1. Introduction
From the start of the oil and gas industry (O&G industry) on the Norwegian continental shelf, safety standards have been established and developed. These national standards and guidelines regulate several industrial safety aspects. This applies both to design and operation of facilities and installations on land and at sea. The industry must operate and maintain its facilities and equipment so that accidents, such as fires and explosions, are prevented.
During the last 3–4 decades, the international O&G industry has experienced several major accidents [
1,
2]. Much work is therefore undertaken to limit the fire and explosion risks associated with processing highly combustible hydrocarbon products. However, severe fires in this industry still occur [
2,
3]. Equipment and piping is often constructed of different steel alloys, being especially vulnerable to impinging jet fires (i.e., the release of hydrocarbon gases under pressure resulting in well mixed high temperature flames of significant momentum). The weakening of exposed pressurized piping, equipment and load bearing constructions when heated may result in escalation of the fire scenario.
In the unfortunate scenario of an industrial fire, active fire protection is very important to prevent escalation of the incident. Among active fire protection measures, fire water systems prevail as outdoor fire protection, with the aim to cool fire exposed objects as well as to cool the flame zone to reduce heat exposure and prevent escalation. The NORSOK S-001 standard devotes a whole section to active fire protection [
4]. For process areas, the design criteria are known to be standardized and general (e.g., 10 L/min·m
2). However, very few studies document cooling efficiency as a function of exposed metal temperature for representative water droplet sizes, which also includes all the relevant droplet cooling/evaporation regimes. The few studies found in the literature are generally devoted to horizontal objects [
5].
Water droplets impinging onto hot surfaces display different boiling regimes, depending mainly on the surface temperature. The German theologist and physician Johann Gottlob Leidenfrost was the first scientist to study this phenomenon [
6]. He noticed that when the temperature of a particular metal object exceeded a certain value, the water droplets were moving about at the hot metal surface with a very low evaporation rate (i.e., a very low cooling rate). Since then, it has become common to call the temperature for the onset of this phenomenon as the Leidenfrost temperature. Different parameters, like the metal itself (thermal properties) or surface roughness (depth of anomalies and pattern), as well as droplet size and deposition method used, exert influence on the observed Leidenfrost temperature. With increasing temperature, the cooling rates of hot metal objects impacted by water droplets go through a maximum in the nucleate boiling regime (i.e., where the critical heat flux is observed) and then decline rapidly in the transition boiling regime as vapor cushions develop below the droplets. At high temperatures, this process results in inefficient cooling, reaching a minimum value at the Leidenfrost temperature for the particular material. The Leidenfrost temperature is not fixed, as the droplet application mode also seems to exert a few K of influence. The importance of surface roughness appears ambiguous [
7].
Bernardin and Mudawar [
8] presented a review on Leidenfrost temperatures for water on heated materials. Liang and Mudawar [
7] conducted a review concerning droplets impinging onto hot metals for all involved boiling regimes. Some researchers studied the behavior of different droplet parameters such as size, impingement velocity [
9], wall material [
10,
11], temperature below the Leidenfrost point [
12], influence of solid–liquid contact time [
13], and evaporation of layers of aqueous salt solution [
14]. Additives may also be used to reduce surface tension and thus enhance heat transfer [
15,
16]. Benedetto et al. [
17] studied the phenomenon called combustion induced rapid phase transition (cRPT), and the effect different mixtures of CH
4/O
2/N
2 have on this behavior. They found cRPT to be most conspicuous at O
2 concentrations above 21 mole%. Bjørge et al. [
5] presented a simple and straightforward method for obtaining the cooling efficiency of droplets impinging onto hot metal discs in the temperature range of 85 °C to 410 °C, covering all the boiling regimes experienced when water droplets are applied to hot metal objects.
The present study aims at analyzing: (a) The cooling efficiency of water droplets with various impingement speed and diameter falling on heated stainless steel discs with varied inclination, and (b) the effect of additives reducing surface tension on water droplet cooling efficiency. Experiments with acetone (additive, two different concentrations) were performed on aluminum discs for different impingement speeds at horizontal disc surface, as well as different surface inclinations for one selected impingement speed. The effect of adding NaCl at concentrations emulating seawater was also studied. The temperature range for all experiments was 85 °C to 410 °C, covering all water-droplet boiling regimes. The recorded droplet cooling efficiency for these different conditions is discussed.
3. Theory: Determining Cooling Efficiency
Droplet impact point and principal convective air flows for upward facing disc surfaces in both horizontal and inclined disc positions are shown in
Figure 3.
Based on differences in cooling rates between dry- and wet-cooling, mass and specific heat of the disc for each orientation, the heat flow to the impinging water droplets can be calculated. Subtracting the respective temperature versus time derivatives for the given disc temperature gives the net water droplets cooling rate,
(K/s). Based on the mass,
m (kg), and the specific heat,
(J/kg K), of the disc as a function of temperature, the water droplet cooling heat flow is given by:
In the present work, the specific heat data for stainless steel and aluminum given by [
5] were used in Equation (1). Ignoring the enthalpy needed to heat the water to 100 °C and heat the steam above 100 °C, the heat required to evaporate droplets at a rate,
(kg/s), is given by:
Representative recordings of temperature versus time for dry-cooling and wet-cooling (by applying water droplets) are shown in
Figure 4. The respective heat losses are shown in
Figure 5. It can be seen from
Figure 4 and
Figure 5 that dry-cooling was faster for the inclined orientation than for the horizontal orientation. Prior to, and after a measurement series, the free-cooling temperature versus time history was therefore always obtained. It should also be noted that the discs loose heat by thermal radiation, which was assumed independent on inclination.
The relative droplet cooling efficiency can then be calculated by:
5. Discussion
When assessing different parameters, such as surface roughness, the expected steam layer thickness is an essential parameter. Using computational methods, Chatzikyriakou et al. [
20] showed that the vapor layer exhibits oscillations for sessile water droplets, eventually settling to a thickness in the range of 20–40
m. This result is also supported by the theoretical value obtained by Wachters et al. [
21] for similar droplet conditions (28.9
m). Some contradictions were presented in [
7,
22] regarding the surface roughness influence on the heat transfer for the transition boiling and film boiling regime. Whereas some researchers describe increasing surface roughness to increase the heat transfer, others claim the opposite. It is believed that this contradiction can be related to the fact that some heat transfer dependences are valid in certain surface roughness ranges. There are also different ways of producing a given surface roughness; for example, the surface pattern could differ significantly, while still giving the same Ra value. This was the main reason for choosing Ra 0.4 (smooth surface) as a primary surface roughness for the experiments with acetone surfactant additives.
In the present work, there was no flame present during the water droplet cooling of the hot discs. Additionally, when applying water droplets, the discs were always the hottest object, hotter than the generated steam from the evaporating droplets. No condensation would therefore occur on the disc. The presented method could therefore not reveal phenomena such as cRPT [
17].
For the horizontally aligned stainless steel discs, the smallest droplets were observed to give a higher cooling efficiency over a wider temperature range. This is probably due to the larger relative contact surface area to the volume of the smaller droplets. The largest droplets demonstrated higher peak efficiency (i.e., higher cooling efficiency at boiling crisis) which was recorded to be in the range of 64–75% for the different configurations. The droplet cooling efficiency increased slightly with increasing impact velocity for 30
and 60
orientation. This is believed to be due to a rise in pressure inside the droplet and slowdown of the steam layer. For horizontal orientation, a higher cooling efficiency over a wider temperature range was observed for the lowest impact velocity. A reason for this could be the two identified boiling regimes/mechanisms of droplet bouncing based on Weber-number (We), as described by Biance et al. [
23]. In the first boiling regime, which represents droplet impingement at high We, drop impact inertia is significantly greater than surface tension. This renders the rebound less elastic. Due to droplet break up, the droplet more easily bounces off the hot metal disc.
For the horizontal configuration, droplet bouncing was more prominent for the largest droplets while the smaller droplets (lower Weber number) tended to attach better to the surface. The droplet cooling efficiency was shown to decrease with increased inclination for the lowest impact velocity (2.2 m/s). For higher droplet velocities, an increase in cooling efficiency for 30° inclination was observed. In line with the results of other researchers [
8], a cooling efficiency maximum was observed at 130 °C to 210 °C. In the film boiling regime, at temperatures above the Leidenfrost temperature (290–300 °C), the cooling efficiency was reduced to about 10%. This result was also in agreement with previous research [
8], with the added value in the present study being a concrete value for the cooling efficiency. For industrial fire water applications, a standard water supply rate of 10 L/min·m
2 gives a cooling capacity of 43 kW/m
2 at 10% cooling efficiency. This cooling heat flux is significantly lower than expected heat fluxes associated with pool and jet fires (250 kW/m
2 and 350 kW/m
2, respectively) [
4].
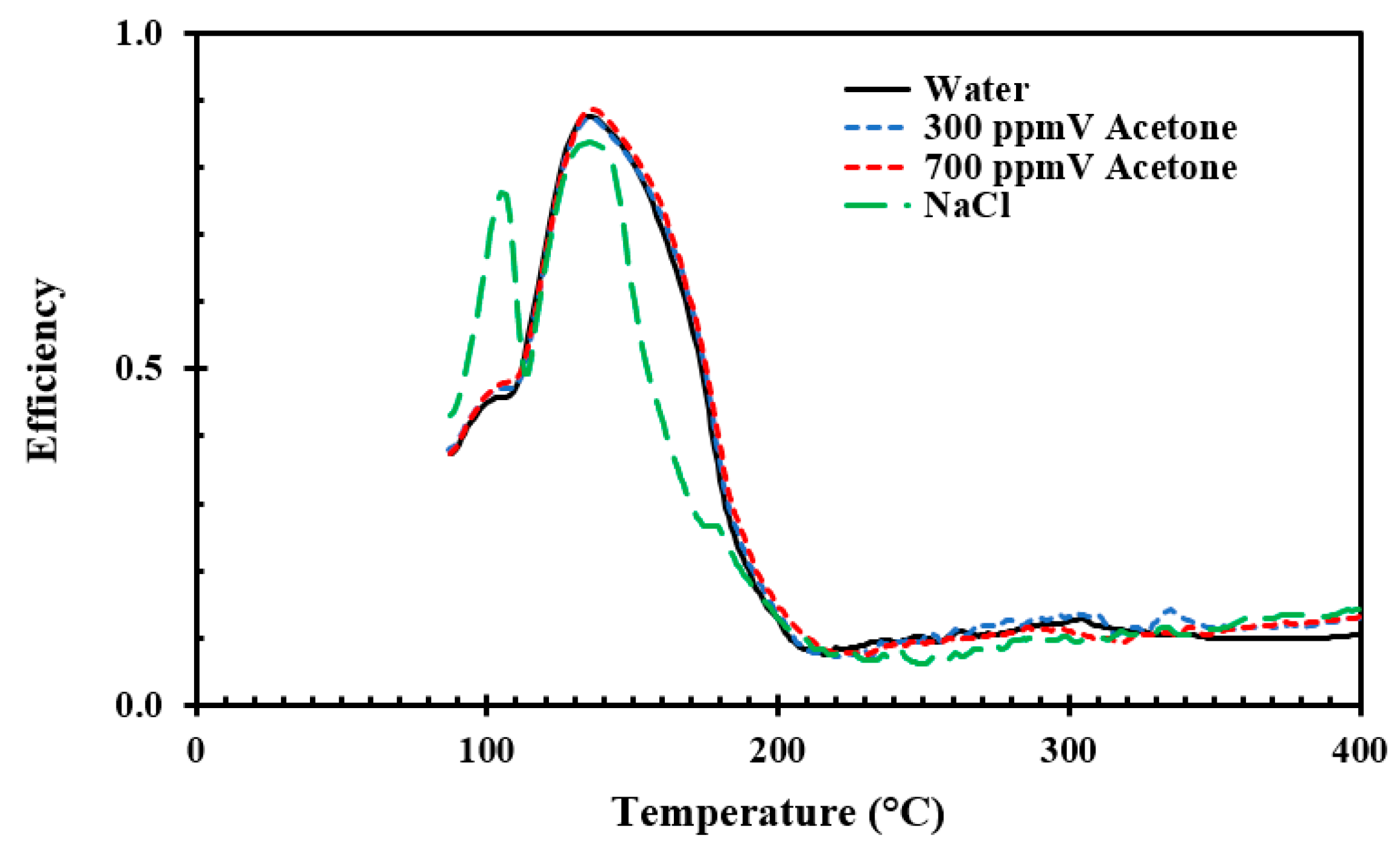
For the aluminum discs, the water droplet cooling efficiency was not significantly altered by adding acetone surfactant. The Leidenfrost point was only marginally changed for the horizontal orientation and the cooling efficiency increased approximately 2% at higher temperatures. However, the cooling efficiency at boiling crisis increased by 12% compared to pure water when using 700 ppm acetone and 25 cm impingement height. A similar trend was not observed in the other configurations. The droplet cooling efficiency increased slightly with a decrease in impact velocity. An increase in droplet velocity gave no conclusive indication at temperatures below the Leidenfrost temperature, however a low velocity gave a higher cooling efficiency in the film boiling regime. A reason for this may be as already mentioned and is supported by the study of Biance et al. [
23]. The observed Leidenfrost temperature was not significantly altered. Bhatt et al. [
16] reported that the droplet speed had to be 13.5–20 m/s for droplets less than 0.45 mm to significantly increase the Leidenfrost temperature when 300 ppm acetone surfactant was added.
A cooling efficiency maximum at 120–140 °C was observed for all aluminum disc tests. This temperature range is significant smaller than observed for the stainless steel tests. This is likely due to an order of magnitude higher thermal conductivity for aluminum versus stainless steel (i.e., 170 W/m·K versus 15 W/m·K). In the film boiling regime, at temperatures above the Leidenfrost temperature (230–240 °C), the cooling efficiency was in the range of 4–10% dependent on the aluminum disc orientation, where increased inclination gave a decrease in cooling efficiency. This is most likely due to impinging droplets more easily bouncing off after their first collision.
In the experiments with emulated seawater (35 g NaCl/kg), two distinct peaks were observed; the first at approximately 110 °C and the second at 130 °C (i.e., the temperature of critical heat flux for the aluminum discs). For the first temperature peak, salt was observed along the edge of the droplet contact area. The salt layer, which started to form on the metal surface, is believed to increase the evaporation rate in the triple-phase (liquid–gas–solid). A similar observation was made by Cui et al. [
24].
The nucleate boiling regime was observed to be narrower for the NaCl solution while the transition boiling regime was prolonged and the Leidenfrost temperature was significantly increased (i.e., about 20–30 K) compared to pure water. This is in agreement with the observations of other researchers [
25].
In fire water piping, there may be alien objects, like gravel, remains of mussels, etc. Such objects may restrict the flow of the system, in the worst cases render the system inoperable. Fire water systems therefore need to be tested regularly for confirming the system functionality. Using seawater for fire water raises concerns due to corrosion under insulation as well as corrosion attacks on cabling and instrumentation. The limited differences in cooling efficiency between pure water and 35 g/kg NaCl solution observed in the present study therefore do not support that seawater should be the preferred fire water supply.
It should be mentioned that the conspicuous nucleate boiling regime peak in NaCl solution cooling efficiency may be a result of the present study test method. If seawater was applied to the hot surface while the surface temperature was increasing rather than decreasing, the results could be altered in favor of salt water given that more salt may be deposited on the surface, increasing the water droplet surface contact. To test this was not possible with the current setup, and was therefore outside the scope of the present study.
For temperatures above the Leidenfrost temperature, the observed cooling efficiency slightly increased with increasing temperature up to 400 °C. This is most likely caused by the increasing temperature difference between the evaporating levitated droplets and the disc surface. The increase was, however, larger for the aluminum discs than for the stainless steel discs. An interpretation of this finding would require further studies.
During the droplet cooling, temperature gradients will be set up in the disc, especially when using stainless steel as the disc material. However, to analyze this was outside the scope of the present study. For future studies, it would be interesting to do backwards numerical analysis based on the recoded heat loss rates to reveal the magnitude of the internal temperature gradients.