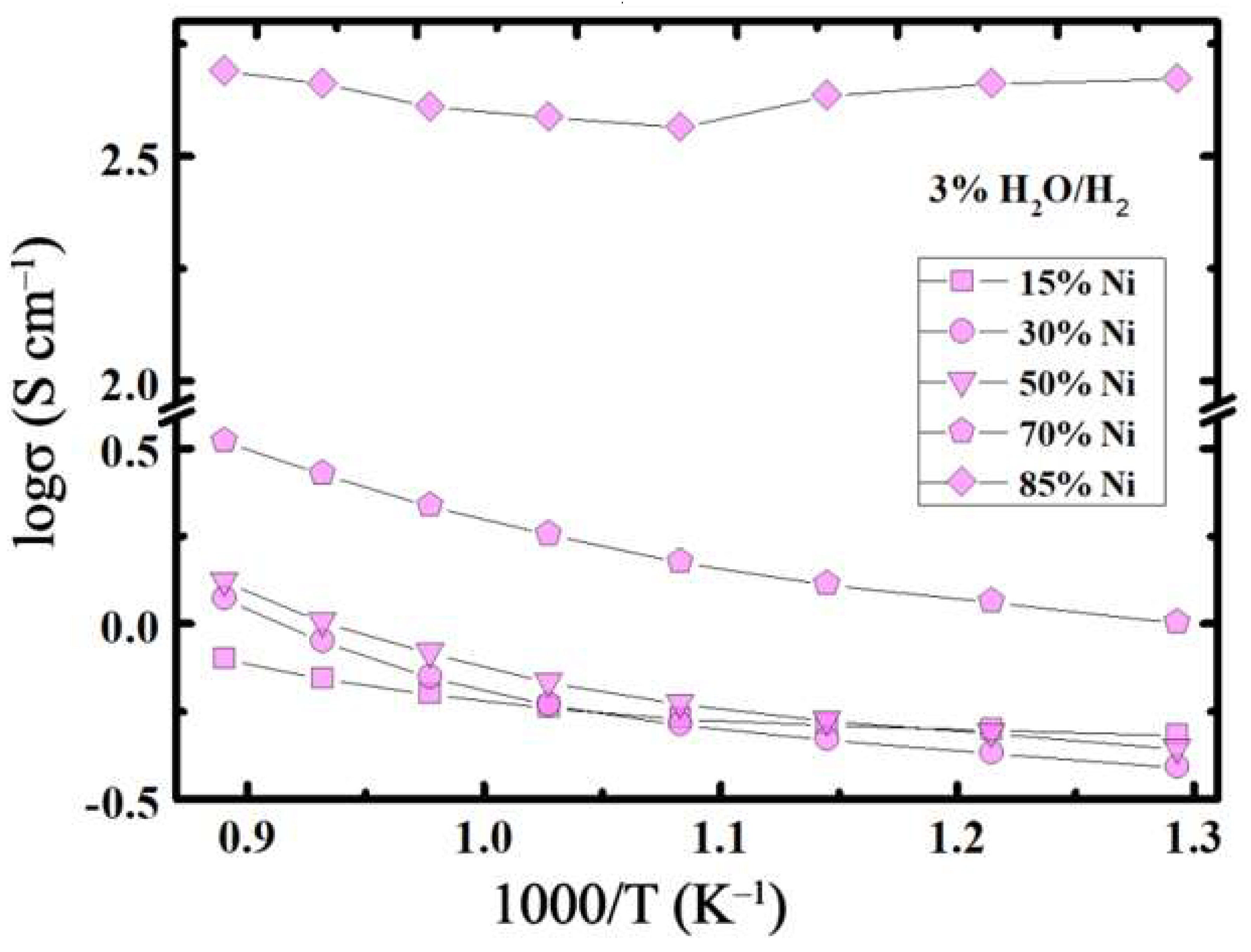
3.3. Conductivity Behaviour
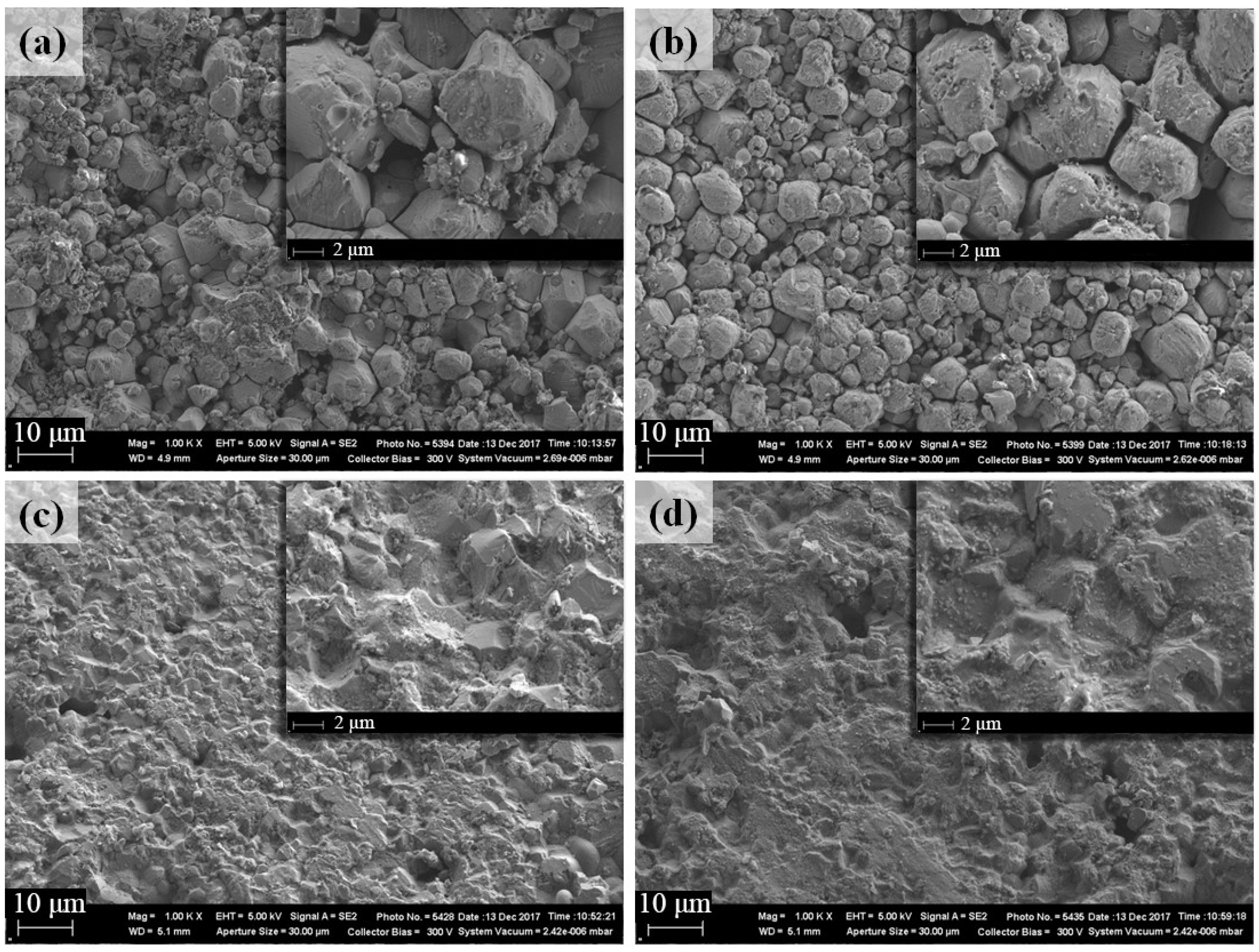
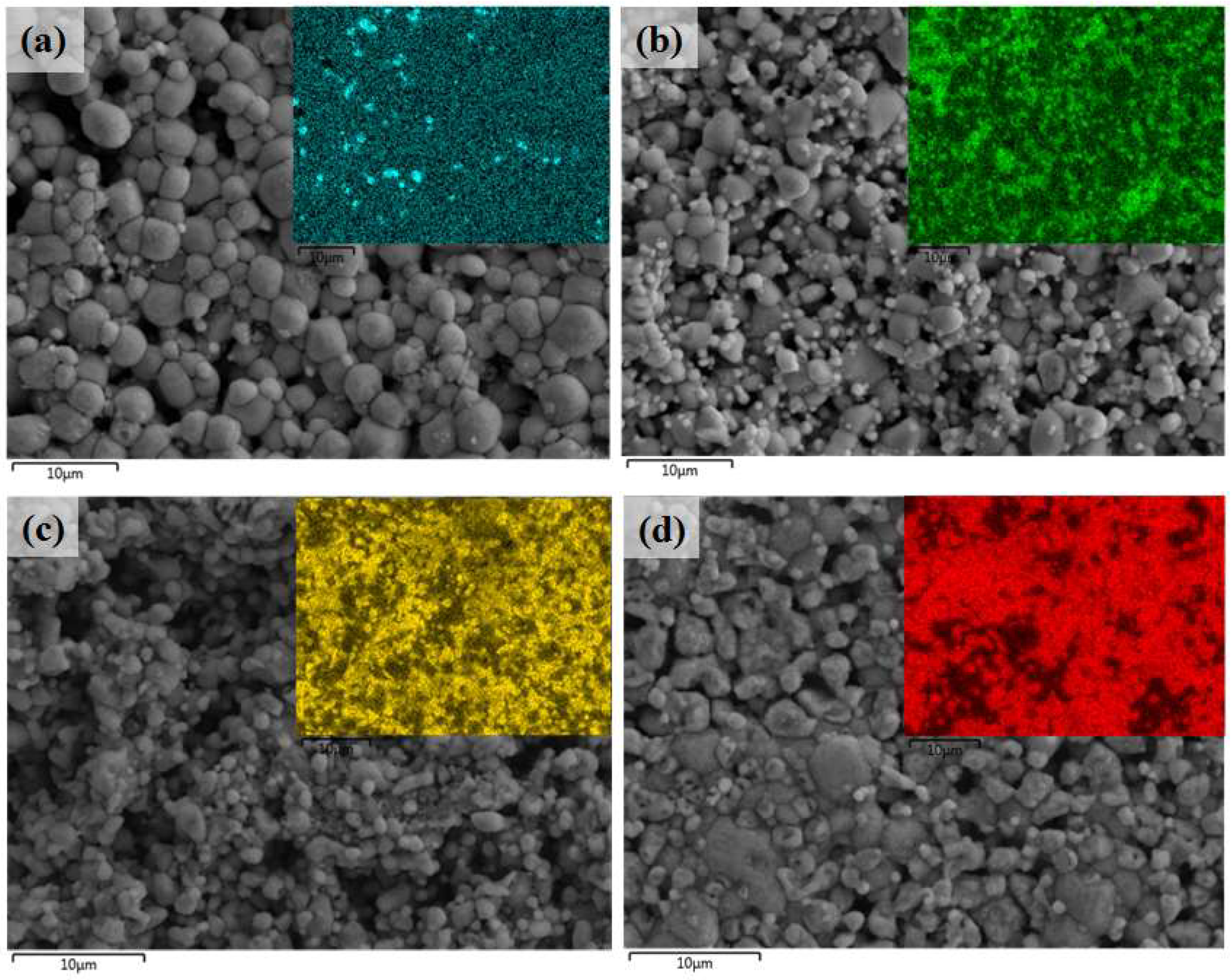
3.4. Microstructural Features
4. Conclusions
- All the materials were stable in both oxidising and reducing atmospheres. The reduced samples were found to comprise dual-phase materials, while an impurity SrMoO4 phase was detected along with two target phases for the oxidised samples.
- Thermal expansion of the studied composite materials was linear over the entire temperature range (200–800 °C); the calculated TECs values remained more or less consistent with a variation in composition, decreasing from the oxidised to the reduced samples.
- The total conductivity of the reduced composites did not exceed 3 S cm−1 at 800 °C at 15 ≤ x, mol.% ≤ 70; whereas, it amounts to 450 S cm−1 for x = 85 mol.% at the same temperature.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization strategies for Solid Oxide Fuel Cell (SOFC) application: Aliterature survey. Renew. Sustain. Energy Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Azad, A.T.; Petra, P.M.I.; Begum, F.; Eriksson, S.G.; Azad, A.K. Nanomaterials for solid oxide fuel cells: A review. Renew. Sustain. Energy Rev. 2018, 82, 353–368. [Google Scholar] [CrossRef]
- Mahato, N.; Banerjee, A.; Gupta, A.; Omar, S.; Balani, K. Progress in material selection for solid oxide fuel cell technology: A review. Prog. Mater. Sci. 2015, 72, 141–337. [Google Scholar] [CrossRef]
- Ruiz-Morales, J.C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Marín Rueda, J.R.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859. [Google Scholar] [CrossRef] [Green Version]
- Minh, N.Q. Solid oxide fuel cell technology-features and applications. Solid State Ion. 2004, 174, 271–277. [Google Scholar] [CrossRef]
- Yamamoto, O. Solid oxide fuel cells: Fundamental aspects and prospects. Electrochim. Acta 2000, 45, 2423–2435. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, M.; Wang, N.; Ma, C.; Wang, J.; Han, M. A short review of cathode poisoning and corrosionin solid oxide fuel cell. Int. J. Hydrogen Energy 2014, 42, 24948–24959. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, S.P. Review—Materials degradation of solid oxide electrolysis cells. J. Electrochem. Soc. 2016, 163, F3070–F3083. [Google Scholar] [CrossRef]
- Torrell, M.; Morata, A.; Kayser, P.; Kendall, M.; Kendall, K.; Tarancón, A. Performance and long term degradation of 7 W micro-tubular solid oxide fuel cells for portable applications. J. Power Sources 2018, 285, 439–448. [Google Scholar] [CrossRef]
- Téllez, H.; Druce, J.; Ishihara, T.; Kilner, J.A. Effects of microstructure on surface segregation: Role of grain boundaries. ECS Trans. 2016, 72, 57–69. [Google Scholar] [CrossRef]
- Kim, S.-D.; Moon, H.; Hyun, S.-H.; Moon, J.; Kim, J.; Lee, H.-W. Ni-YSZ cermet anode fabricated from NiO-YSZ composite powder for high-performance and durability of solid oxide fuel cells. Solid State Ion. 2007, 178, 1304–1309. [Google Scholar] [CrossRef]
- Faes, A.; Hessler-Wyser, A.; Zryd, A. A review of RedOx cycling of solid oxide fuel cells anode. Membranes 2012, 2, 585–664. [Google Scholar] [CrossRef]
- Mogensen, M.; Høgh, J.; Hansena, K.V.; Jacobsen, T. A critical review of models of the H2/H2O/Ni/SZ electrode kinetics. ECS Trans. 2007, 7, 1329–1338. [Google Scholar]
- Khan, M.S.; Lee, S.-B.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J. Fundamental mechanisms involved in the degradation of nickel–yttria stabilized zirconia (Ni–YSZ) anode during solid oxide fuel cells operation: A review. Ceram. Int. 2016, 42, 35–48. [Google Scholar] [CrossRef]
- Rafique, M.; Nawaz, H.; Shahid Rafique, M.; Bilal Tahir, M.; Nabi, G.; Khalid, N.R. Material and method selection for efficient solid oxide fuel cell anode: Recent advancements and reviews. Int. J. Energy Res. 2019, 43, 2423–2446. [Google Scholar] [CrossRef]
- Acosta, M.; Baiutti, F.; Tarancón, A.; MacManus-Driscoll, J.L. Nanostructured materials and interfaces for advanced ionic electronic conducting oxides. Adv. Mater. Interfaces 2019. [Google Scholar] [CrossRef]
- Wei, K.; Wang, X.; Budiman, R.A.; Kang, J.; Lin, B.; Zhou, F.; Ling, Y. Progress in Ni-based anode materials for direct hydrocarbon solid oxide fuel cells. J. Mater. Sci. 2018, 53, 8747–8765. [Google Scholar] [CrossRef]
- Istomin, S.Ya.; Kotova, A.I.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Pr5Mo3O16+δ: A new anode material for solid oxide fuel cells. Russ. J. Inorg. Chem. 2018, 63, 1291–1296. [Google Scholar] [CrossRef]
- Istomin, S.Ya.; Morozov, A.V.; Abdullayev, M.M.; Batuk, M.; Hadermann, J.; Kazakov, S.M.; Sobolev, A.V.; Presniakov, I.A.; Antipov, E.V. High-temperature properties of (La,Ca)(Fe,Mg,Mo)O3–δ perovskites as prospective electrode materials for symmetrical SOFC. J. Solid State Chem. 2018, 258, 1–10. [Google Scholar] [CrossRef]
- Vasala, S.; Lehtimäki, M.; Huang, Y.H.; Yamauchi, H.; Goodenough, J.B.; Karppinen, M. Degree of order and redox balance in B-site ordered double-perovskite oxides Sr2MMoO6-δ (M = Mg, Mn, Fe, Co, Ni, Zn). J. Solid State Chem. 2010, 183, 1007–1012. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Allix, M.; Bridges, C.A.; Claridge, J.B.; Rosseinsky, M.J. Sr2MgMoO6: Structure, phase stability and cation site order control of reduction. Chem. Mater. 2007, 19, 1035–1043. [Google Scholar] [CrossRef]
- Wei, T.; Ji, Y.; Meng, X.; Zhang, Y. Sr2NiMoO6−δ as anode material for LaGaO3-based solid oxide fuel cell. Electrochem. Commun. 2008, 10, 1369–1372. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Zhao, N.; Liu, Y.; He, B.; Hu, F.; Chen, C. Structure properties and catalytic performance in methane combustion of double perovskites Sr2Mg1−xMnxMoO6−δ. Appl. Catal. B Environ. 2010, 102, 78–84. [Google Scholar] [CrossRef]
- Li, C.; Wang, W.; Xu, C.; Liu, Y.; He, B.; Chen, C. Double perovskite oxides Sr2Mg1−xFexMoO6−δ for catalytic oxidation of methane. J. Nat. Gas Chem. 2011, 156, 345–349. [Google Scholar] [CrossRef]
- Wang, Z.; Tian, Y.; Li, Y. Direct CH4 fuel cell using Sr2FeMoO6 as an anode material. J. Power Sources 2011, 196, 6104–6109. [Google Scholar] [CrossRef]
- Escudero, M.; Gómez de Parada, I.; Fuerte, A.; Daza, L. Study of Sr2Mg(Mo0.8Nb0.2)O6−δ as anode material for solid oxide fuel cells using hydrocarbons as fuel. J. Power Sources 2013, 243, 654–660. [Google Scholar] [CrossRef]
- Howell, T.; Kuhnell, C.; Reitz, T. A2MgMoO6 (A = Sr, Ba) for use as sulfur tolerant anodes. J. Power Sources 2013, 231, 279–284. [Google Scholar] [CrossRef]
- Zheng, К.; Swierczek, K.; Zając, W.; Klimkowicz, A. Rock salt ordered-type double perovskite anode materials for solid oxide fuel cells. Solid State Ion. 2014, 257, 9–16. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Yang, X.; Feng, T.; He, T. Resisting coking and sulfur poisoning of double perovskite Sr2TiFe0.5Mo0.5O6−δ anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 3280–3290. [Google Scholar] [CrossRef]
- Gwan, M.A.; Yun, J.W. Carbon tolerance effects of Sr2NiMoO6−δ as an alternative anode in solid oxide fuel cell under methane fuel condition. J. Electroceramics 2018, 40, 171–179. [Google Scholar] [CrossRef]
- Filonova, E.A.; Dmitriev, A.S.; Pikalov, P.S.; Medvedev, D.A.; Pikalova, E.Yu. The structural and electrical properties of Sr2Ni0.75Mg0.25MoO6 and its compatibility with solid state electrolytes. Solid State Ion. 2014, 262, 365–369. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Du, Z.; Chen, T.; Chen, N. Electrical, chemical, and electrochemical properties of double perovskite oxides Sr2Mg1−xNixMoO6−δ as anode materials for solid oxide fuel cells. J. Phys. Chem. C 2014, 118, 18853–18860. [Google Scholar] [CrossRef]
- Sereda, V.V.; Tsvetkov, D.S.; Sednev, A.L.; Druzhinina, A.I.; Malyshkin, D.A.; Zuev, A.Y. Thermodynamics of Sr2NiMoO6 and Sr2CoMoO6 and their stability under reducing conditions. Phys. Chem. Chem. Phys. 2018, 20, 20108–20116. [Google Scholar] [CrossRef] [PubMed]
- Skutina, L.S.; Vylkov, A.I.; Medvedev, D.A.; Filonova, E.A. Features of structural, thermal and electrical properties of Mo-based composite materials as fuel electrodes for high-temperature applications. J. Alloys Compd. 2017, 705, 854–861. [Google Scholar] [CrossRef]
- Niu, B.; Jin, F.; Fu, R.; Feng, T.; Shen, Y.; Liu, J.; He, T. Pd-impregnated Sr1.9VMoO6+δ double perovskite as an efficient and stable anode for solid-oxide fuel cells operating on sulfur-containing syngas. Electrochim. Acta 2018, 274, 91–102. [Google Scholar] [CrossRef]
- Xiao, G.; Chen, F. Ni modified ceramic anodes for direct-methane solid oxide fuel cells. Electrochem. Commun. 2011, 13, 57–59. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Sud, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Available online: http://www.ihte.uran.ru/?page_id=3154 (accessed on 21 June 2019).
- Available online: https://nanocenter.urfu.ru/en (accessed on 21 June 2019).
- Available online: https://zirconiaproject.wordpress.com/devices/zirconia-318/ (accessed on 21 June 2019).
- Osinkin, D.A.; Zabolotskaya, E.V.; Kellerman, D.G.; Suntsov, A.Yu. The physical properties and electrochemical performance of Ca-doped Sr2MgMoO6−δ as perspective anode for solid oxide fuel cells. J. Solid State Electrochem. 2018, 22, 1209–1215. [Google Scholar] [CrossRef]
- Filonova, E.A.; Russkikh, O.V.; Skutina, L.S.; Kochetova, N.A.; Korona, D.V.; Ostroushko, A.A. Influence of synthesis conditions on phase formation and functional properties of prospective anode material Sr2Ni0.75Mg0.25MoO6−δ. J. Alloys Compd. 2018, 748, 671–678. [Google Scholar] [CrossRef]
- Merkulov, O.V.; Markov, A.A.; Patrakeev, M.V.; Leonidov, I.A.; Shalaeva, E.V.; Tyutyunnik, A.P.; Kozhevnikov, V.L. Structural features and high-temperature transport in SrFe0.7Mo0.3O3−δ. J. Solid State Chem. 2018, 258, 447–452. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Steparuk, A.S.; Zuev, A.Y. The defect structure and chemical lattice strain of the double perovskites Sr2BMoO6−δ (B = Mg, Fe). Dalton Trans. 2016, 45, 12906–12913. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.R.; Marrocchelli, D.; Chatzichristodoulou, C.; Perry, N.H.; Mogensen, M.B.; Tuller, H.L.; Wachsman, E.D. Chemical expansion: Implications for electrochemical energy storage and conversion devices. Annu. Rev. Mater. Res. 2014, 44, 205–239. [Google Scholar] [CrossRef]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and chemical expansion in proton ceramic electrolytes and compatible electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Coors, W.G.; Manerbino, A. Characterization of composite cermet with 68 wt.% NiO and BaCe0.2Zr0.6Y0.2O3−δ. J. Membr. Sci. 2011, 376, 50–55. [Google Scholar] [CrossRef]
- Mori, M.; Yamamoto, T.; Itoh, H.; Inaba, H.; Tagawa, H. Thermal expansion of nickel-zirconia anodes in solid oxide fuel cells during fabrication and operation. J. Electrochem. Soc. 1998, 145, 1374–1381. [Google Scholar] [CrossRef]
- Marrero-López, D.; Peña-Martínez, J.; Ruiz-Morales, J.C.; Gabás, M.; Núñez, P.; Aranda, M.A.G.; Ramos-Barrado, J.R. Redox behaviour, chemical compatibility and electrochemical performance of Sr2MgMoO6−δ as SOFC anode. Solid State Ion. 2010, 180, 1672–1682. [Google Scholar] [CrossRef]
- Marrero-Lopez, D.; Pena-Martinez, J.; Ruiz-Morales, J.C.; Perez-Coll, D.; Aranda, M.A.G.; Nunez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6−δ anode. Mater. Res. Bull. 2008, 43, 2441–2450. [Google Scholar] [CrossRef]
- Kong, L.; Liu, B.; Zhao, J.; Gu, Y.; Zhang, Y. Synthesis of nano-crystalline Sr2MgMoO6−δ anode material by a sol–gel thermolysis method. J. Power Sources 2009, 188, 114–117. [Google Scholar] [CrossRef]
- Niakolas, D.K. Sulfur poisoning of Ni-based anodes for Solid Oxide Fuel Cells in H/C-based fuels. Appl. Catal. A Gen. 2014, 486, 123–142. [Google Scholar] [CrossRef]
- Wang, W.; Su, C.; Wu, Y.; Ran, R.; Shao, Z. Progress in solid oxide fuel cells with nickel-based anodes operating on methane and related fuels. Chem. Rev. 2013, 113, 8104–8151. [Google Scholar] [CrossRef] [PubMed]
| x in (1−x)Sr2Mg0.25Ni0.75MoO6−δ + xNiO | αox·106, К−1 | x in (1−x)Sr2Mg0.25Ni0.75MoO6−δ + xNi | αred·106, К−1 |
|---|---|---|---|
| 0 | 14.6 [34] | 0 | 14.0 [34] |
| 15 | 15.1 | 15 | 13.8 |
| 30 | 15.6 | 30 | 13.9 |
| 50 | 15.6 | 50 | 14.0 |
| 70 | 15.6 | 70 | 13.9 |
| 85 | 15.4 | 85 | 14.5 |
| 100 | 23.1 (200–235 °C) 16.6 (235–800 °C) | 100 | 17.0 (200–580 °C) 21.2 (580–800 °C) |
| x, Ni content | σ, S cm−1 | Ea, eV |
|---|---|---|
| 15 | 0.79 | 0.11 (500–650 °C), 0.23 (650–800 °C) |
| 30 | 1.18 | 0.18 (500–650 °C), 0.33 (650–800 °C) |
| 50 | 1.01 | 0.19 (500–650 °C), 0.30 (650–800 °C) |
| 70 | 2.66 | 0.23 (500–650 °C), 0.43 (650–800 °C) |
| 85 | 458 | – (500–600 °C), 0.21 (650–800 °C) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skutina, L.S.; Vylkov, A.A.; Kuznetsov, D.K.; Medvedev, D.A.; Shur, V.Y. Tailoring Ni and Sr2Mg0.25Ni0.75MoO6−δ Cermet Compositions for Designing the Fuel Electrodes of Solid Oxide Electrochemical Cells. Energies 2019, 12, 2394. https://doi.org/10.3390/en12122394
Skutina LS, Vylkov AA, Kuznetsov DK, Medvedev DA, Shur VY. Tailoring Ni and Sr2Mg0.25Ni0.75MoO6−δ Cermet Compositions for Designing the Fuel Electrodes of Solid Oxide Electrochemical Cells. Energies. 2019; 12(12):2394. https://doi.org/10.3390/en12122394
Chicago/Turabian StyleSkutina, Lubov S., Aleksey A. Vylkov, Dmitry K. Kuznetsov, Dmitry A. Medvedev, and Vladimir Ya. Shur. 2019. "Tailoring Ni and Sr2Mg0.25Ni0.75MoO6−δ Cermet Compositions for Designing the Fuel Electrodes of Solid Oxide Electrochemical Cells" Energies 12, no. 12: 2394. https://doi.org/10.3390/en12122394