Formation of Conductive Oxide Scale on 33NK and 47ND Interconnector Alloys for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Experimental Procedure
3. Results and Discussion
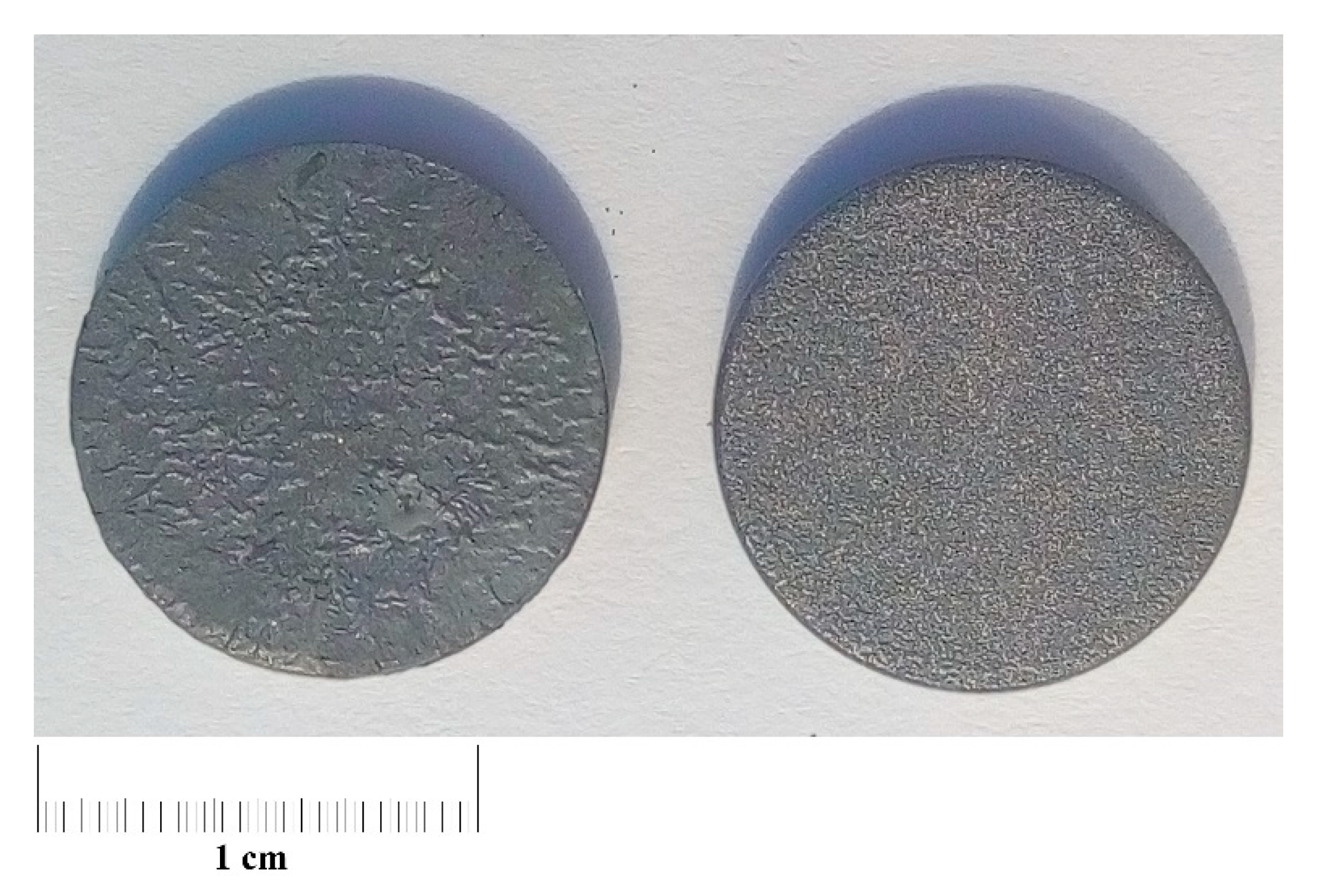
3.1. Features of the Studied Samples
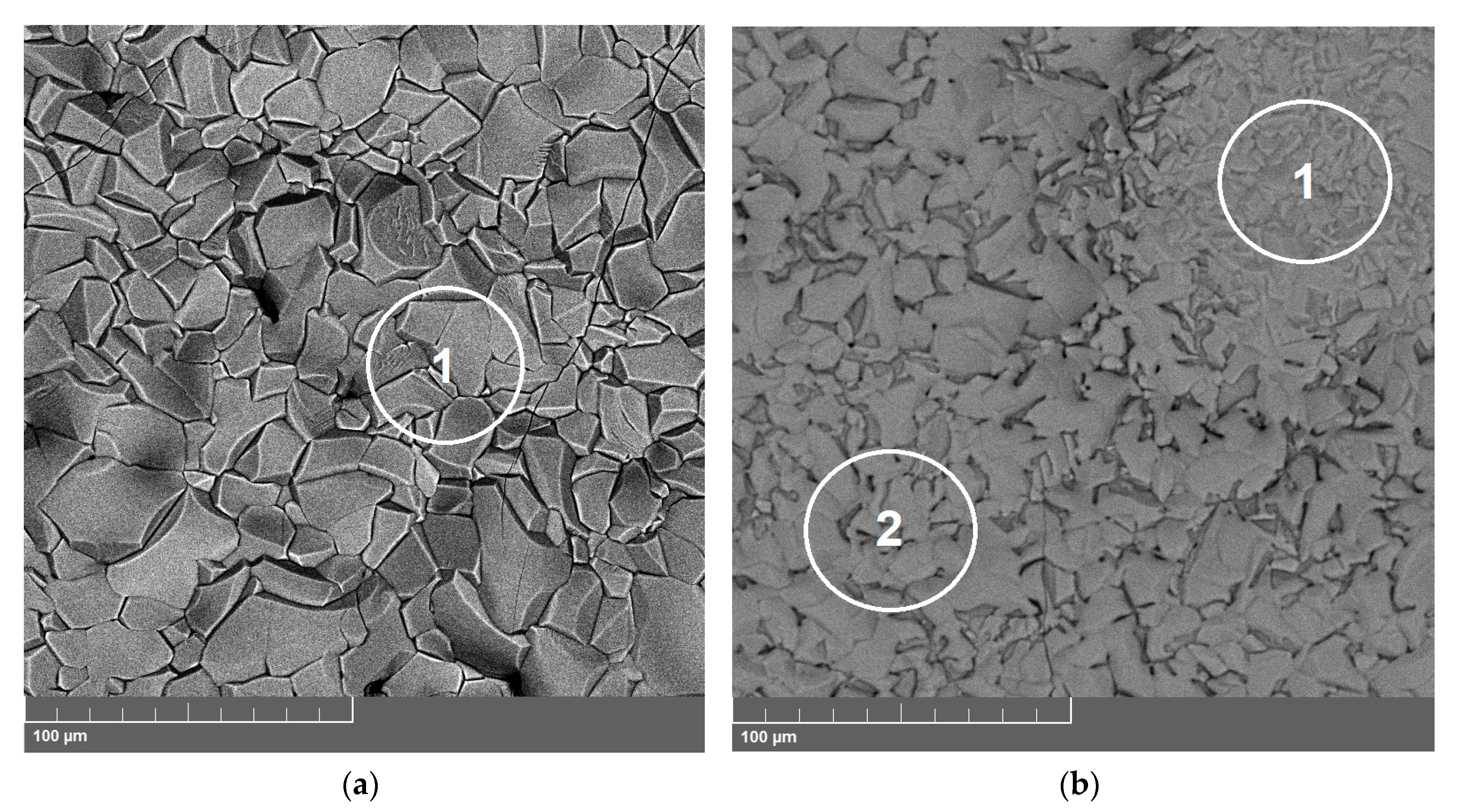
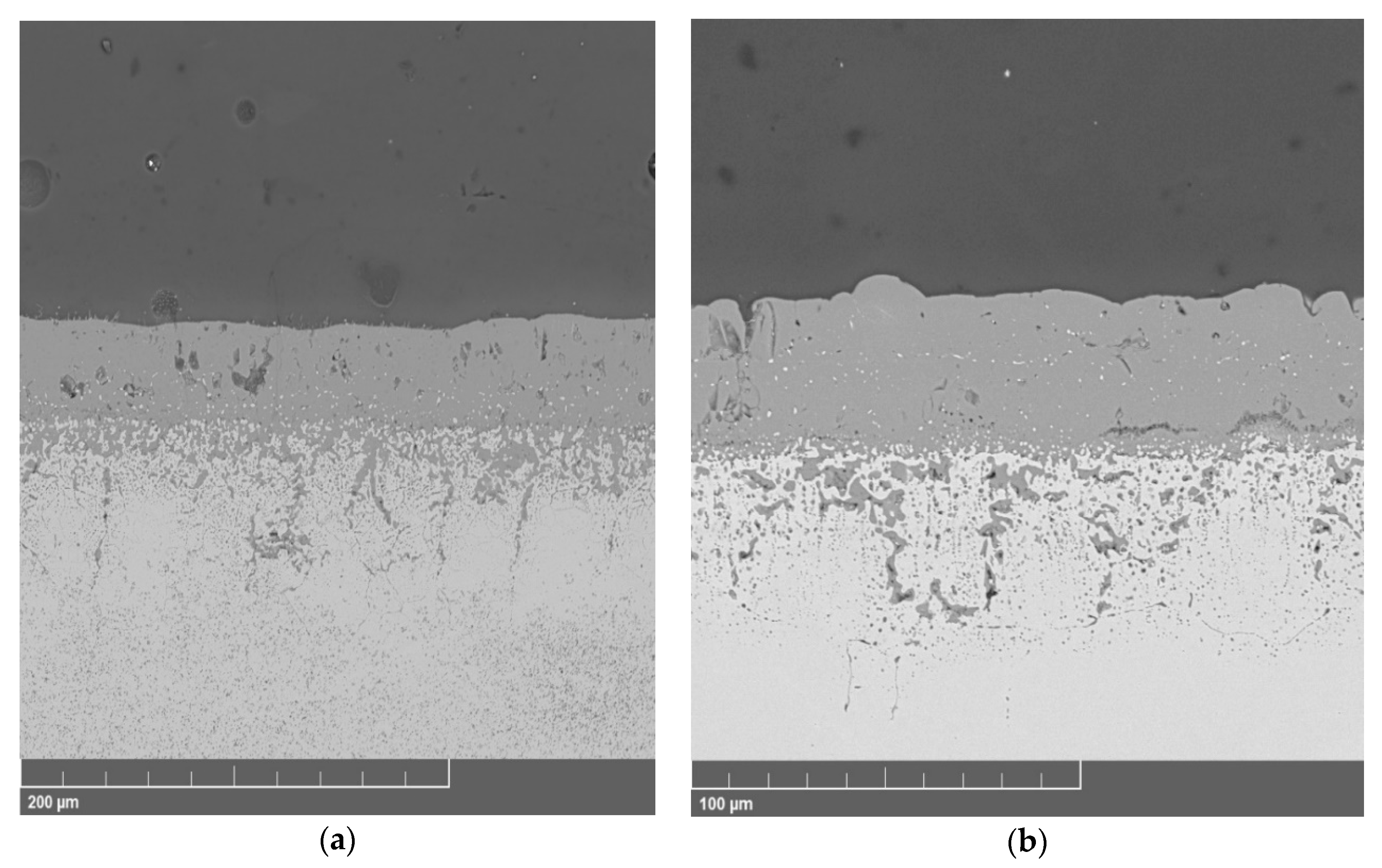
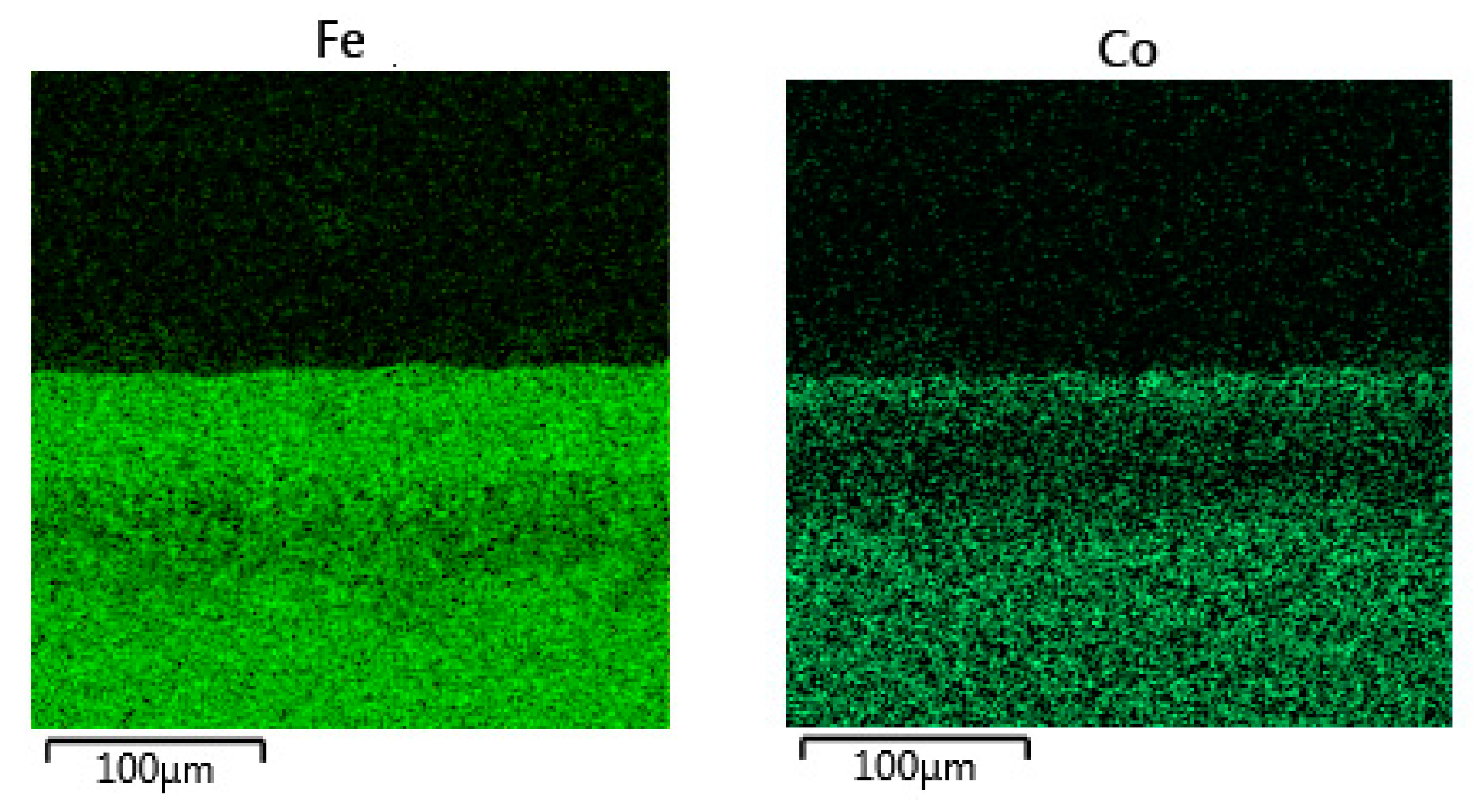
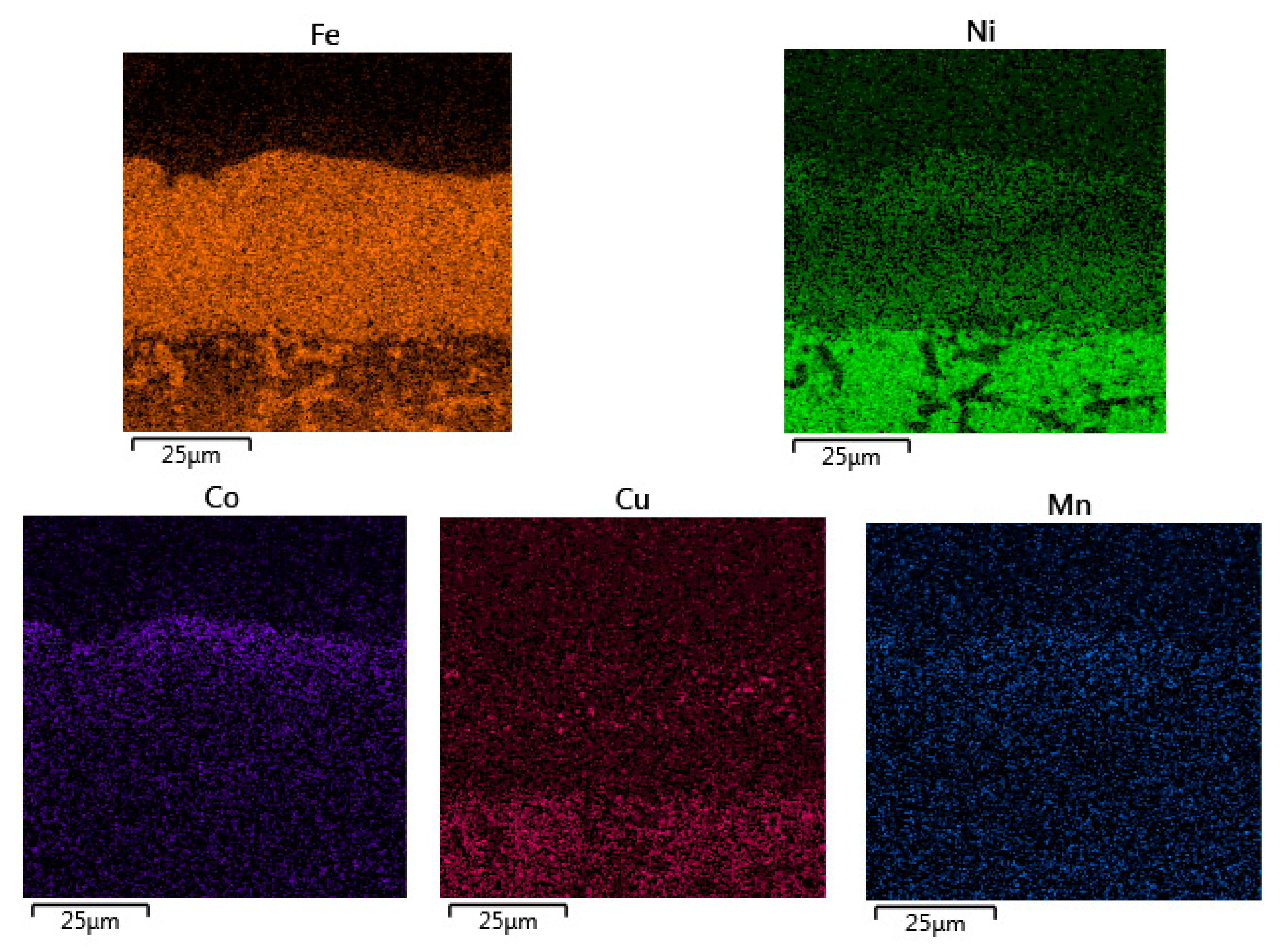
3.2. Microstructure of the Oxide Scales
3.2.1. 33NK Alloy
3.2.2. 47ND Alloy
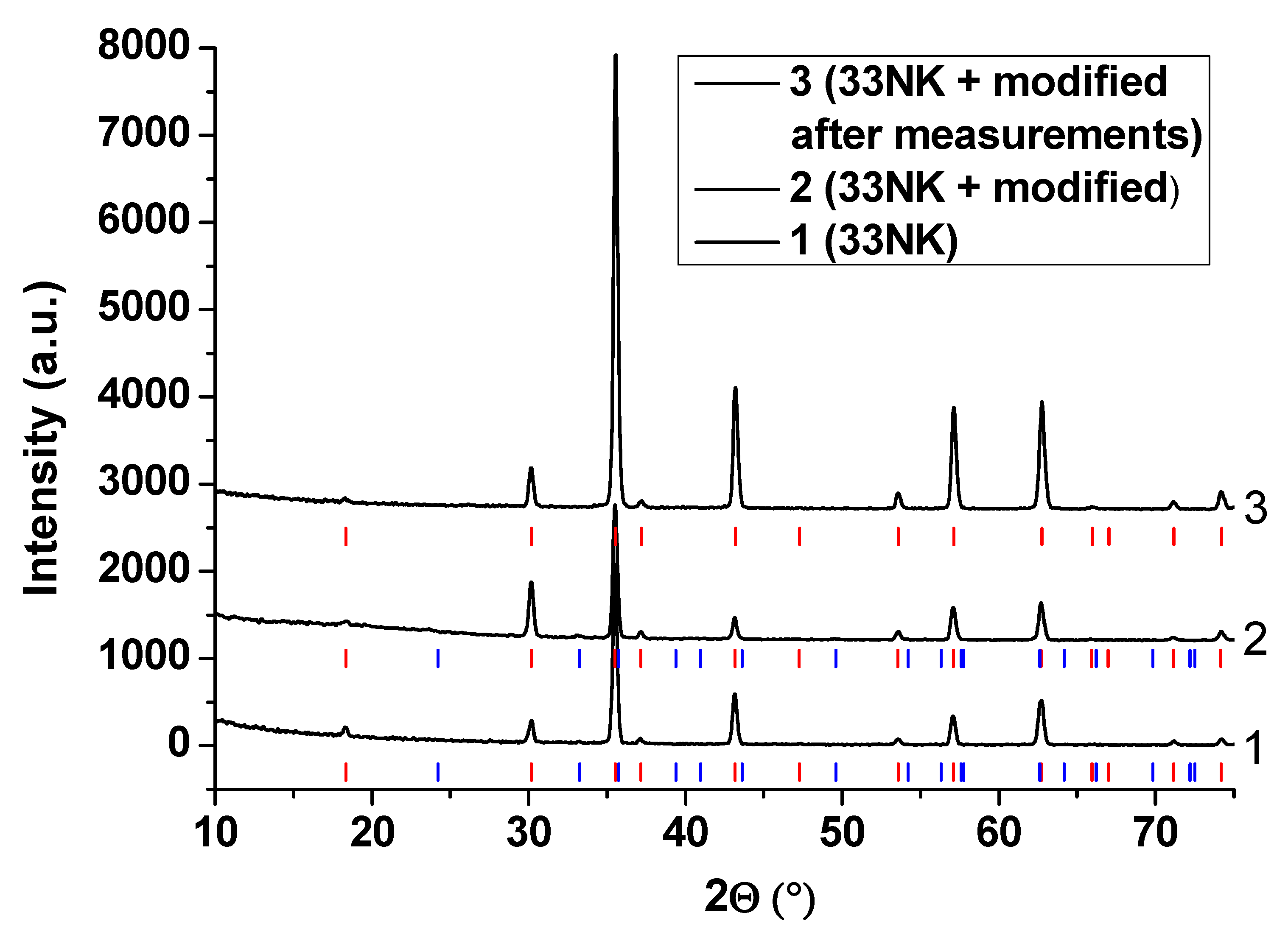
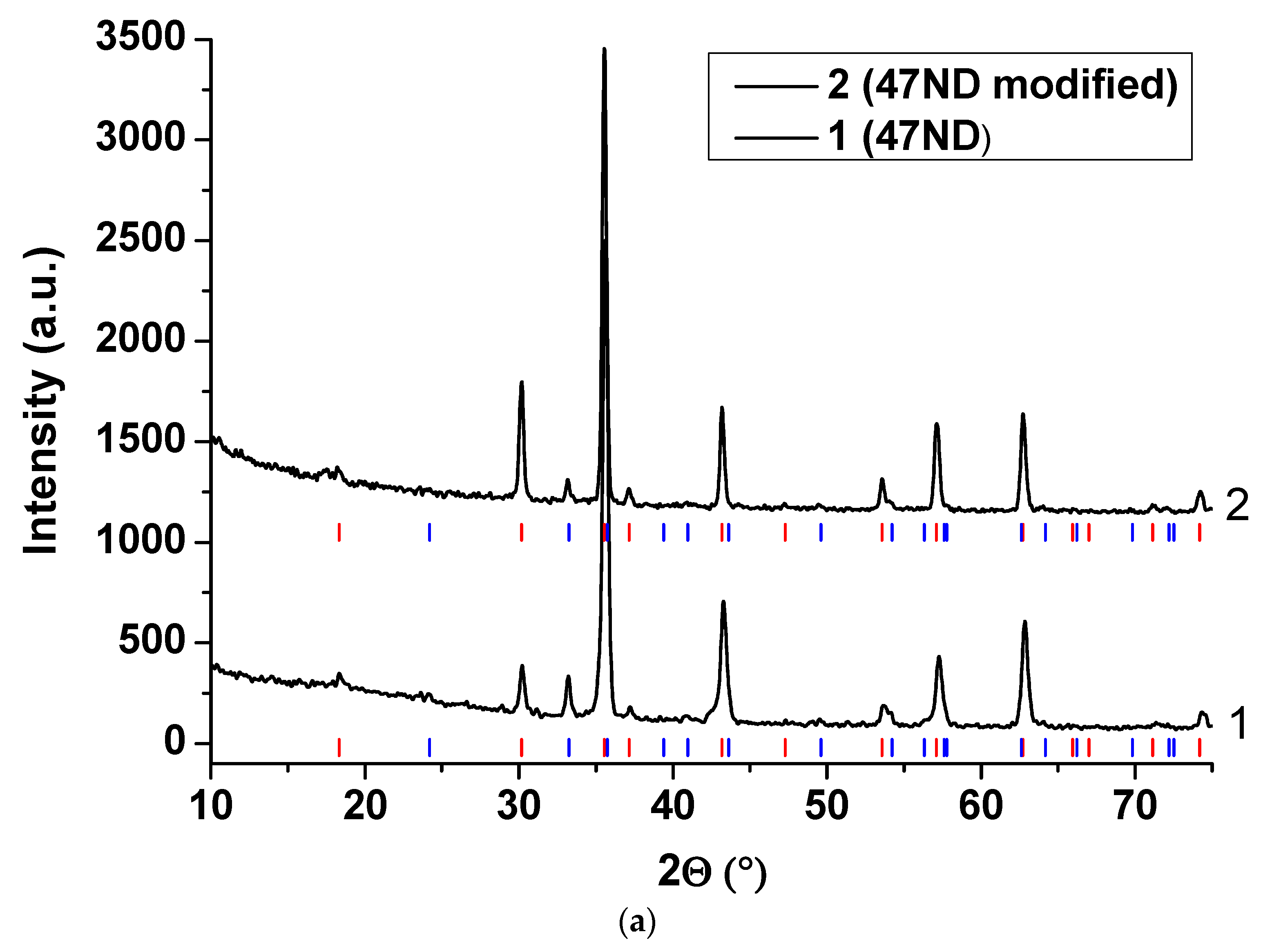
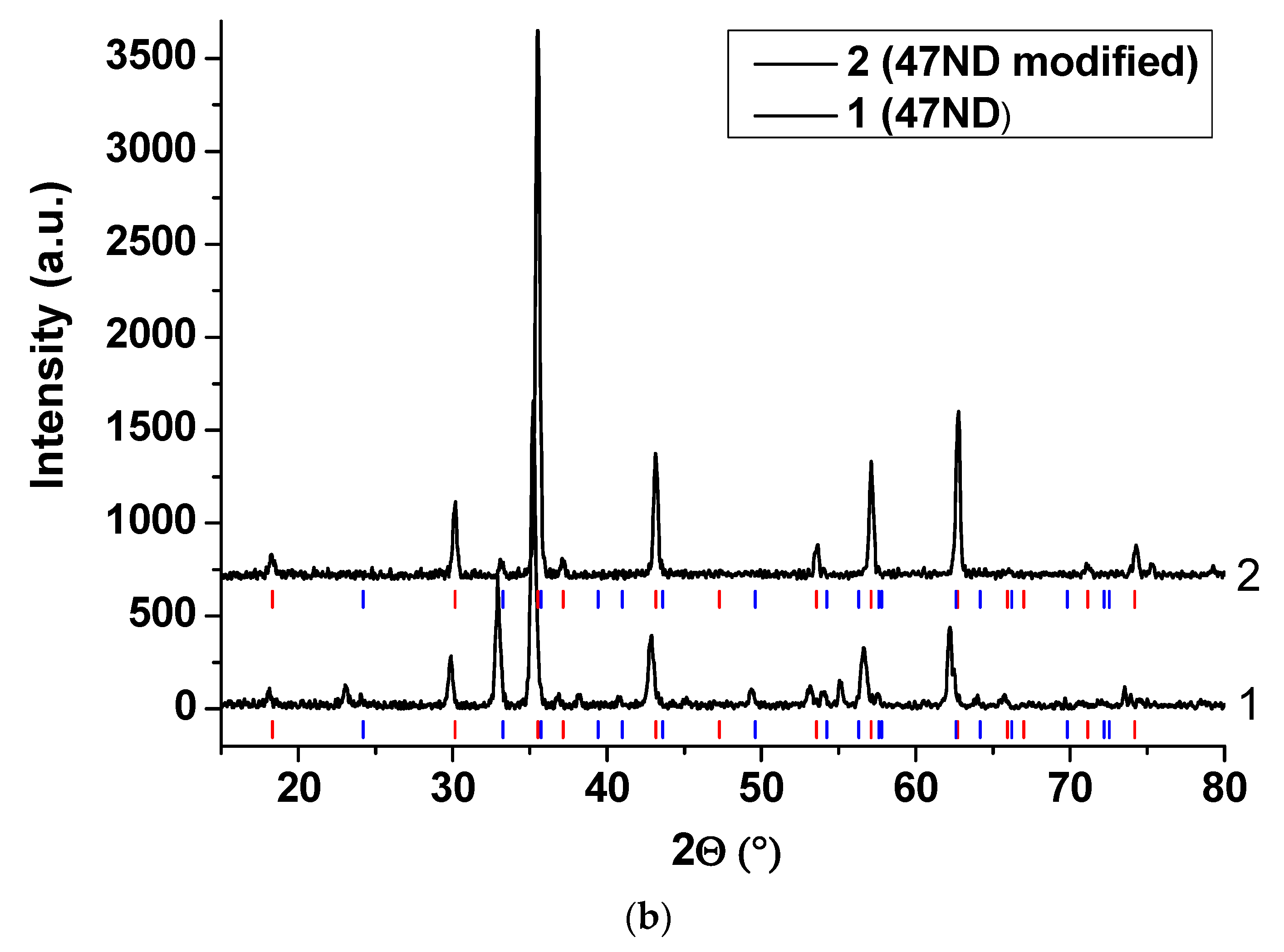
3.3. XRD Analysis of the Oxide Scales
3.3.1. 33NK Alloy
3.3.2. 47ND Alloy
3.4. Compatibility of the Oxide Scales with the Glass Sealant
3.5. Electrical Conductivity
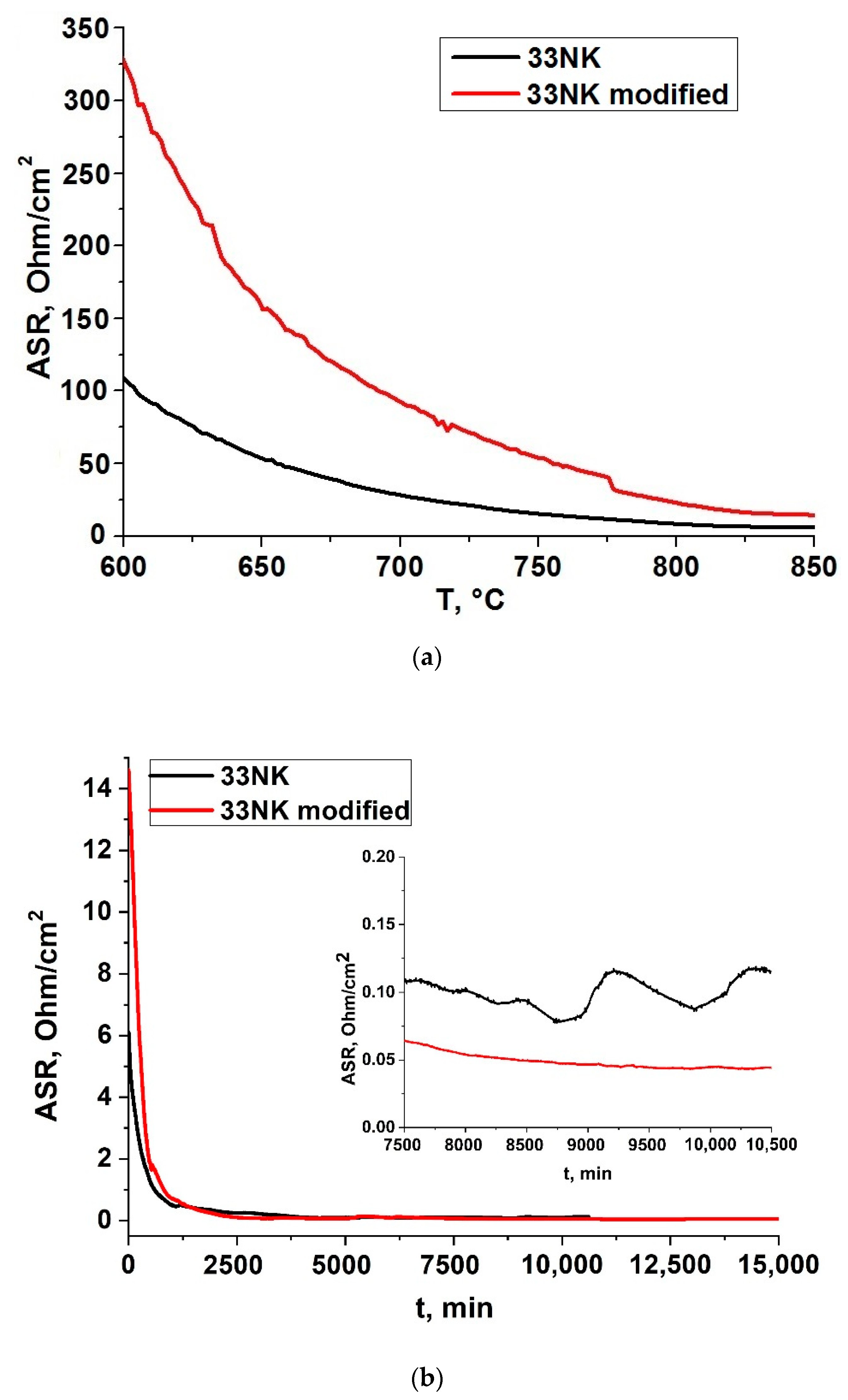
3.5.1. 33NK Alloy
3.5.2. 47ND Alloy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boaro, M.; Aricò, A.S. (Eds.) Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; CISM International Centre for Mechanical Sciences: Udine, Italy, 2017; Volume 574. [Google Scholar]
- Fergus, J.W. Lanthanum chromite-based materials for solid oxide fuel cell interconnects. Solid State Ion. 2004, 171, 1–15. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, M. Perspectives on the metallic interconnects for solid oxide fuel cells. J. Zhejiang Univ. Sci. 2004, 5, 1471–1503. [Google Scholar] [CrossRef]
- Fergus, J.W. Metallic interconnects for solid oxide fuel cells. Mater. Sci. Eng. 2005, 397, 271–283. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Piron-Abellan, J.; Shemet, V.; Singheiser, L. Metallic interconnectors for solid oxide fuel cells: A review. Mater. High Temp. 2003, 20, 115–127. [Google Scholar]
- Geng, S.; Zhu, J. Promising alloys for intermediate-temperature solid oxide fuel cell interconnect application. J. Power Sources 2006, 160, 1009–1016. [Google Scholar] [CrossRef]
- Hua, B.; Pu, J.; Lu, F.; Zhang, J.; Chi, B.; Jian, L. Development of a Fe–Cr alloy for interconnect application in intermediate temperature solid oxide fuel cells. J. Power Sources 2010, 195, 2782–2788. [Google Scholar] [CrossRef]
- Toji, A.; Uehara, T. Stability of oxidation resistance of ferritic Fe-Cr alloy for SOFC interconnects. ECS Trans. 2007, 7, 2117–2124. [Google Scholar]
- Sachitanand, R.; Sattari, M.; Svensson, J.E.; Froitzheim, J. Evaluation of the oxidation and Cr evaporation properties of selected Fe-Cr alloys used as SOFC interconnects. Int. J. Hydrog. Energy 2013, 38, 15328–15334. [Google Scholar] [CrossRef]
- Jablonski, P.D.; Alman, D.E. Oxidation resistance of novel ferritic stainless steels alloyed with titanium for SOFC interconnect applications. J. Power Sources 2008, 180, 433–439. [Google Scholar] [CrossRef]
- Seo, H.S.; Yun, D.W.; Kim, K.Y. Effect of Ti addition on the electric and ionic property of the oxide scale formed on the ferritic stainless steel for SOFC interconnect. Int. J. Hydrog. Energy 2012, 37, 16151–16160. [Google Scholar] [CrossRef]
- Hosseini, N.; Karimzadeh, F.; Abbasi, M.H.; Choi, G.M. Correlation between microstructure and electrical properties of Cu1.3Mn1.7O4/La2O3 composite-coated ferritic stainless steel interconnects. J. Alloys Compd. 2016, 673, 249–257. [Google Scholar] [CrossRef]
- Shaigan, N.; Qu, W.; Ivey, D.G.; Chen, W. A review of recent progress in coatings, surface modifications and alloy developments for solid oxide fuel cell ferritic stainless steel interconnects. J. Power Sources 2010, 195, 1529–1542. [Google Scholar] [CrossRef]
- Garcia-Fresnillo, L.; Shemet, V.; Chyrkin, A.; de Haart, L.G.J. Long-term behaviour of solid oxide fuel cell interconnect materials in contact with Ni-mesh during exposure in simulated anode gas at 700 and 800 °C. J. Power Sources 2014, 271, 213–222. [Google Scholar] [CrossRef]
- England, D.M.; Virkar, A. Oxidation kinetics of some nickel-based superalloy foils and electronic resistance of the oxide scale formed in air. Part 1. J. Electrochem. Soc. 1999, 146, 3196–3202. [Google Scholar] [CrossRef]
- England, D.M.; Virkar, A. Oxidation kinetics of some nickel-based superalloy foils and electronic resistance of the oxide scale formed in air. Part 2. J. Electrochem. Soc. 2001, 148, 330–338. [Google Scholar] [CrossRef]
- Zhu, W.Z.; Deevi, S.C. Development of interconnect materials for solid oxide fuel cells. Mater. Sci. Eng. 2003, 348, 227–243. [Google Scholar] [CrossRef]
- GOST (State Standard) 10994-74. Precision Alloys. Brands. USSR, 1974. Available online: http://docs.cntd.ru/document/1200009057 (accessed on 16 December 2019).
- Ananyev, M.V.; Solodyankin, A.A.; Eremin, V.A.; Farlenkov, A.S.; Khodimchuk, A.V.; Fetisov, A.V.; Chernik, A.A.; Yaskelychik, V.V.; Ostanina, T.N.; Zaikov, Y.P. Protective Coatings La–Mn–Cu–O for Stainless-Steel Interconnector 08X17T for SOFC, Obtained by the Electrocrystallization Method from Non-Aqueous Solutions. Russ. J. Non-Ferr. Met. 2018, 59, 102–110. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Eremin, V.A.; Solodyankin, A.A.; Yaskelchik, V.V. Electrochemical Method of Applying Conductive Oxide Protective Coating of Interconnector. Russian Federation Patent 2,016,139,988, 9 September 2016. [Google Scholar]
- Krainova, D.A.; Saetova, N.S.; Kuzmin, A.V.; Raskovalov, A.A.; Eremin, V.A.; Ananyev, M.V.; Steinberger-Wilckens, R. Non-Crystallising Glass Sealants for SOFC: Effect of Y2O3 Addition. Ceram. Int. 2020, in press. [Google Scholar] [CrossRef]







| Alloy Grade | Content, wt % | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | S | P | Ni | Co | Cu | Fe | ||
| 33NK | <0.05 | <0.30 | <0.40 | <0.015 | <0.015 | 32.5–33.5 | 16.5–17.5 | - | basic | [18] |
| 47ND | <0.05 | <0.30 | <0.40 | <0.015 | <0.015 | 46.0–48.0 | - | 4.5–5.5 | basic | [18] |
| Material | CTE, °C−1 |
|---|---|
| steel 08H17T | 12.8 × 10−6 (20–870 °C) |
| steel Crofer 22APU | 12.8 × 10−6 (20–870 °C) |
| alloy 33NK | 7.7 × 10−6 (20–500 °C) 17.6 × 10−6 (500–870 °C) |
| alloy 47ND | 10.1 × 10−6 (20–870 °C) |
| glass-sealant [21] | 9.1 × 10−6 (20–870 °C) |
| YSZ electrolyte | 10.4 × 10−6 (20–870 °C) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eremin, V.A.; Solodyankin, A.A.; Belyakov, S.A.; Khodimchuk, A.V.; Farlenkov, A.S.; Krainova, D.A.; Saetova, N.S.; Kuzmin, A.V.; Artamonov, A.S.; Steinberger-Wilckens, R.; et al. Formation of Conductive Oxide Scale on 33NK and 47ND Interconnector Alloys for Solid Oxide Fuel Cells. Energies 2019, 12, 4795. https://doi.org/10.3390/en12244795
Eremin VA, Solodyankin AA, Belyakov SA, Khodimchuk AV, Farlenkov AS, Krainova DA, Saetova NS, Kuzmin AV, Artamonov AS, Steinberger-Wilckens R, et al. Formation of Conductive Oxide Scale on 33NK and 47ND Interconnector Alloys for Solid Oxide Fuel Cells. Energies. 2019; 12(24):4795. https://doi.org/10.3390/en12244795
Chicago/Turabian StyleEremin, V.A., A.A. Solodyankin, S.A. Belyakov, A.V. Khodimchuk, A.S. Farlenkov, D.A. Krainova, N.S. Saetova, A.V. Kuzmin, A.S. Artamonov, R. Steinberger-Wilckens, and et al. 2019. "Formation of Conductive Oxide Scale on 33NK and 47ND Interconnector Alloys for Solid Oxide Fuel Cells" Energies 12, no. 24: 4795. https://doi.org/10.3390/en12244795
APA StyleEremin, V. A., Solodyankin, A. A., Belyakov, S. A., Khodimchuk, A. V., Farlenkov, A. S., Krainova, D. A., Saetova, N. S., Kuzmin, A. V., Artamonov, A. S., Steinberger-Wilckens, R., & Ananyev, M. V. (2019). Formation of Conductive Oxide Scale on 33NK and 47ND Interconnector Alloys for Solid Oxide Fuel Cells. Energies, 12(24), 4795. https://doi.org/10.3390/en12244795







