Experimental Investigation of the Thermal Performance of a Wickless Heat Pipe Operating with Different Fluids: Water, Ethanol, and SES36. Analysis of Influences of Instability Processes at Working Operation Parameters
Abstract
1. Introduction
- (1)
- Obtaining temperature profiles of the axial wall temperature distribution;
- (2)
- Recognizing the effects of geyser boiling and boiling limit on the performance of the thermosyphon;
- (3)
- Recognizing the effects of the fill ratio and power input on the thermal performance of TPCTs;
- (4)
- Recognizing the effect of geometrical parameters on the thermal resistance of TPCTs;
- (5)
- Checking the ability of several literature correlations to predict experimental heat transfer coefficients.
2. Experimental Setup
3. Mathematical Model
4. Experimental Results and Discussion
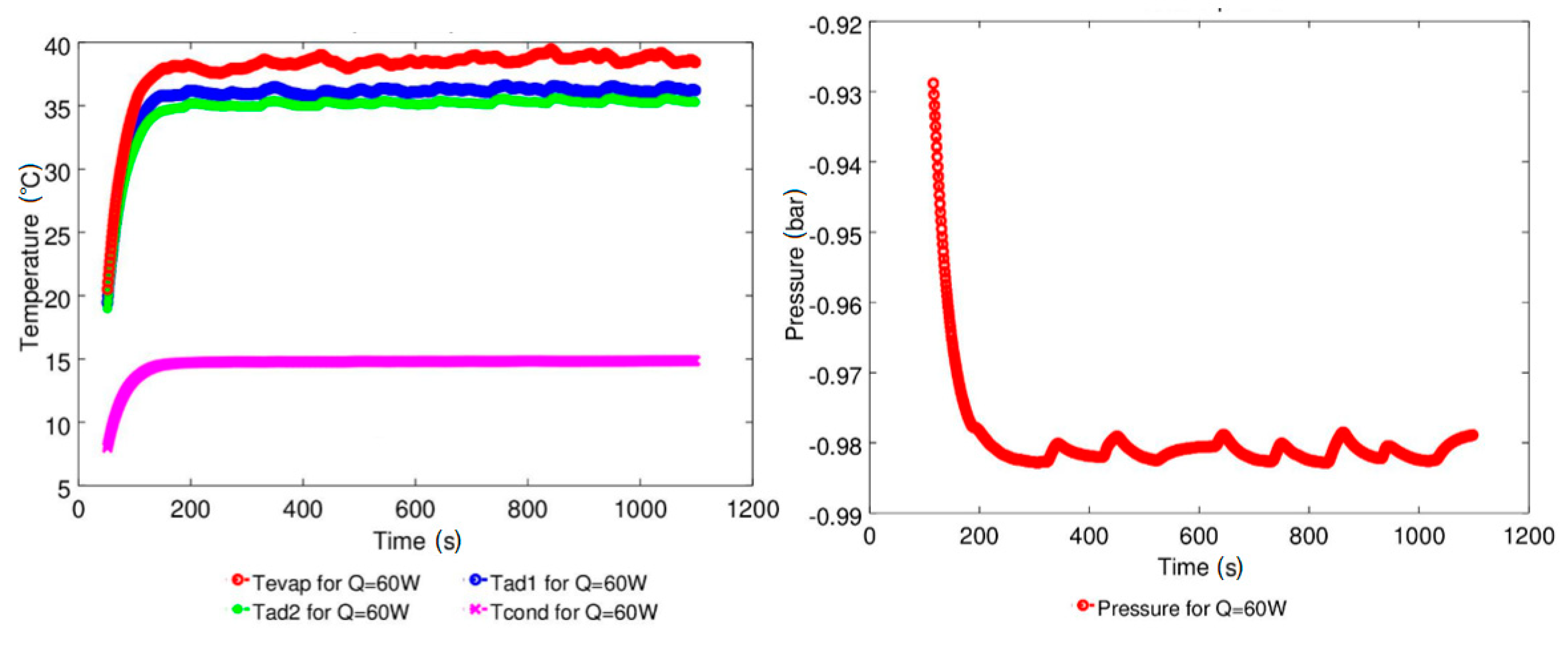
4.1. Effect of Geyser Boiling Influences and Boiling Limit on Operating Parameters
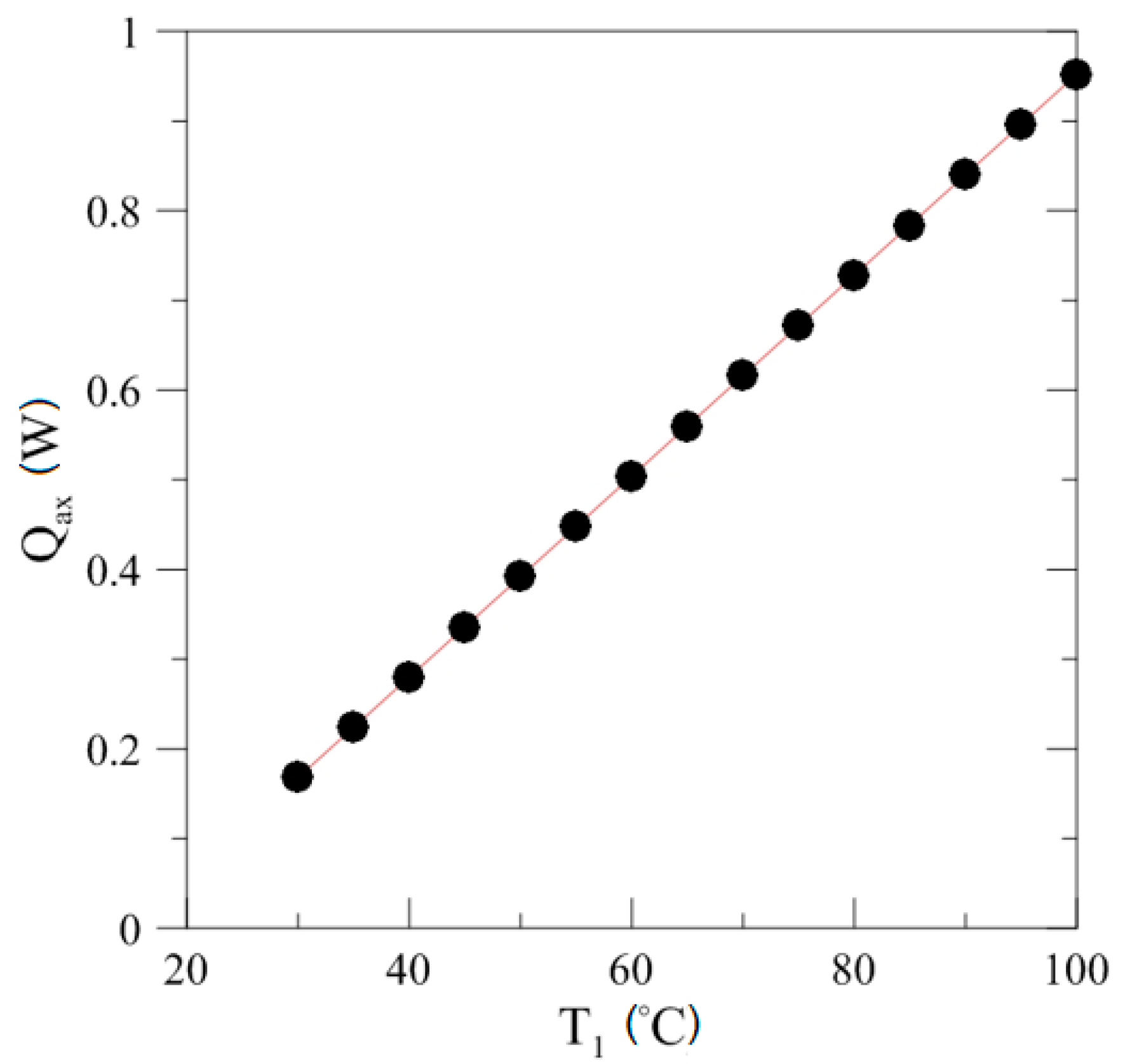
4.2. Effect of Power Input on Axial Temperature Distribution
4.3. Effect of Heating Power on Thermal Resistance
4.4. Heat Transfer
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | heat transfer surface area (m2) |
| AR | aspect ratio (-) |
| Cp | specific heat (J/kg·K) |
| D | inside thermosyphon diameter (m) |
| FR | fill ratio (-) |
| g | acceleration due to gravity (m/s2) |
| h | heat transfer coefficient (W/m2·K) |
| hfg | heat of vapor (kJ/kg) |
| I | current (A) |
| L | length of thermosyphon (m) |
| MAD | mean absolute deviation (-) |
| cold water mass flow (kg/s) | |
| electric power (W) | |
| operating pressure inside thermosyphon (bar) | |
| atmospheric pressure (Pa) | |
| vapor phase pressure (Pa) | |
| Prl | Prandtl number for liquid phase (-) |
| ΔP | pressure drop (Pa) |
| axial conducted heat flux (W) | |
| heat flux absorbed by cold water (W) | |
| electric heat flux (W) | |
| q | heat flux density (W/m2) |
| R | thermal resistance (K/W) |
| S | cross-section area of the tube (m2) |
| T | temperature (K) |
| T1 | temperature of cold end (K) |
| T2 | temperature of hot end (K) |
| U | voltage (V) |
| w | velocity (m/s) |
| W | fluid heat capacity rate (W/K) |
| volume (m3) | |
| z | axial coordinate (m) |
| Greek symbols | |
| ρ | density (kg/m3) |
| λ | thermal conductivity (W/m·K) |
| μ | dynamic viscosity (Pas) |
| Subscripts | |
| Atm | atmospheric |
| ad1 | beginning of adiabatic section |
| ad2 | end of adiabatic section |
| cond | end of condensation section |
| c | condenser |
| cu | copper |
| evap | evaporator |
| exp | experimental |
| f | fluid |
| in | inlet |
| l | liquid phase |
| out | outlet |
| sat | saturation |
| sur | surface |
| th | theoretical |
| w | water |
| v | vapor |
References
- Ong, K.-S.; Lim, C. Performance of water filled thermosyphons between 30–150 °C. Front. Heat Pipes 2015, 6. [Google Scholar] [CrossRef]
- Ong, K.S.; Goh, G.; Tshai, K.H.; Chin, W.M. Thermal resistance of a thermosyphon filled with R410A operating at low evaporator temperature. Appl. Therm. Eng. 2016, 106, 1345–1351. [Google Scholar] [CrossRef]
- Jouhara, H.; Robinson, A.J. Experimental investigation of small diameter two-phase closed thermosyphons charged with water, FC-84, FC-77 and FC-3283. Appl. Therm. Eng. 2010, 30, 201–211. [Google Scholar] [CrossRef]
- Ong, K.S. Effects of Inclination and Fill Ratio on R134a and R410a Thermosyphon. J. Energy Heat Mass Transf. 2011, 33, 143–152. [Google Scholar]
- Jafari, D.; Filippeschi, S.; Franco, A.; Di Marco, P. Unsteady experimental and numerical analysis of a two-phase closed thermosyphon at different filling ratios. Exp. Therm. Fluid Sci. 2017, 81, 164–174. [Google Scholar] [CrossRef]
- Heris, S.Z.; Fallahi, M.; Shanbedi, M.; Amiri, A. Heat transfer performance of two-phase closed thermosyphon with oxidized CNT/water nanofluids. Heat Mass Transf. 2016, 52, 85–93. [Google Scholar] [CrossRef]
- Eidan, A.A.; Najim, S.E.; Jalil, J.M. Experimental and numerical investigation of thermosyphone performance in HVAC system applications. Heat Mass Transf. 2016, 52, 2879–2893. [Google Scholar] [CrossRef]
- Aghel, B.; Rahimi, M.; Almasi, S. Heat-transfer enhancement of two-phase closed thermosyphon using a novel cross-flow condenser. Heat Mass Transf. 2017, 53, 765–773. [Google Scholar] [CrossRef]
- Wits, W.W.; te Riele, G.J. Modelling and performance of heat pipes with long evaporator sections. Heat Mass Transf. 2017, 53, 3341–3351. [Google Scholar] [CrossRef]
- Ong, K.S.; Hamlaoui, A. Experimental Observations of Water-filled and R134a-filled Thermosyphons Operating at Low Temperatures. J. Energy Heat Mass Transf. 2002, 24, 235–254. [Google Scholar]
- Alammar, A.A.; Al-Dadah, R.K.; Mahmoud, S.M. Experimental investigation of the influence of the geyser boiling phenomenon on the thermal performance of a two-phase closed thermosyphon. J. Clean. Prod. 2018, 172, 2531–2543. [Google Scholar] [CrossRef]
- Tahani, M.; Javan, S.; Biglari, M. A comprehensive study on waste heat recovery from internal combustion engines using organic Rankine cycle. Therm. Sci. 2013, 17, 611–624. [Google Scholar] [CrossRef]
- Riva, M.; Flohr, F.; Fröba, A. New fluid for high temperature applications. In International Refrigeration and Air Conditioning Conference; Purdue University: West Lafayette, IN, USA, 2006. [Google Scholar]
- Bell, I.H.; Quoilin, S.; Wronski, J.; Lemort, V. Coolprop: An open-source reference-quality thermophysical property library. In Proceedings of the ASME ORC 2nd International Seminar on ORC Power Systems, Rotterdam, The Netherlands, 7–8 October 2013. [Google Scholar]
- Zhang, L.; Zhang, Y.-F. Research on energy saving potential for dedicated ventilation systems based on heat recovery technology. Energies 2014, 7, 4261–4280. [Google Scholar] [CrossRef]
- Gedik, E.; Yılmaz, M.; Kurt, H. Experimental investigation on the thermal performance of heat recovery system with gravity assisted heat pipe charged with R134a and R410A. Appl. Therm. Eng. 2016, 99, 334–342. [Google Scholar] [CrossRef]
- Noie-Baghban, S.H.; Majideian, G.R. Waste heat recovery using heat pipe heat exchanger (HPHE) for surgery rooms in hospitals. Appl. Therm. Eng. 2000, 20, 1271–1282. [Google Scholar] [CrossRef]
- Jafari, D.; Franco, A.; Filippeschi, S.; Di Marco, P. Two-phase closed thermosyphons: A review of studies and solar applications. Renew. Sustain. Energy Rev. 2016, 53, 575–593. [Google Scholar] [CrossRef]
- Andrzejczyk, R.; Muszynski, T. Thermodynamic and geometrical characteristics of mixed convection heat transfer in the shell and coil tube heat exchanger with baffles. Appl. Therm. Eng. 2017, 121, 115–125. [Google Scholar] [CrossRef]
- Andrzejczyk, R.; Muszynski, T. An Experimental investigation on the effect of NEW continuous core-baffle geometry on the mixed convection heat transfer in shell and coil heat exchanger. Appl. Therm. Eng. 2018, 136, 237–251. [Google Scholar] [CrossRef]
- Andrzejczyk, R.; Muszynski, T.; Gosz, M. Experimental investigations on heat transfer enhancement in shell coil heat exchanger with variable baffles geometry. Chem. Eng. Process. Process Intensif. 2018, 132, 114–126. [Google Scholar] [CrossRef]
- Zhao, D.; Qian, X.; Gu, X.; Jajja, S.A.; Yang, R. Measurement techniques for thermal conductivity and interfacial thermal conductance of bulk and thin film materials. J. Electron. Packag. 2016, 138, 040802. [Google Scholar] [CrossRef]
- Jaśkiewicz, P. Pomiar przewodnictwa cieplnego i temperaturowego metali metodą Angströma. Available online: www.if.pw.edu.pl/~labfiz1p/cmsimple2_4/1instrukcje_pdf/38.pdf (accessed on 27 December 2018).
- Zhao, Z.; Zhang, Y.; Zhang, Y.; Zhou, Y.; Hu, H. Numerical Study on the Transient Thermal Performance of a Two-Phase Closed Thermosyphon. Energies 2018, 11, 1433. [Google Scholar] [CrossRef]
- Wang, D.; Liu, Z.; Shen, J.; Jiang, C.; Chen, B.; Yang, J.; Tu, Z.; Liu, W. Experimental study of the loop heat pipe with a flat disk-shaped evaporator. Exp. Therm. Fluid Sci. 2014, 57, 157–164. [Google Scholar] [CrossRef]
- Lin, T.F.; Lin, W.T.; Tsay, Y.L.; Wu, J.C.; Shyu, R.J. Experimental investigation of geyser boiling in an annular two-phase closed thermosyphon. Int. J. Heat Mass Transf. 1995, 38, 295–307. [Google Scholar] [CrossRef]
- Casarosa, C.; Latrofa, E.; Shelginski, A. The geyser effect in a two-phase thermosyphon. Int. J. Heat Mass Transf. 1983, 26, 933–941. [Google Scholar] [CrossRef]
- Khazaee, I.; Hosseini, R.; Noie, S.H. Experimental investigation of effective parameters and correlation of geyser boiling in a two-phase closed thermosyphon. Appl. Therm. Eng. 2010, 30, 406–412. [Google Scholar] [CrossRef]
- Cengel, Y.A. Introduction to Thermodynamics and Heat Transfer; McGraw-Hill: New York, NY, USA, 1997. [Google Scholar]
- Khazaee, I. Experimental investigation and comparison of heat transfer coefficient of a two phase closed thermosyphon. Int. J. Energy Environ. 2014, 5, 495–505. [Google Scholar]
- Andrzejczyk, R.; Muszyński, T. The performance of H2O, R134a, SES36, ethanol, and HFE7100 two-phase closed thermosyphons for varying operating parameters and geometry. Arch. Thermodyn. 2017, 3, 3–21. [Google Scholar] [CrossRef]
- Baojin, Q.; Li, Z.; Hong, X.; Yan, S. Heat transfer characteristics of titanium/water two-phase closed thermosyphon. Energy Convers. Manag. 2009, 50, 2174–2179. [Google Scholar] [CrossRef]
- Andrzejczyk, R.; Muszynski, T.; Dorao, C.A. Experimental investigations on adiabatic frictional pressure drops of R134a during flow in 5 mm diameter channel. Exp. Therm. Fluid Sci. 2017, 83. [Google Scholar] [CrossRef]
- Shabgard, H.; Xiao, B.; Faghri, A.; Gupta, R.; Weissman, W. Thermal characteristics of a closed thermosyphon under various filling conditions. Int. J. Heat Mass Transf. 2014, 70, 91–102. [Google Scholar] [CrossRef]
- Táboas, F.; Valles, M.; Bourouis, M.; Coronas, A. Pool boiling of ammonia/water and its pure components: Comparison of experimental data in the literature with the predictions of standard correlations. Int. J. Refrig. 2007, 30, 778–788. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Karayiannis, T.G. Heat transfer correlation for flow boiling in small to micro tubes. Int. J. Heat Mass Transf. 2013, 66, 553–574. [Google Scholar] [CrossRef]
- Kandlikar, S.; Garimella, S.; Li, D.; Colin, S.; King, M.R. Heat Transfer and Fluid Flow in Minichannels and Microchannels; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Imura, H.; Sasaguchi, K.; Kozai, H.; Numata, S. Critical heat flux in a closed two-phase thermosyphon. Int. J. Heat Mass Transf. 1983, 26, 1181–1188. [Google Scholar] [CrossRef]
- Shiraishi, M.; Kikuchi, K.; Yamanishi, T. Investigation of heat transfer characteristics of a two-phase closed thermosyphon. In Advances in Heat Pipe Technology; Elsevier: Amsterdam, The Netherlands, 1982; pp. 95–104. [Google Scholar]
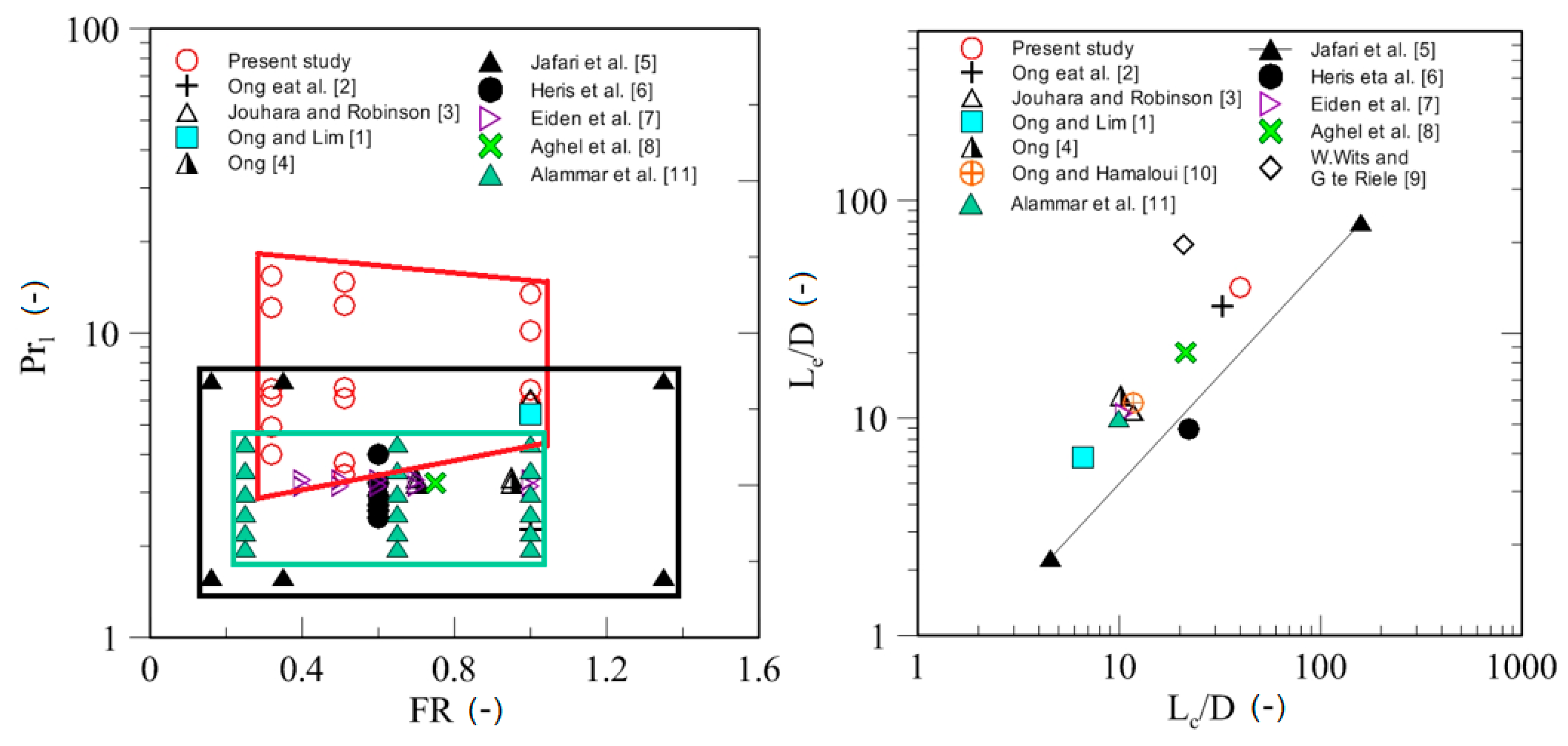

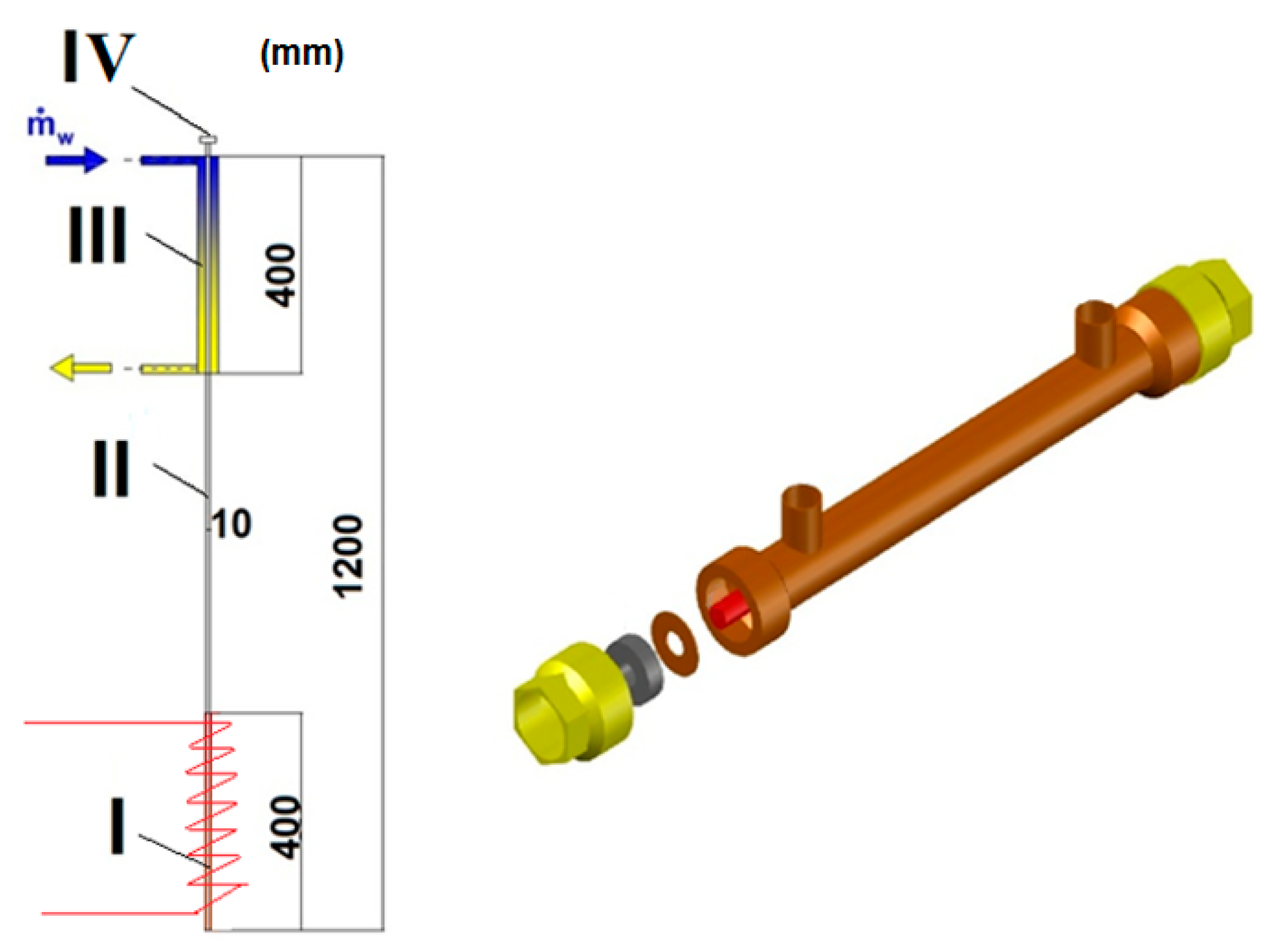

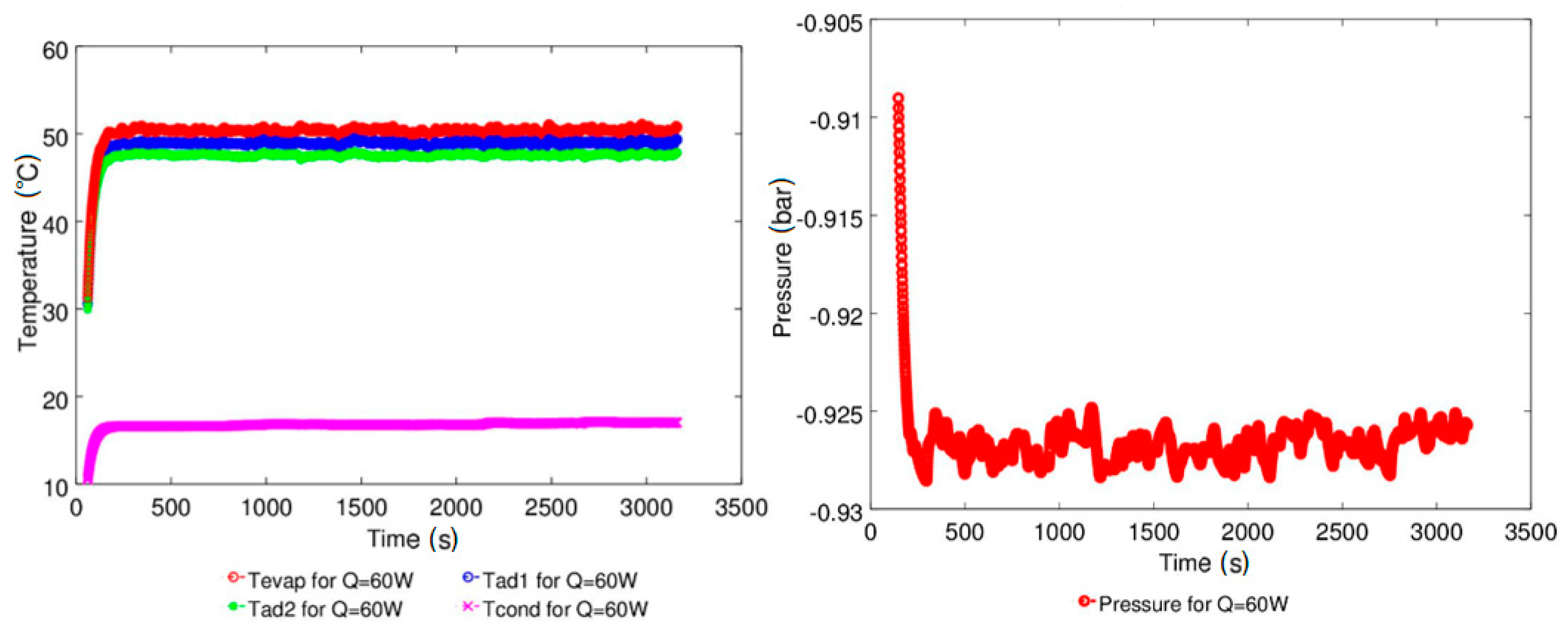
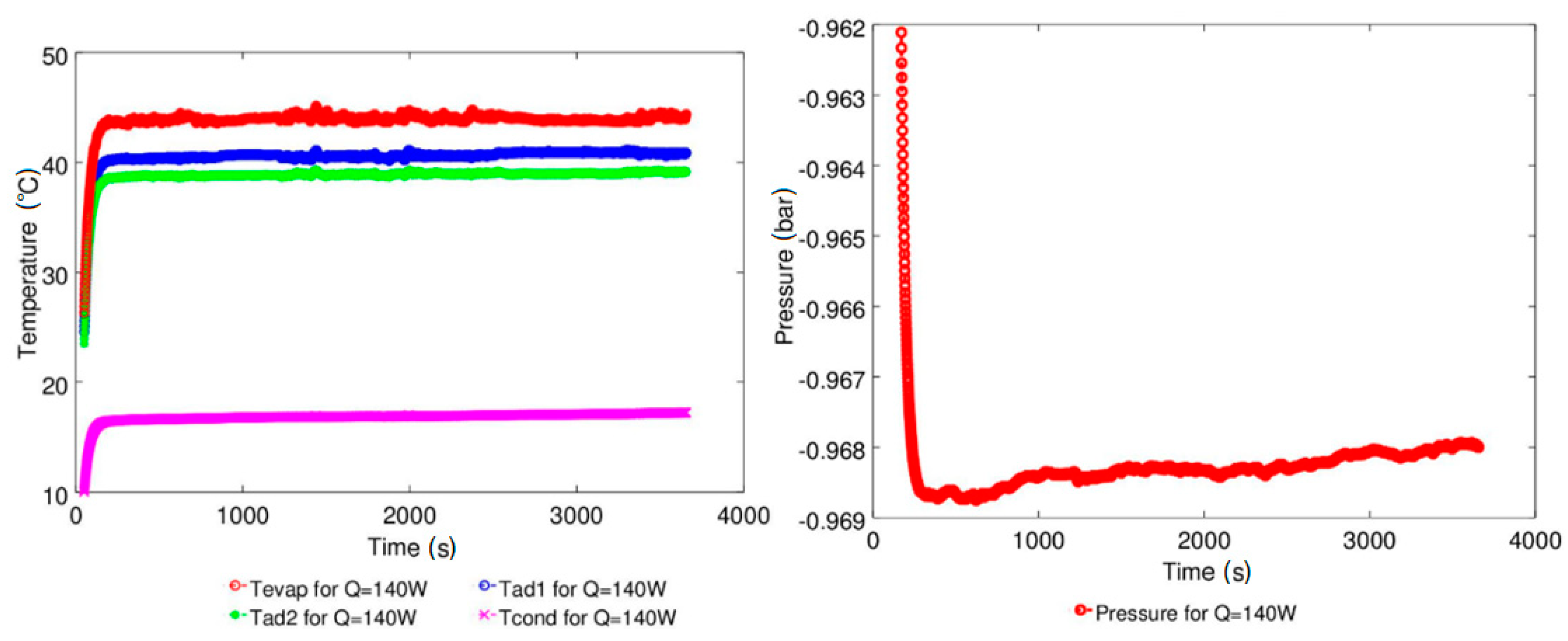
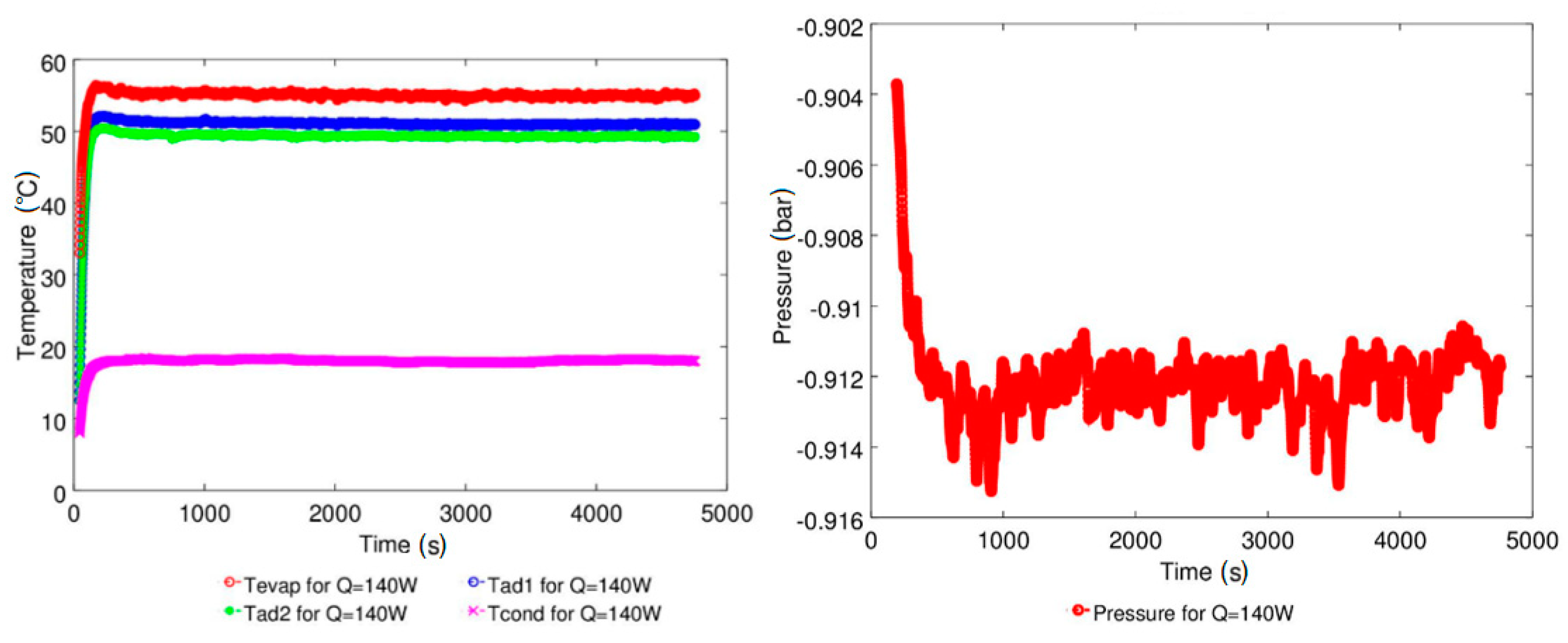
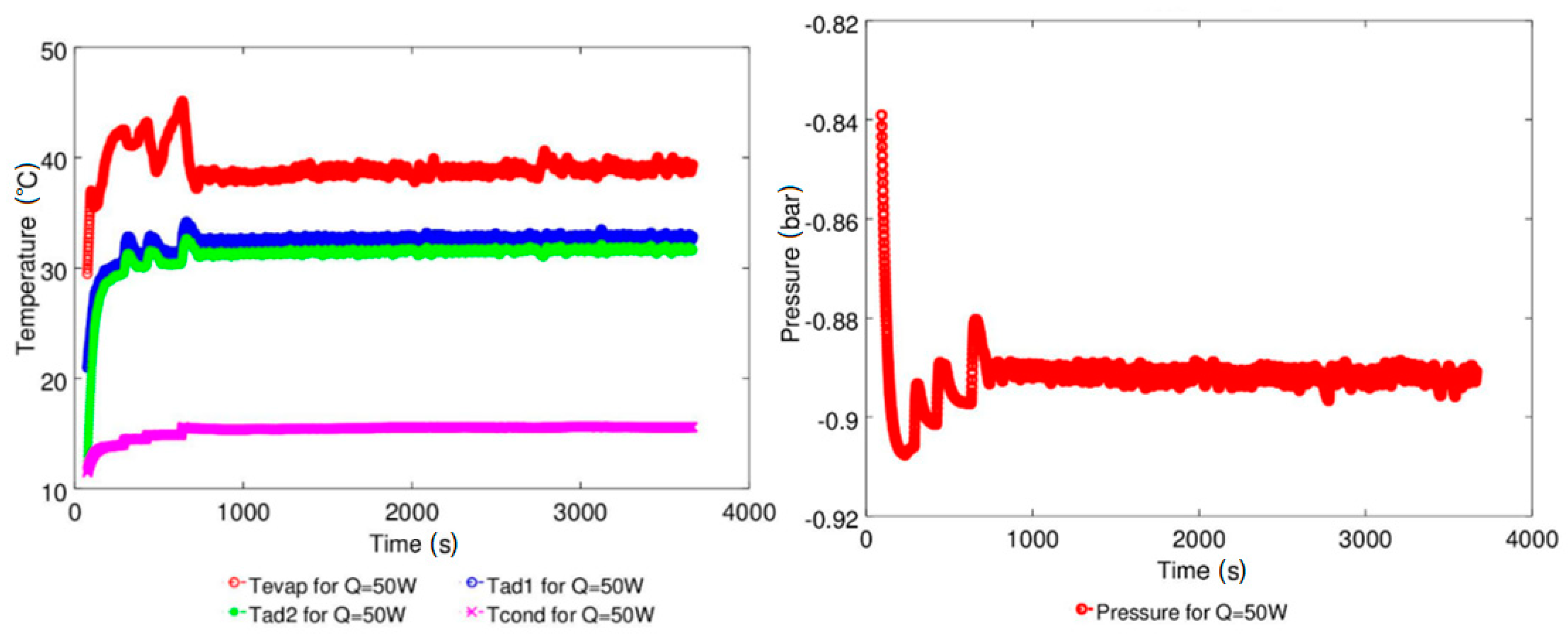
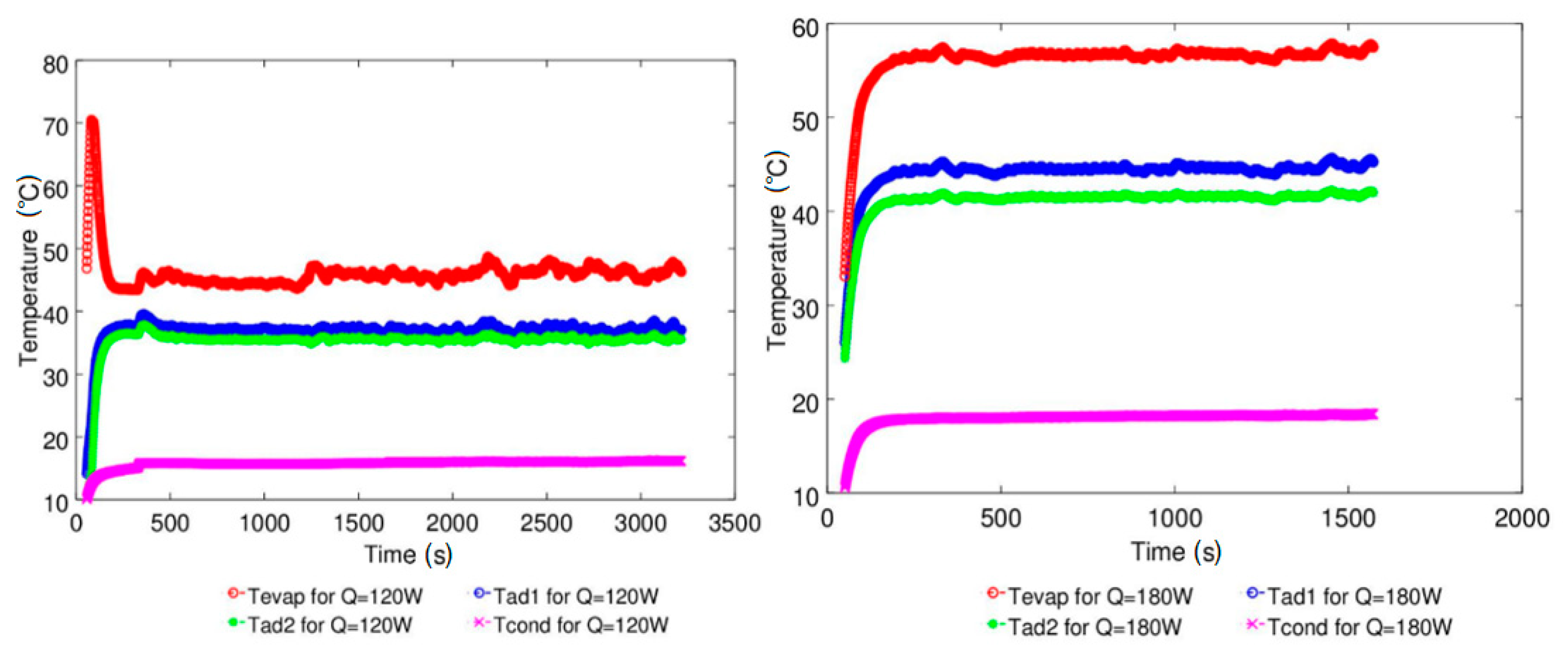
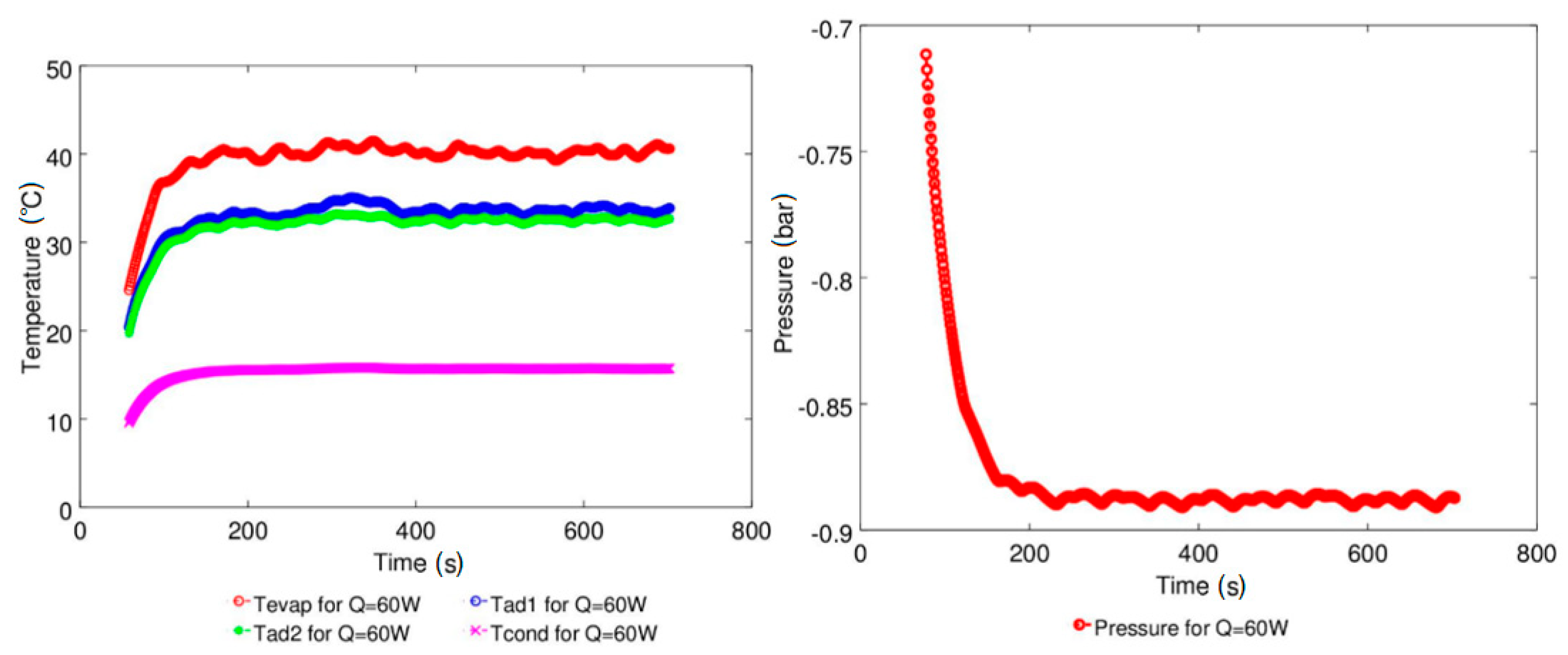
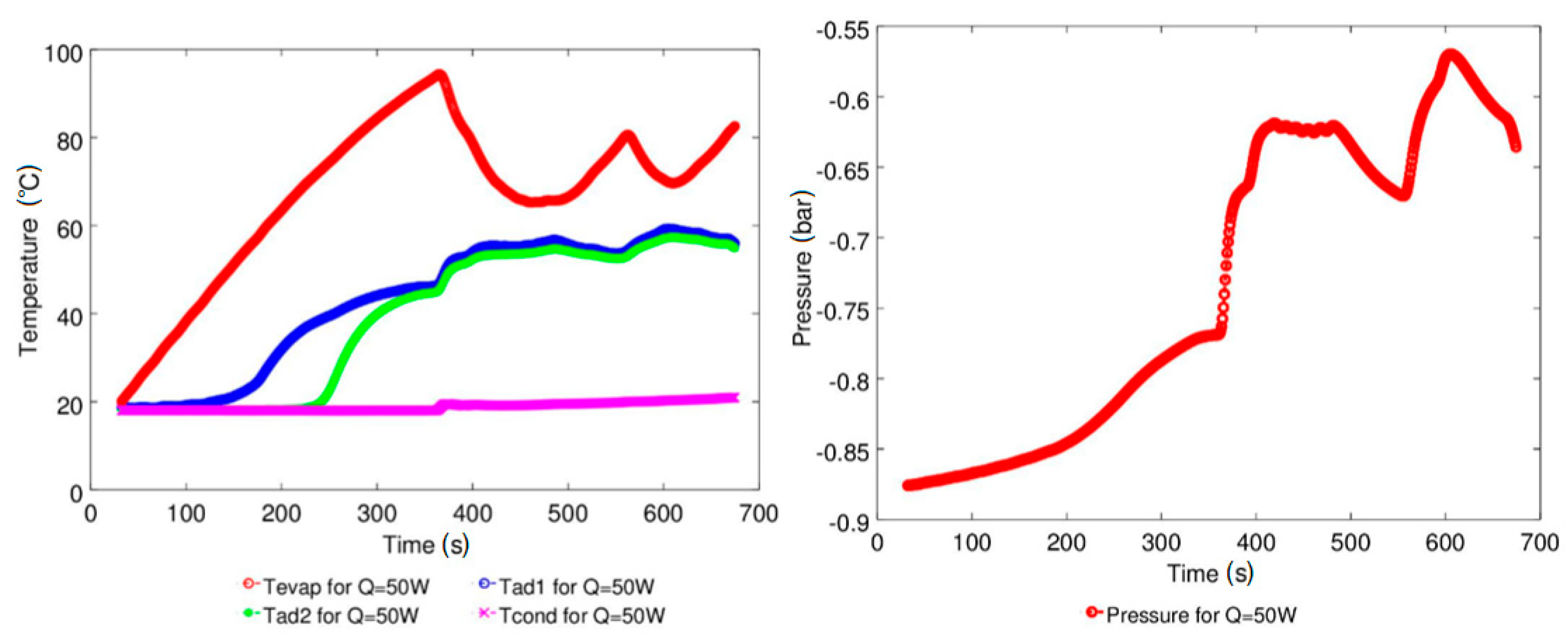
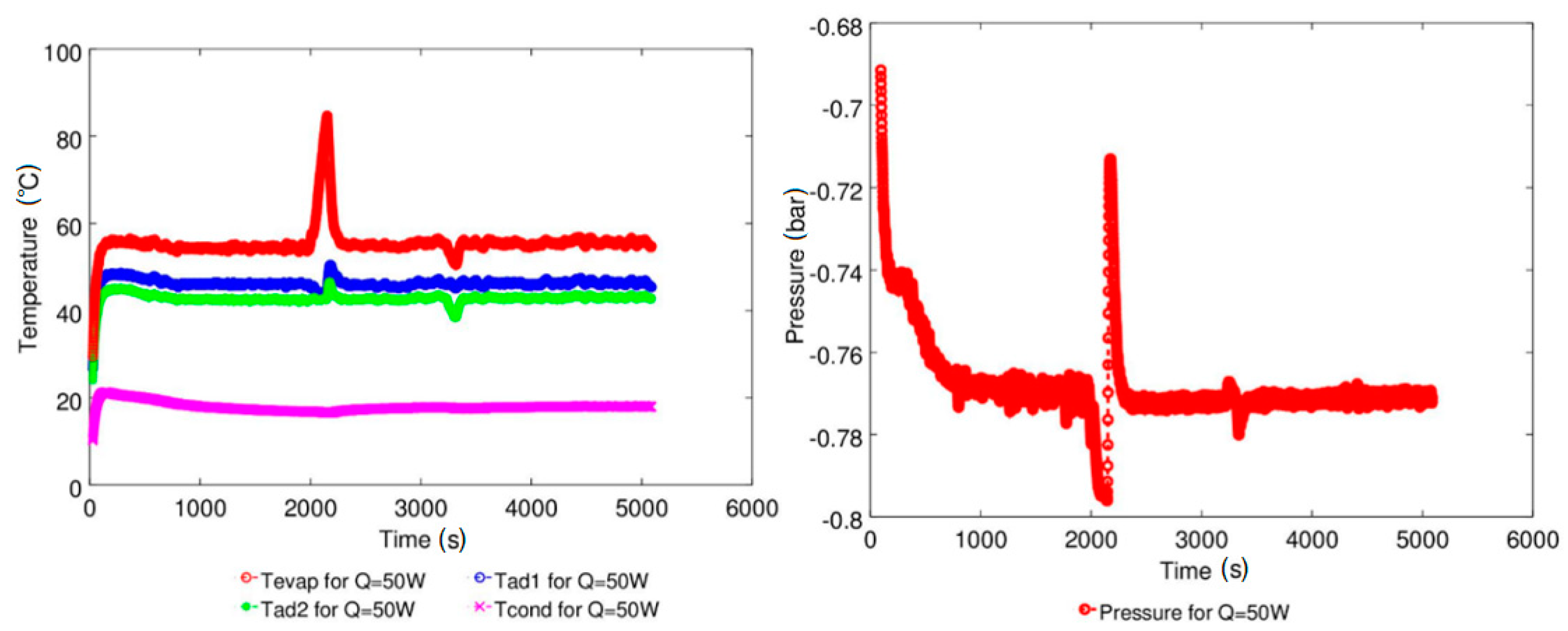
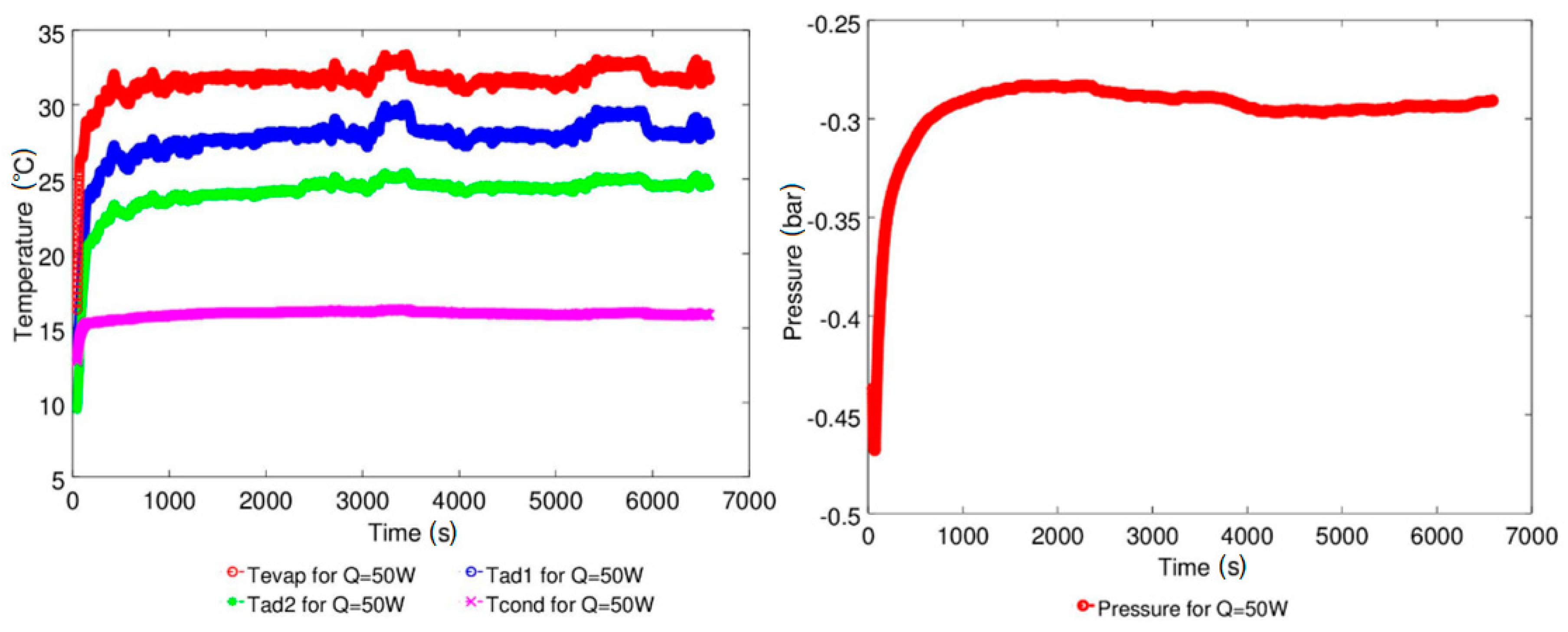
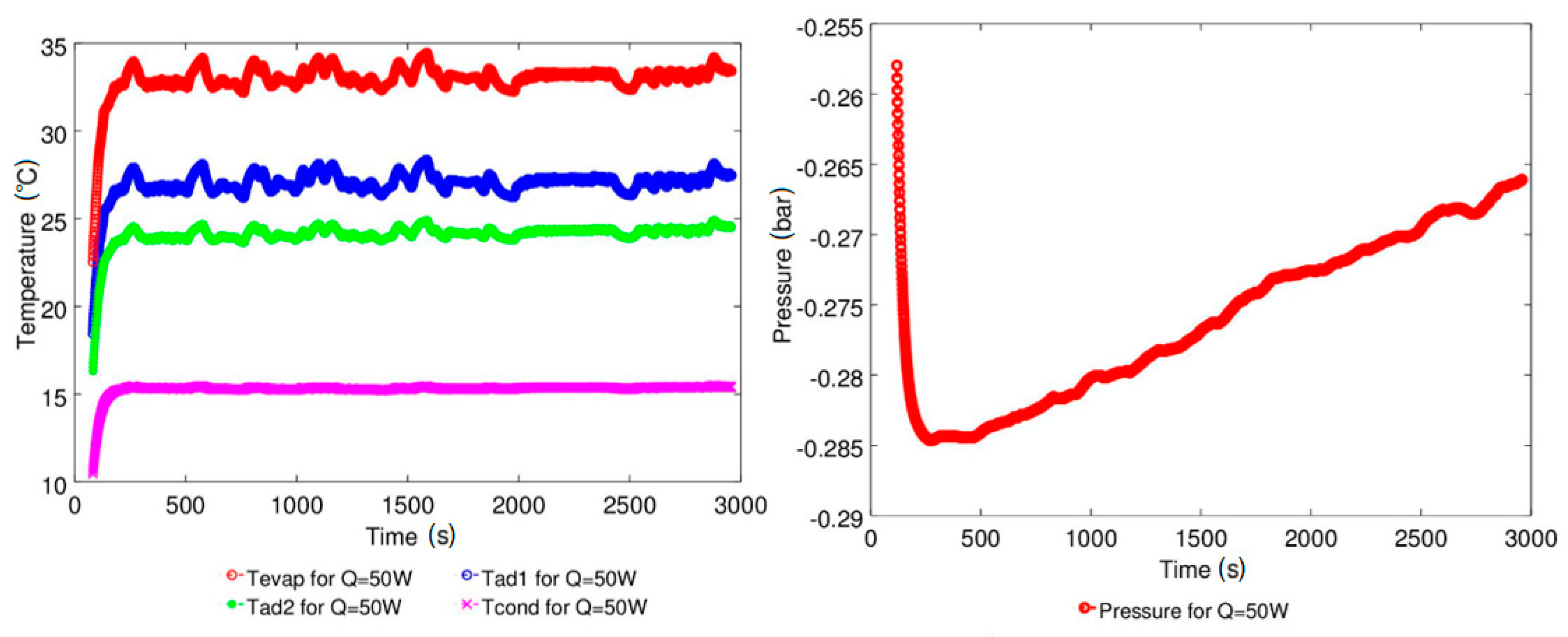
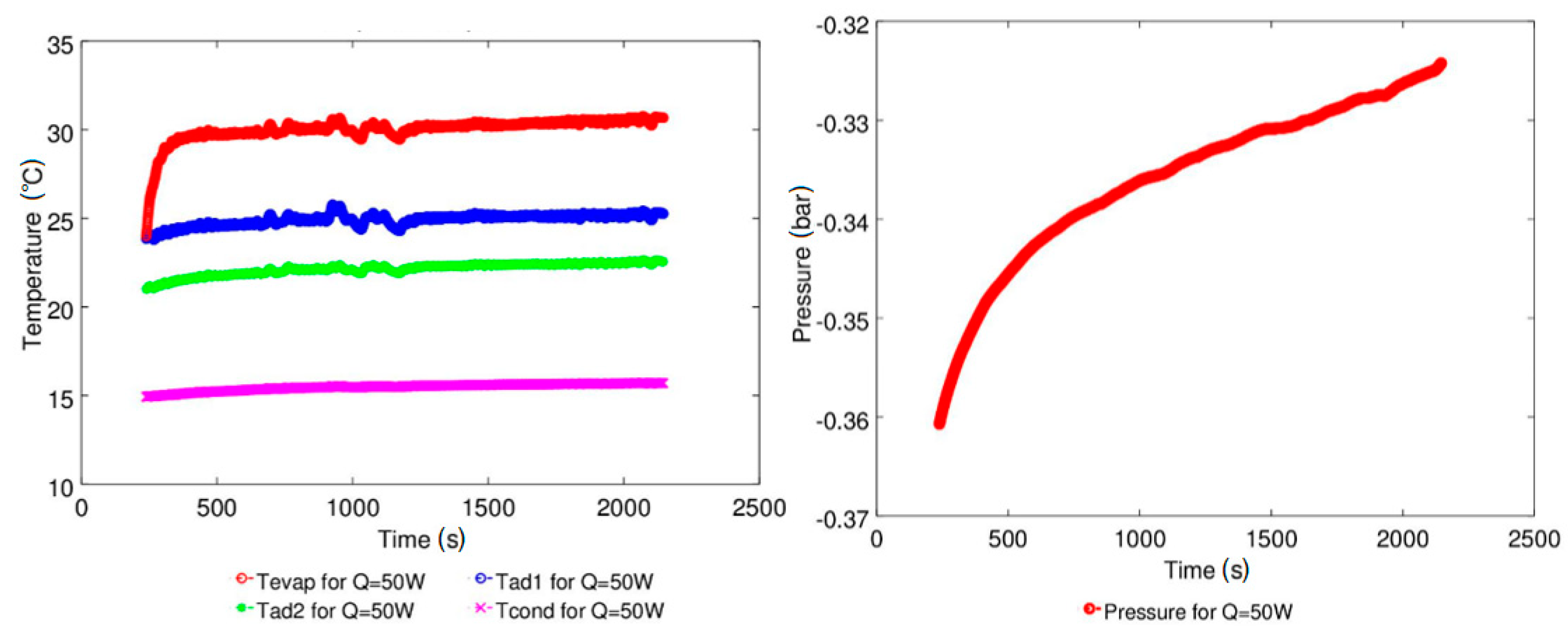
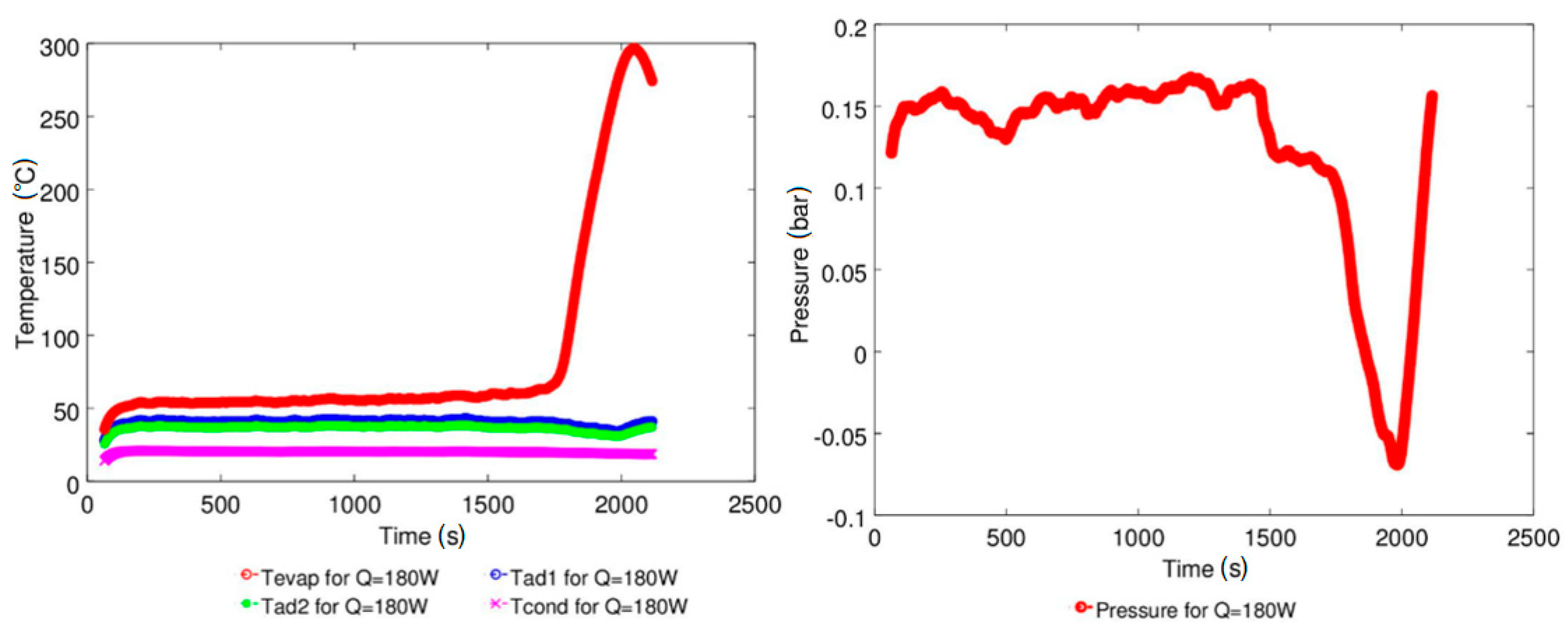
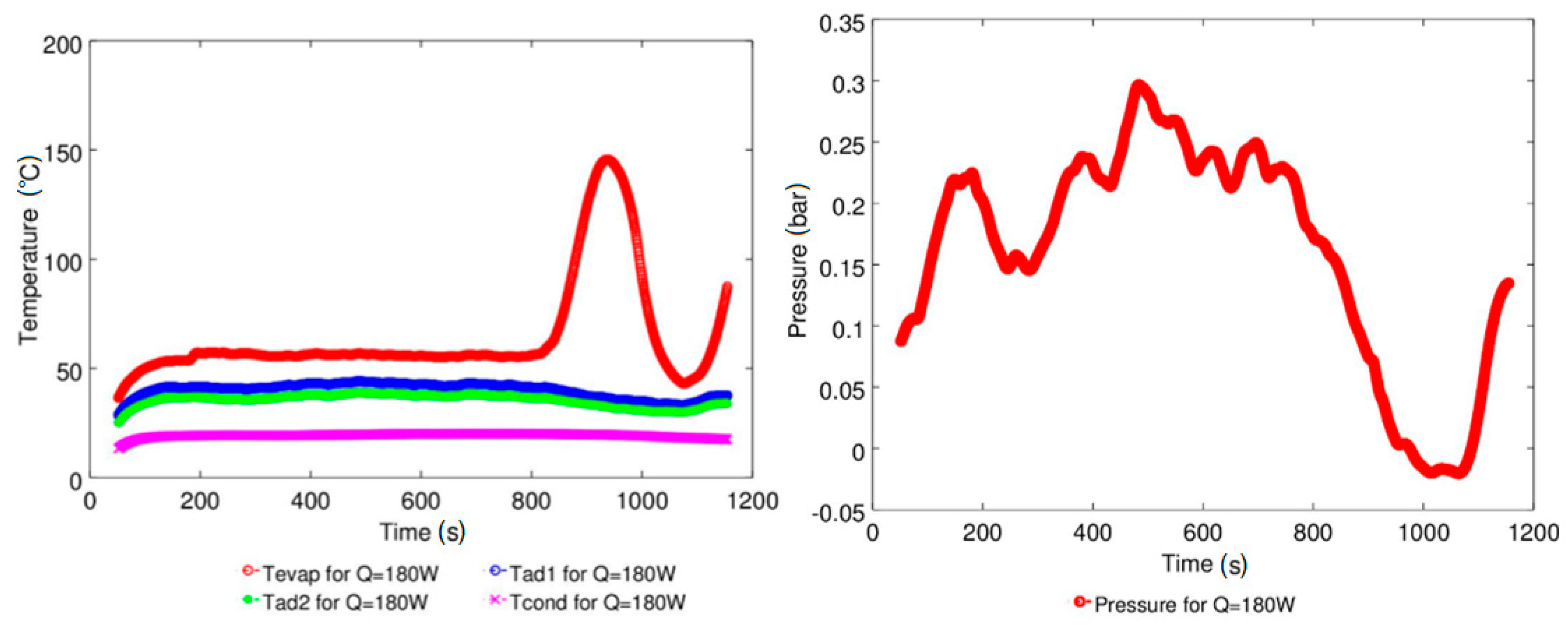
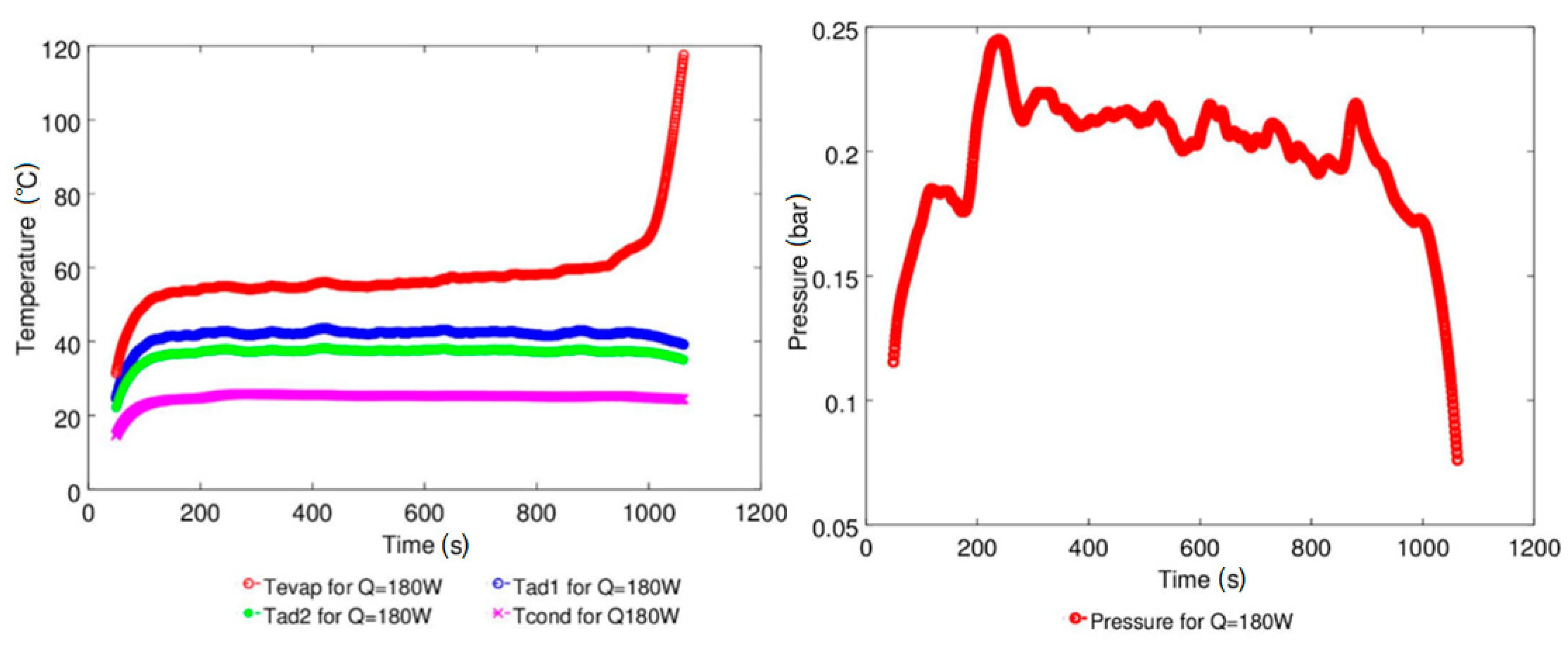
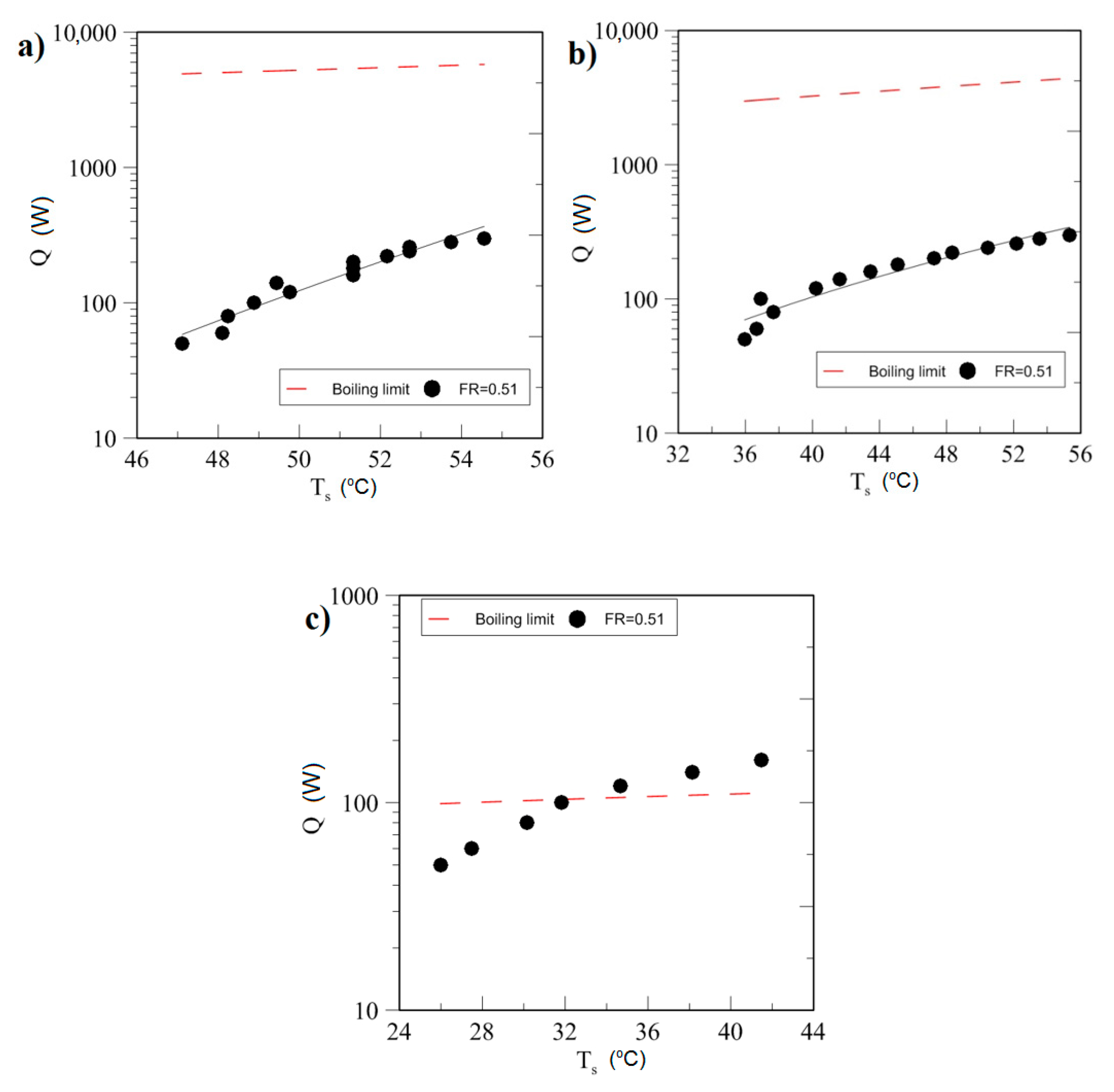
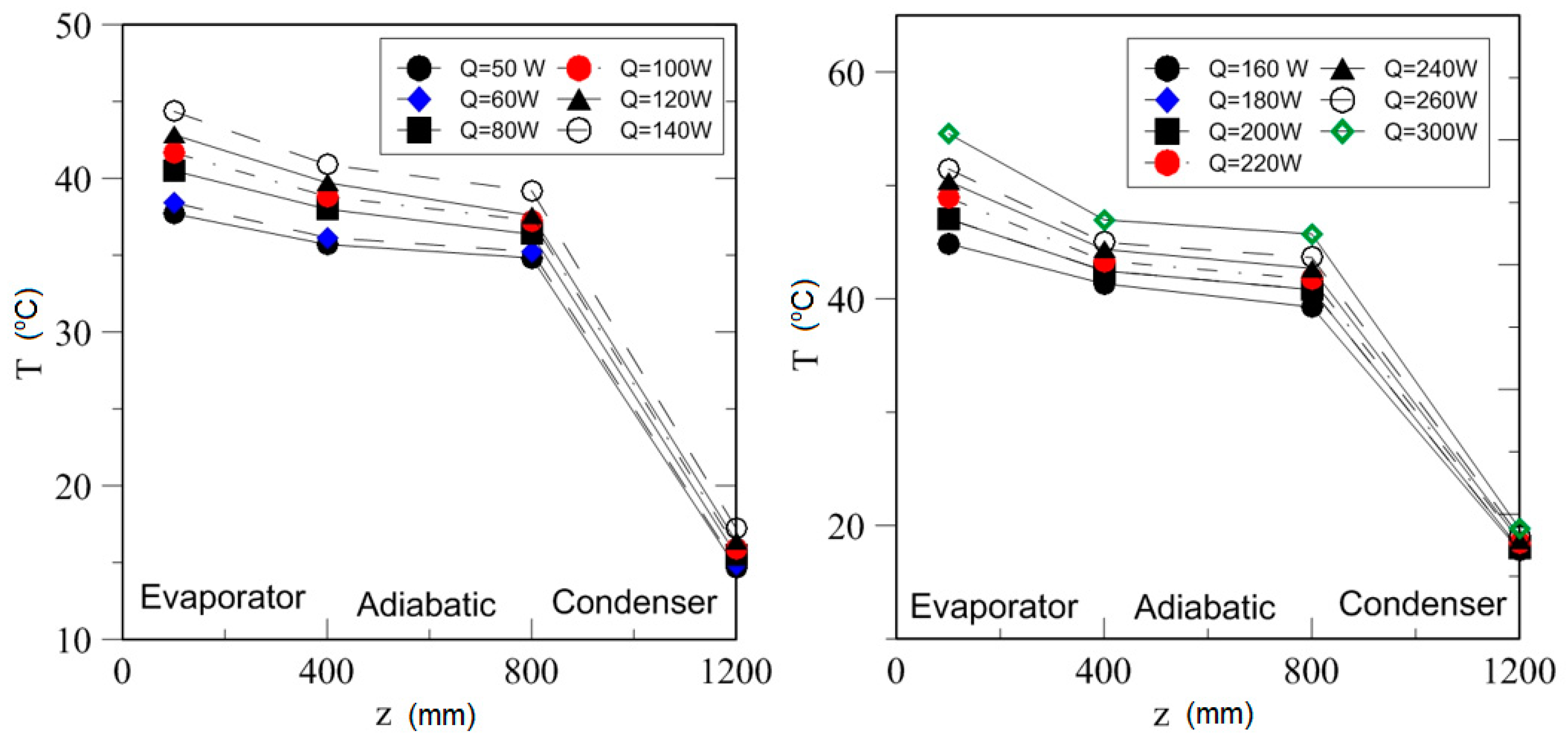
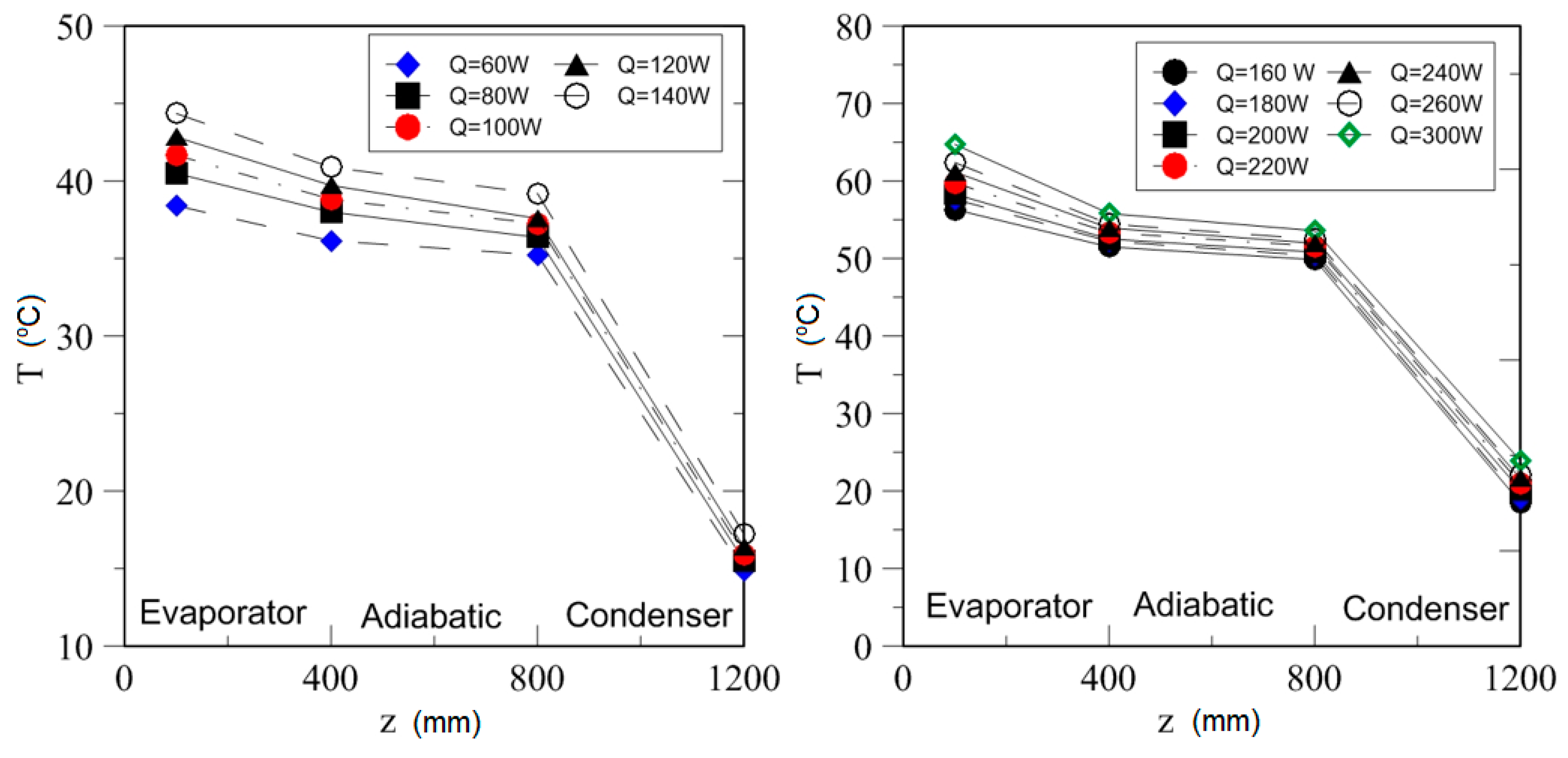
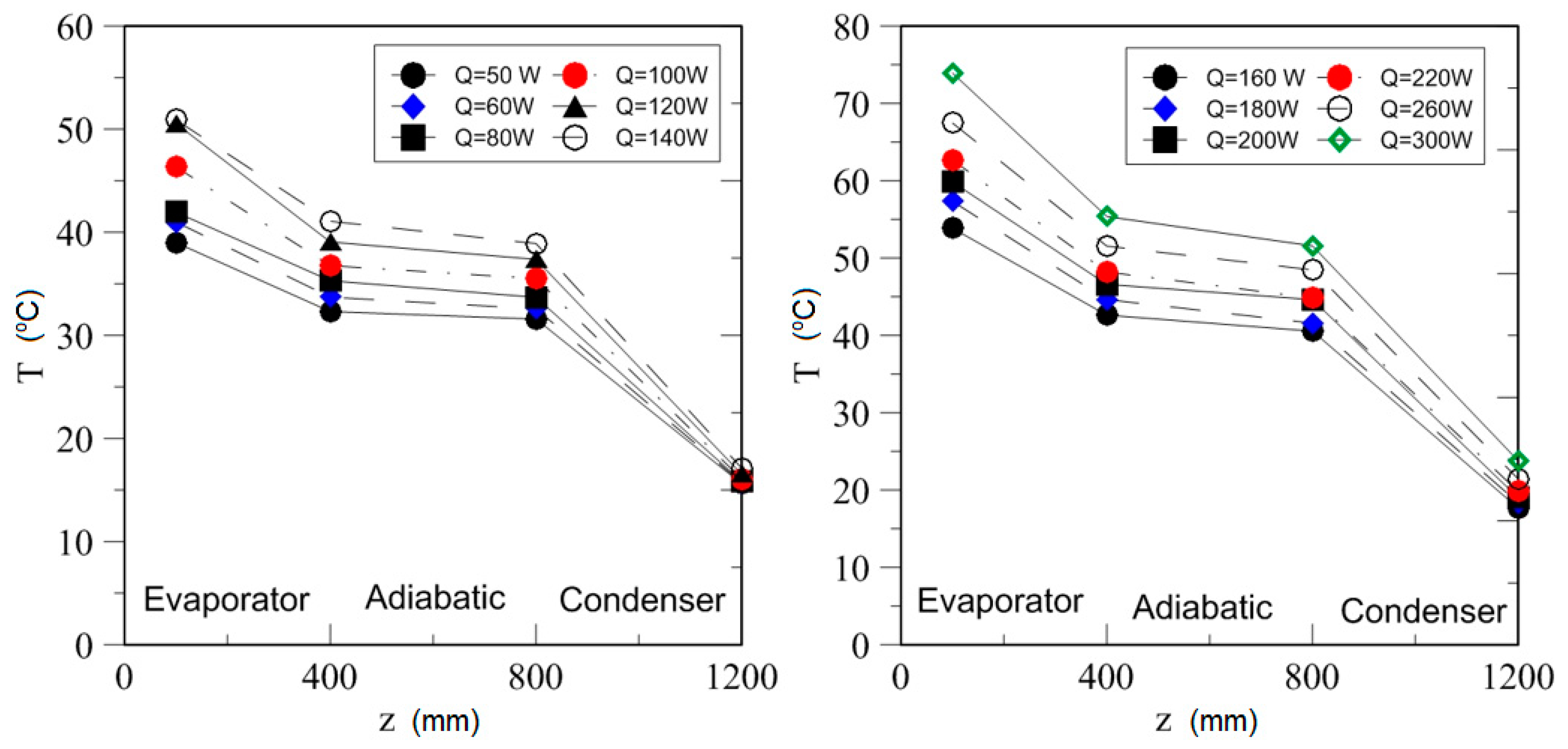
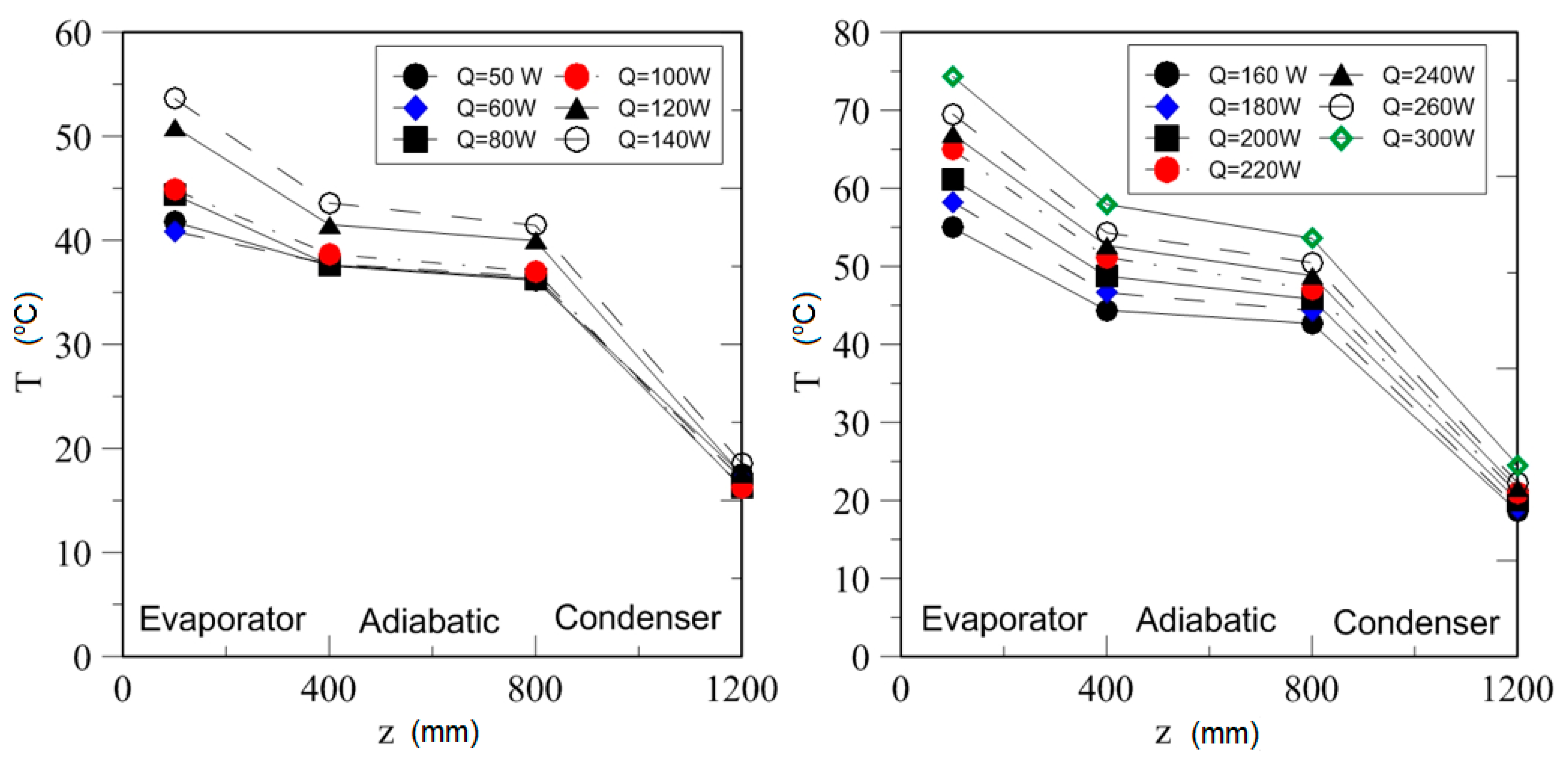
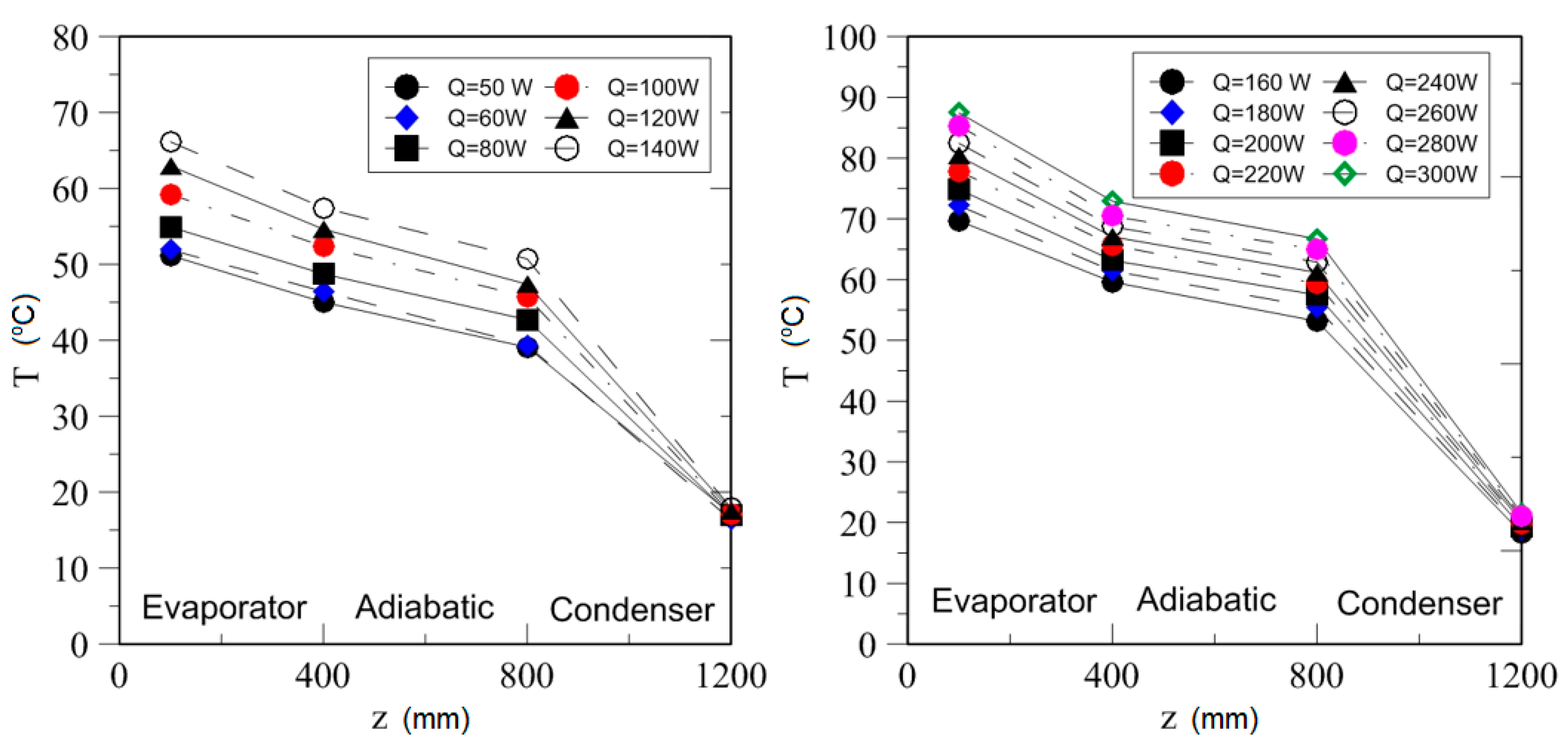

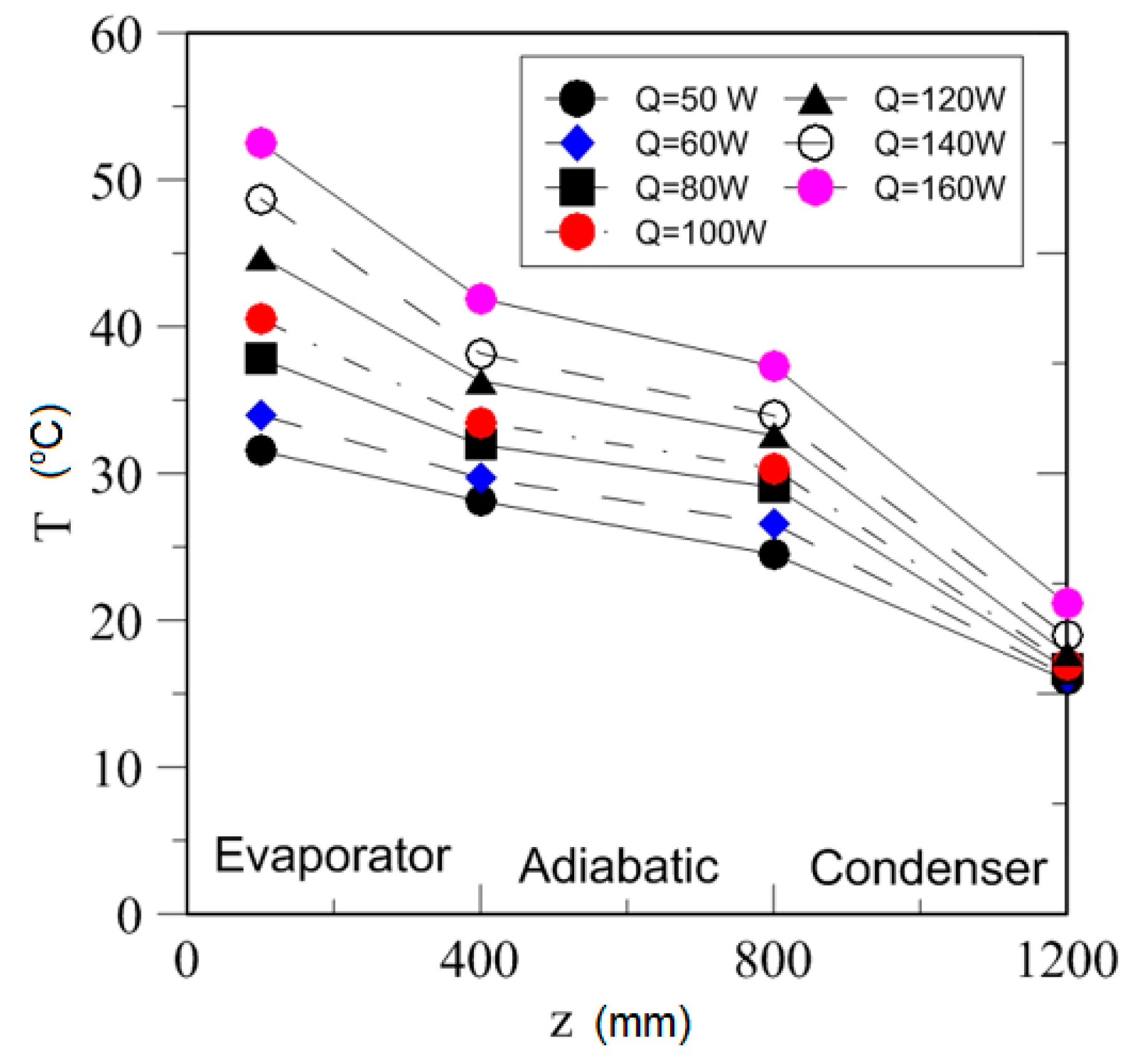

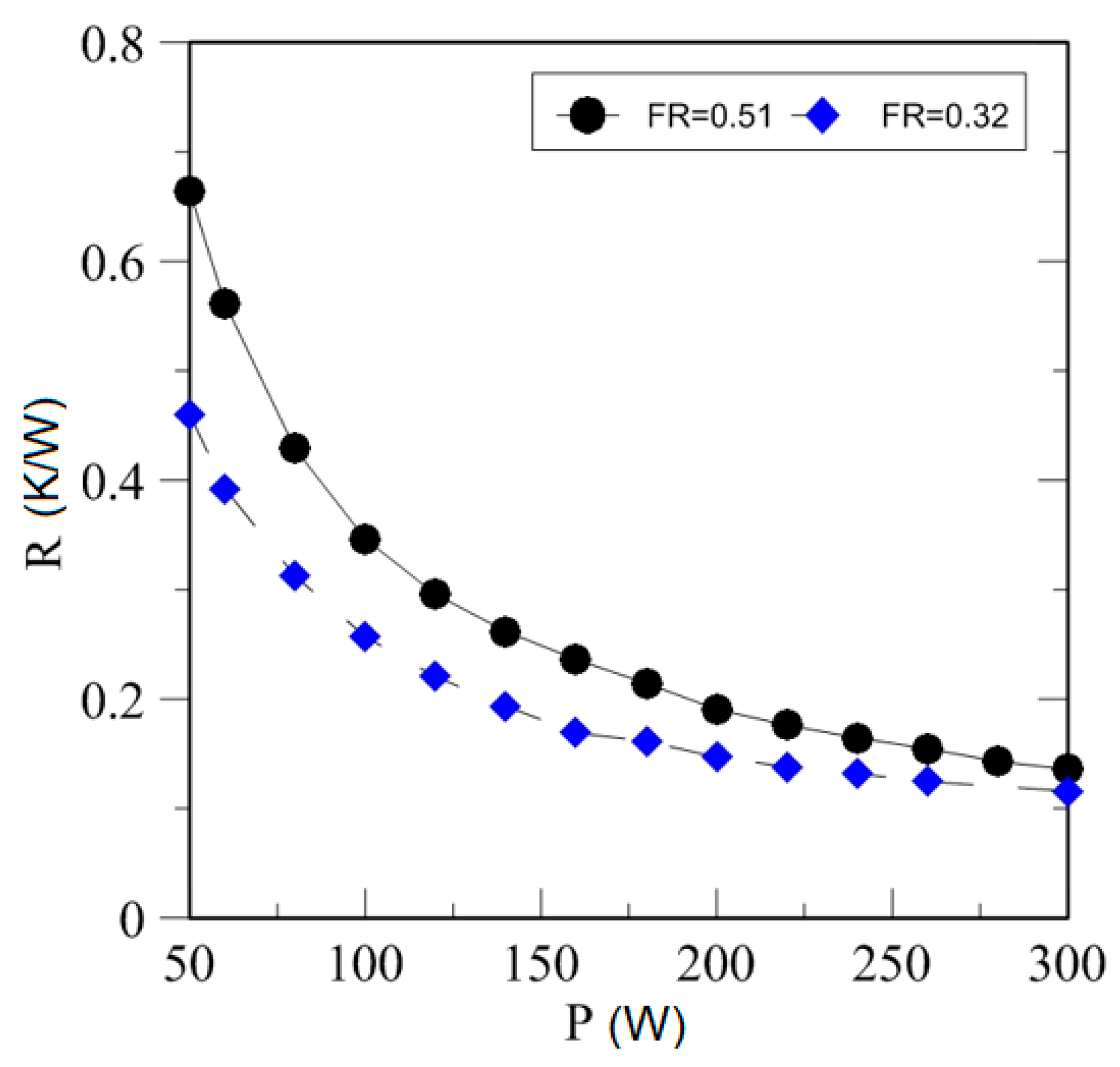
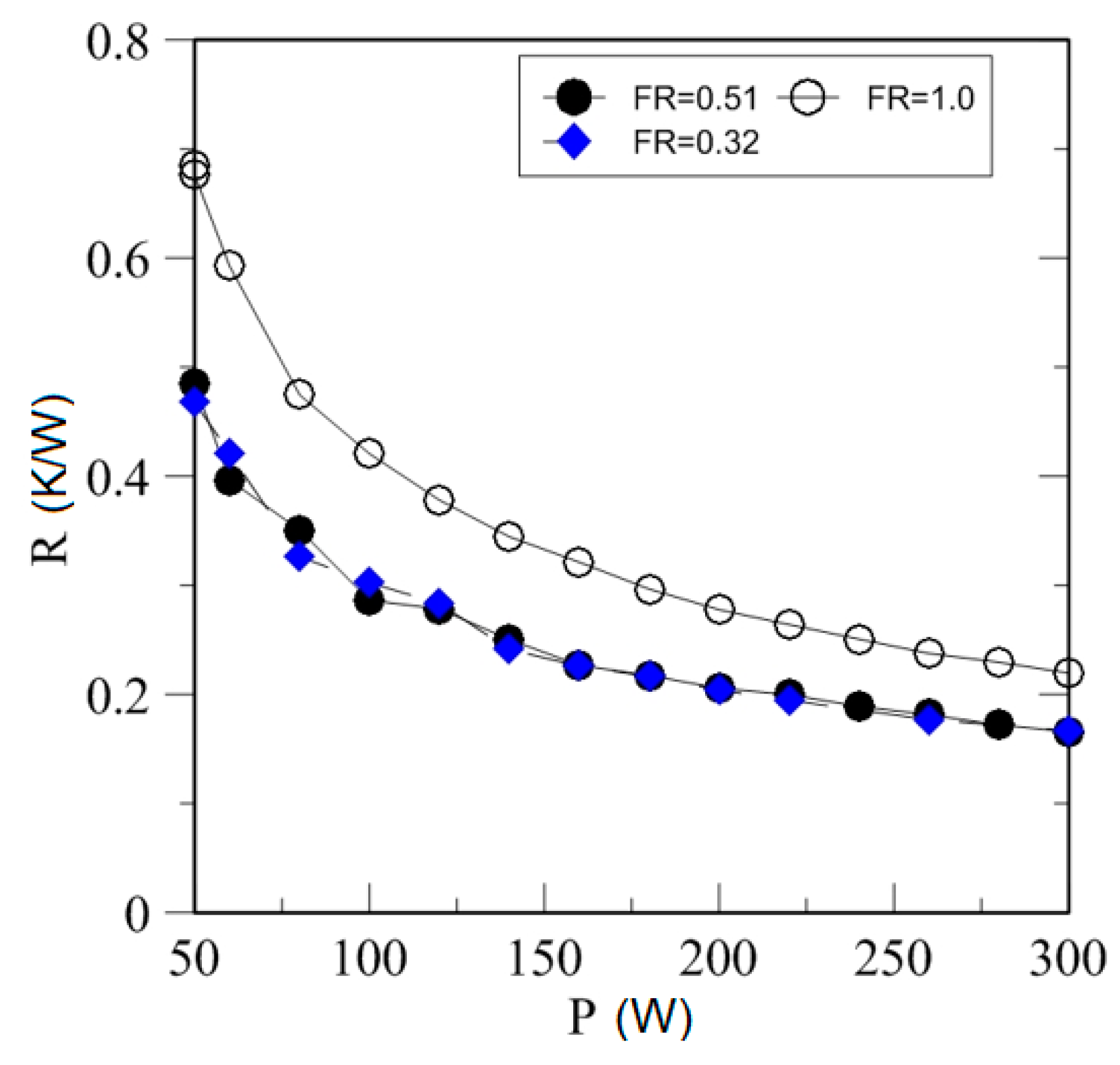
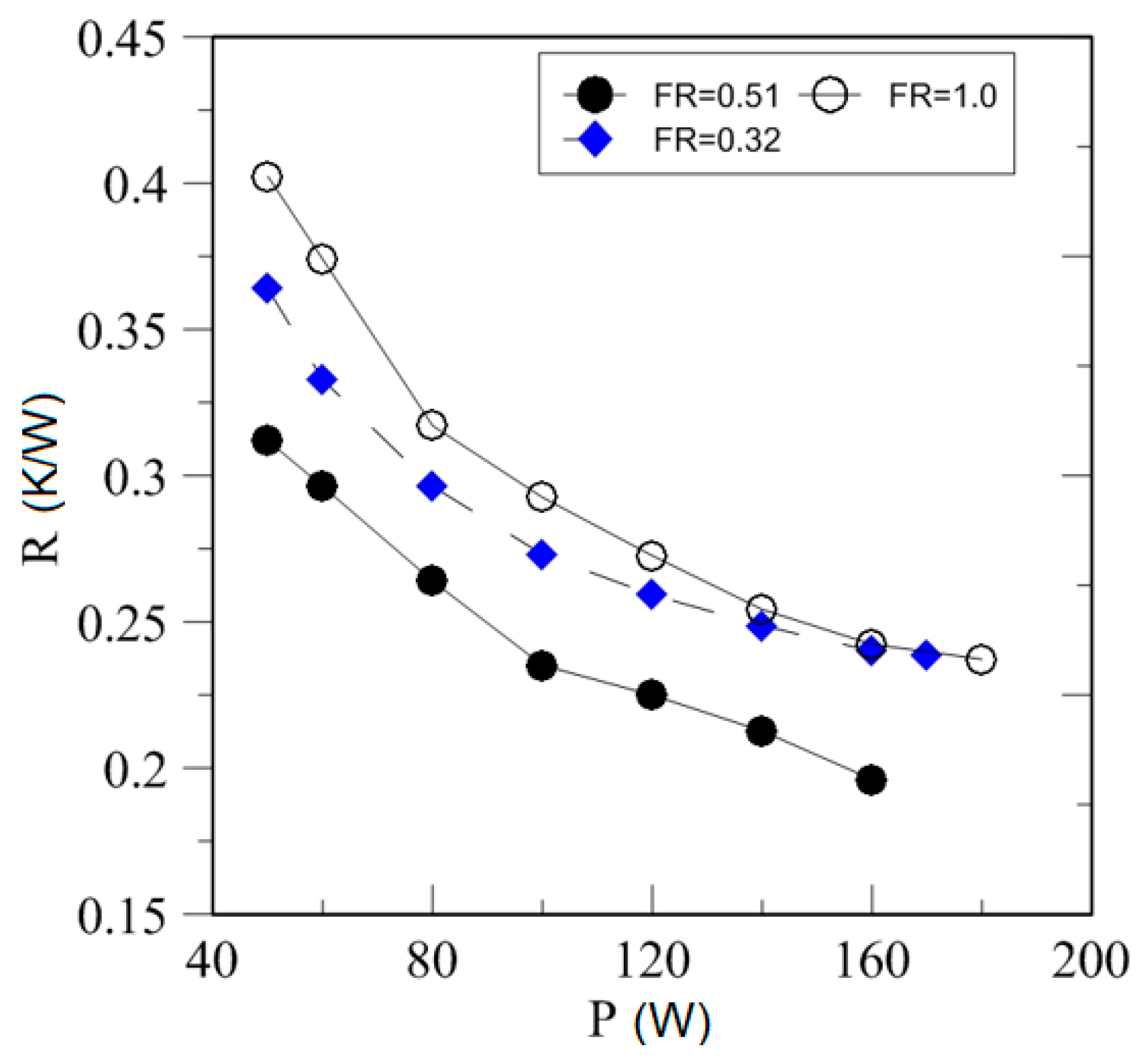
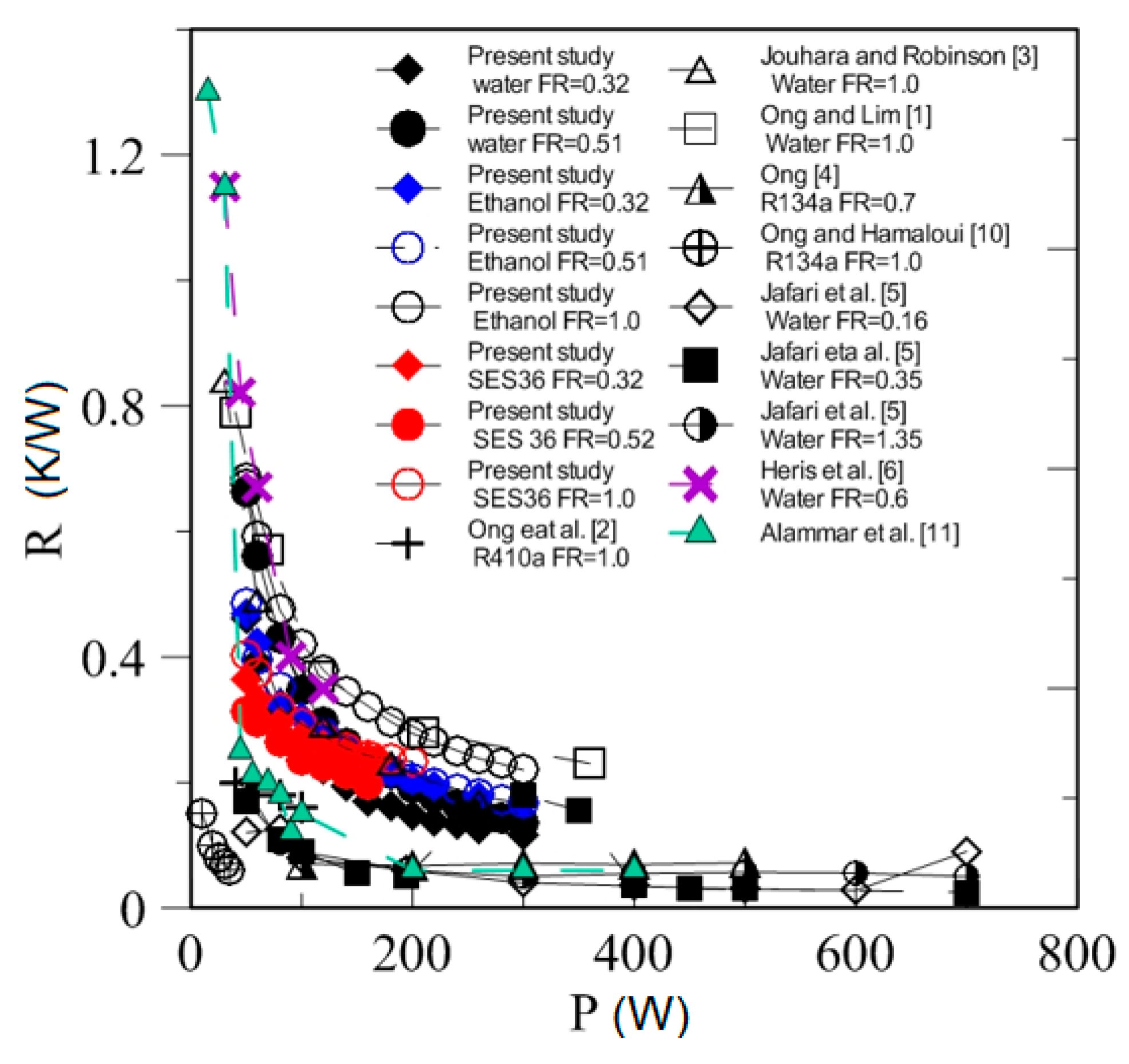
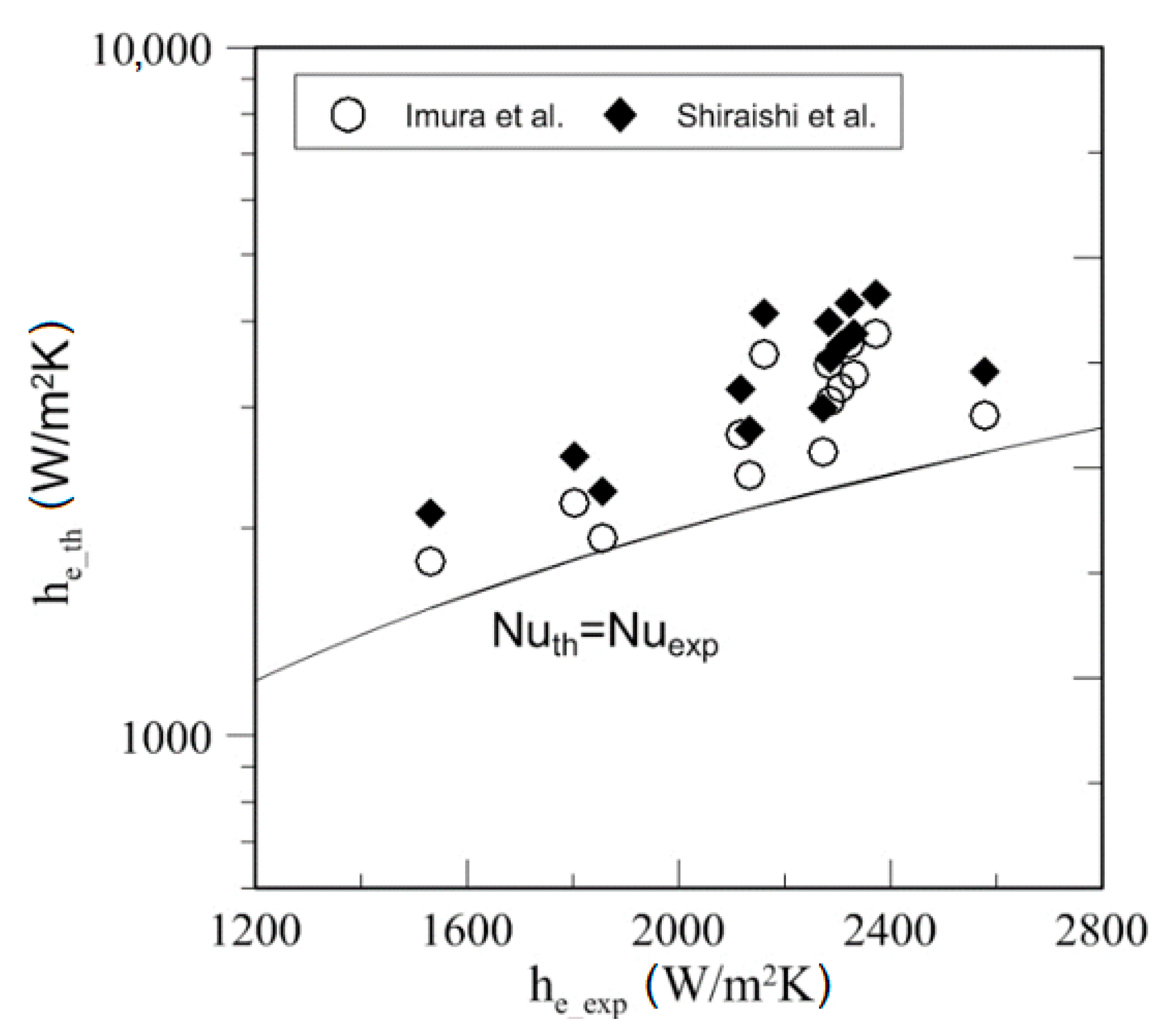
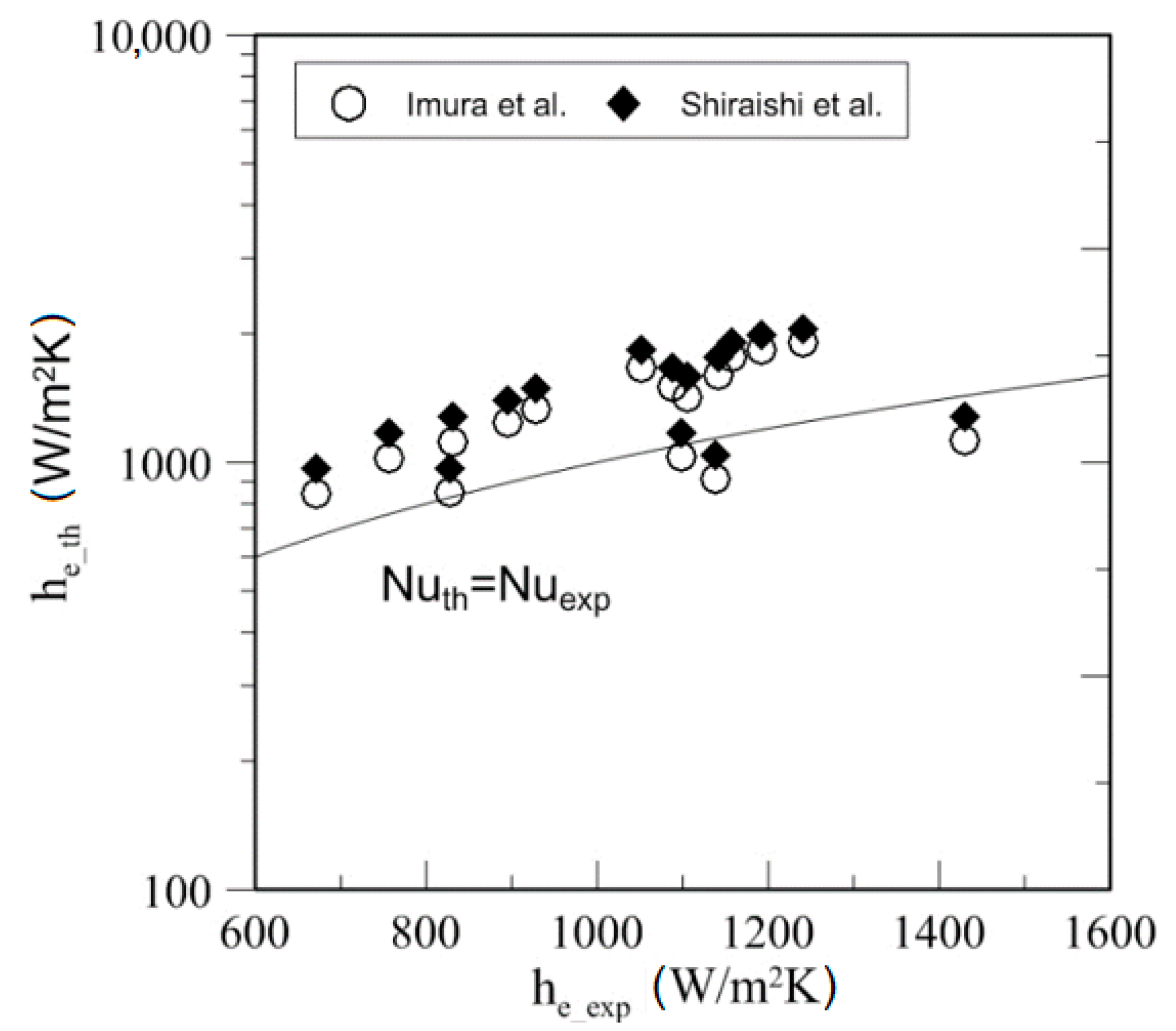
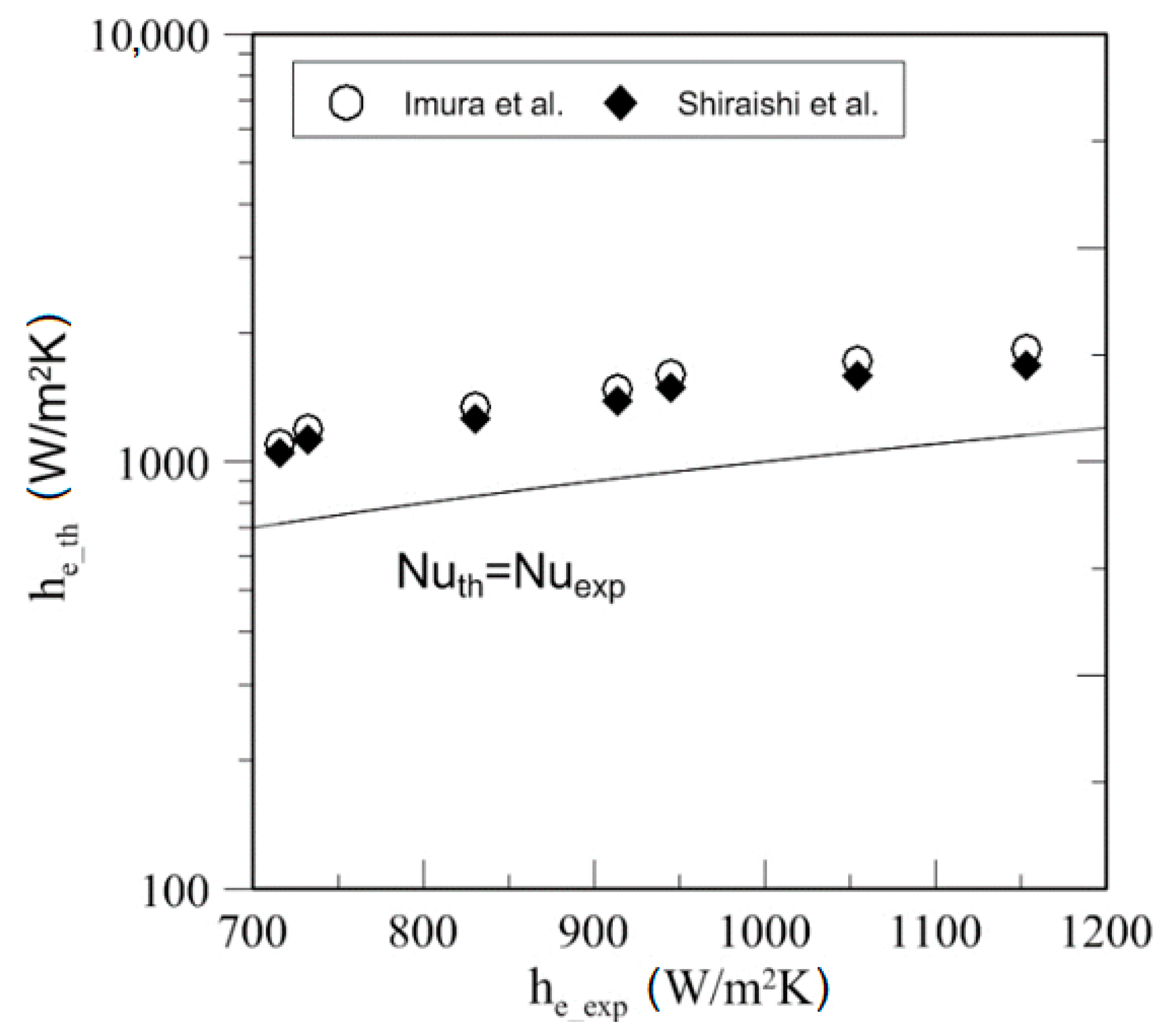
| Study | D (mm) | Le (mm) | Lc (mm) | Le/D (-) | Lc/D (-) | Test Fluid, Prl | FR (-) |
|---|---|---|---|---|---|---|---|
| Ong and Lim [1] | 19.1 | 127 | 127 | 6.6 | 6.6 | Prl = 6.6 Water | 1.0 |
| Ong et al. [2] | 9.5 | 310 | 310 | 32.6 | 32.6 | R410A 2.26 < Prl < 2.44 | 1.0 |
| Jouhara and Robinson [3] | 6 | 64 | 70 | 10.6 | 11.7 | Water Prl = 6.13 | 1.0 |
| Ong [4] | 32 | 405 | 327 | 12.7 | 10.2 | R134a 3.23 <Pr < 3.35 | 0.7 |
| Jafari et al. [5] | 35 | 79.5 | 159 | 2.27 | 4.55 | Water 1.58< Prl < 7 | 0.16, 0.35, 1.35 |
| Heris et al. [6] | 18 | 160 | 200 | 8.88 | 22.22 | Water 2.48 < Prl < 4.0 | 0.6 |
| Eidan et al. [7] | 14.2 | 150 | 150 | 10.56 | 10.56 | R134a 3.15 < Prl < 3.29 | 0.4, 0.5, 0.6, 0.7, 1.0 |
| Aghel et al. [8] | 14 | 280 | 300 | 20 | 21.42 | Water Prl = 3.23 | 0.75 |
| W. Wits and G. te Riele [9] | 12 | 750 | 250 | 62.5 | 20.83 | Water Prl = 3.50 | No data |
| Ong and Hamlaoui [10] | 25.5 | 300 | 300 | 11.8 | 11.8 | R134a | 0.95 |
| Alammar et al. [11] | 20.2 | 200 | 200 | 9.9 | 9.9 | Water 4.32 < Prl < 7.48 | 0.26, 0.65, 1.0 |
| Present study | 10 | 400 | 400 | 40 | 40 | Water, Ethanol, SES36 3.42 < Prl < 15.46 | 0.32, 0.51, 1.0 |
| Properties | Unit | Water | Ethanol | SES36 |
|---|---|---|---|---|
| Flash point | - | No flammability | Flammable | No flammability |
| Toxicity | - | No | Low Toxicity | No |
| GWP (global warming potential) | - | 0 | <25 | 4121 |
| ODP (ozone depletion potential) | - | 0 | 0 | None |
| Freezing point at normal pressure | °C | 0 | −114.14 | −40 |
| Boiling point at normal pressure | °C | 99.98 | 78.24 | 36 |
| Critical temperature | °C | 373.94 | 240.75 | 177.6 |
| Critical pressure | MPa | 22.06 | 6.148 | 2.85 |
| Parameter | Unit | Operating Range | Uncertainty |
|---|---|---|---|
| Di | mm | 10 | 0.003 |
| Do | mm | 12 | 0.003 |
| mw | kg/s | 0.01–0.03 | 0.3% |
| Tw | °C | 10 | 0.1 |
| Tsur | °C | 10–300 | 0.1 |
| W | 10–1000 | ±1% | |
| h | W/m2·K | 600–1200 | ±2.5% |
| ΔP | mbar | 2–10 | ±0.065% |
| Pin | mbar | −9 to 4 | ±0.5% |
| Correlations | References |
|---|---|
| Imura et al. [38] | |
| Shiraishi et al. [39] |
| Kind of Fluid | Source | MAD (%) | % of Data within 50% |
|---|---|---|---|
| Water | Imura et al. [38] | 14.99 | 100 |
| Shiraishi et al. [39] | 24.28 | 100 | |
| Ethanol | Imura et al. [38] | 14.72 | 75 |
| Shiraishi et al. [39] | 22.37 | 75 | |
| SES36 | Imura et al. [38] | 61.22 | 30 |
| Shiraishi et al. [39] | 50.89 | 30 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrzejczyk, R. Experimental Investigation of the Thermal Performance of a Wickless Heat Pipe Operating with Different Fluids: Water, Ethanol, and SES36. Analysis of Influences of Instability Processes at Working Operation Parameters. Energies 2019, 12, 80. https://doi.org/10.3390/en12010080
Andrzejczyk R. Experimental Investigation of the Thermal Performance of a Wickless Heat Pipe Operating with Different Fluids: Water, Ethanol, and SES36. Analysis of Influences of Instability Processes at Working Operation Parameters. Energies. 2019; 12(1):80. https://doi.org/10.3390/en12010080
Chicago/Turabian StyleAndrzejczyk, Rafal. 2019. "Experimental Investigation of the Thermal Performance of a Wickless Heat Pipe Operating with Different Fluids: Water, Ethanol, and SES36. Analysis of Influences of Instability Processes at Working Operation Parameters" Energies 12, no. 1: 80. https://doi.org/10.3390/en12010080
APA StyleAndrzejczyk, R. (2019). Experimental Investigation of the Thermal Performance of a Wickless Heat Pipe Operating with Different Fluids: Water, Ethanol, and SES36. Analysis of Influences of Instability Processes at Working Operation Parameters. Energies, 12(1), 80. https://doi.org/10.3390/en12010080




