A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. TEM-EDS and XPS Study under Accelerated Aging Conditions
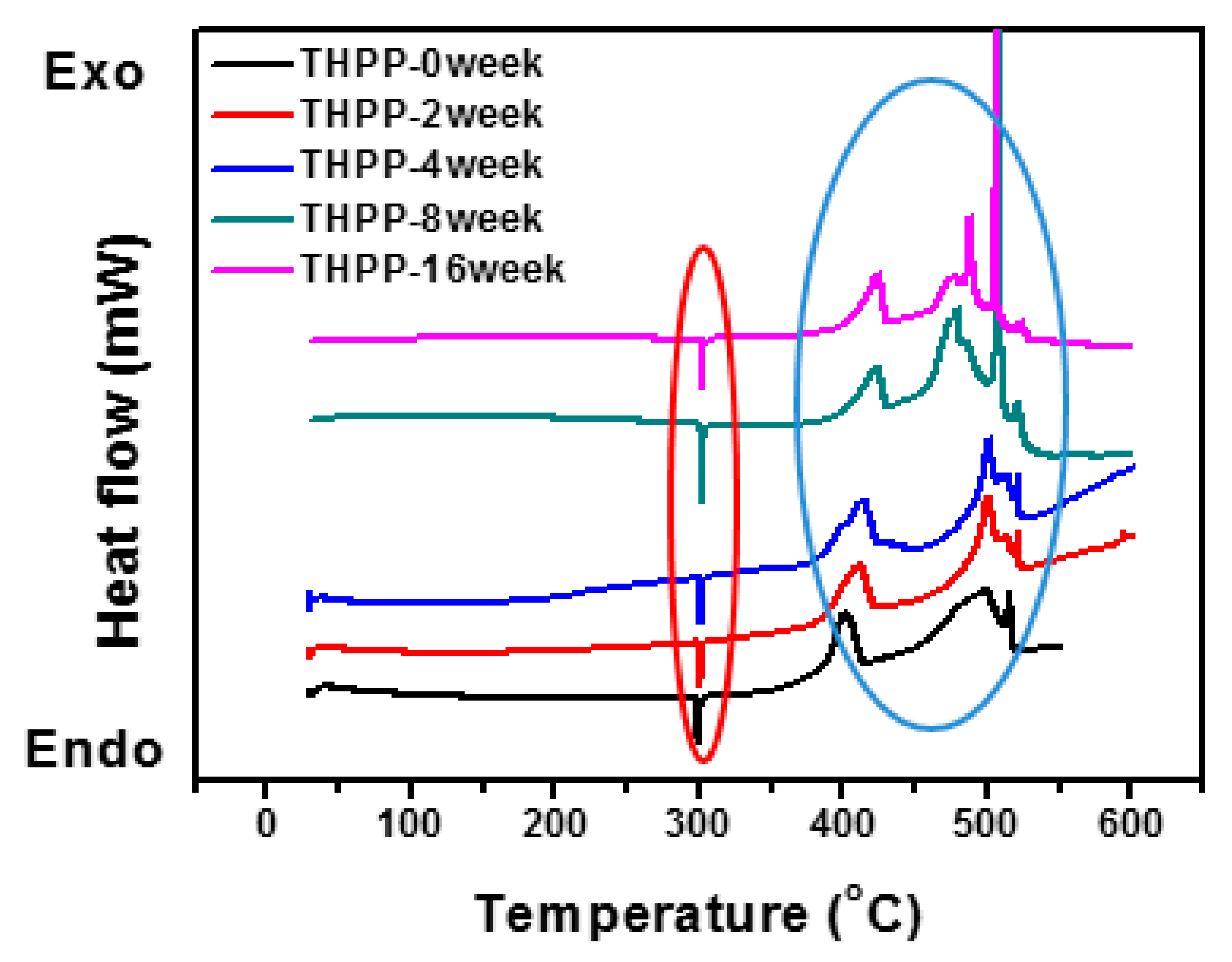
2.2. DSC Measurements
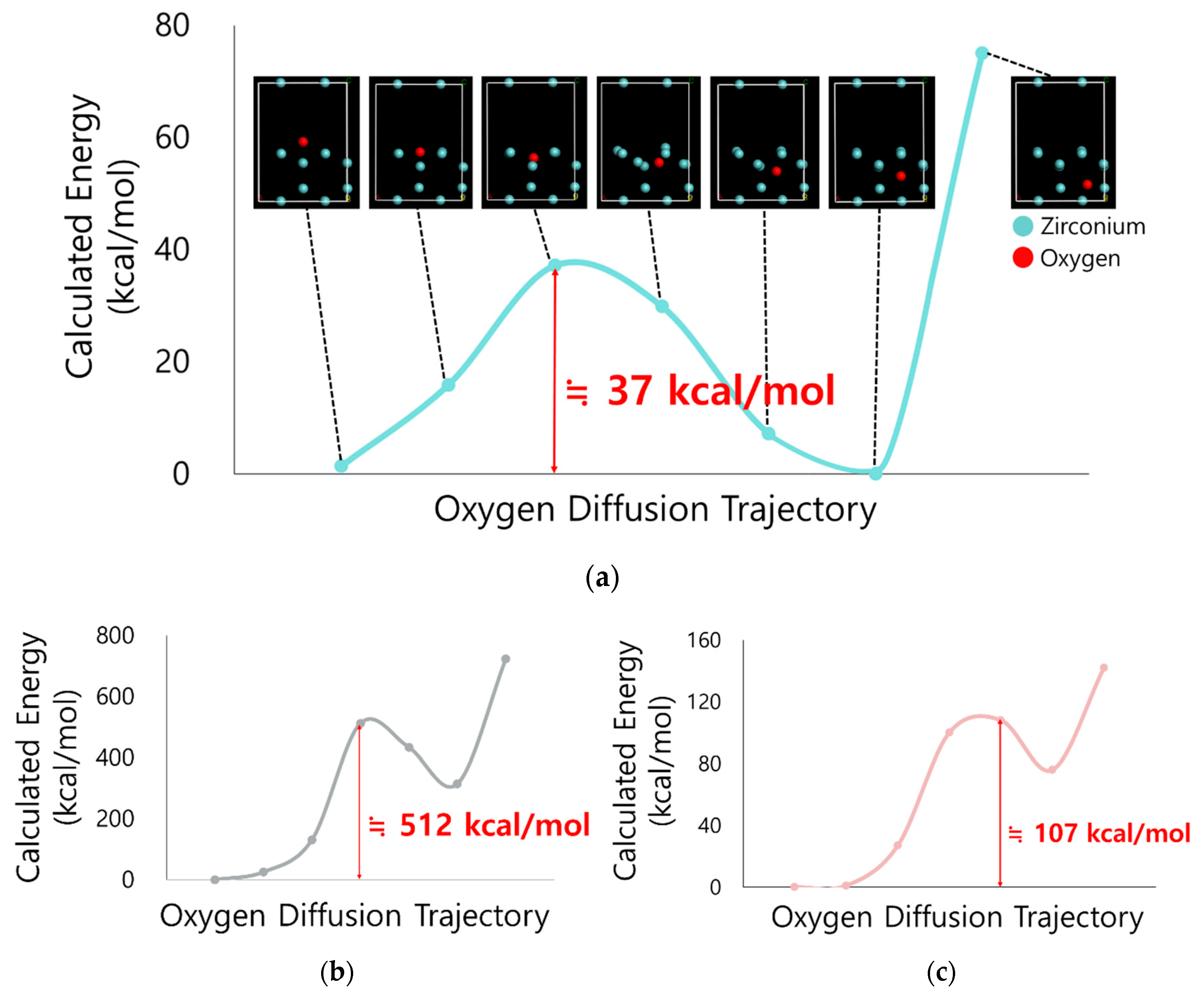
2.3. DFT-Based Computational Calculation
3. Results and Discussion
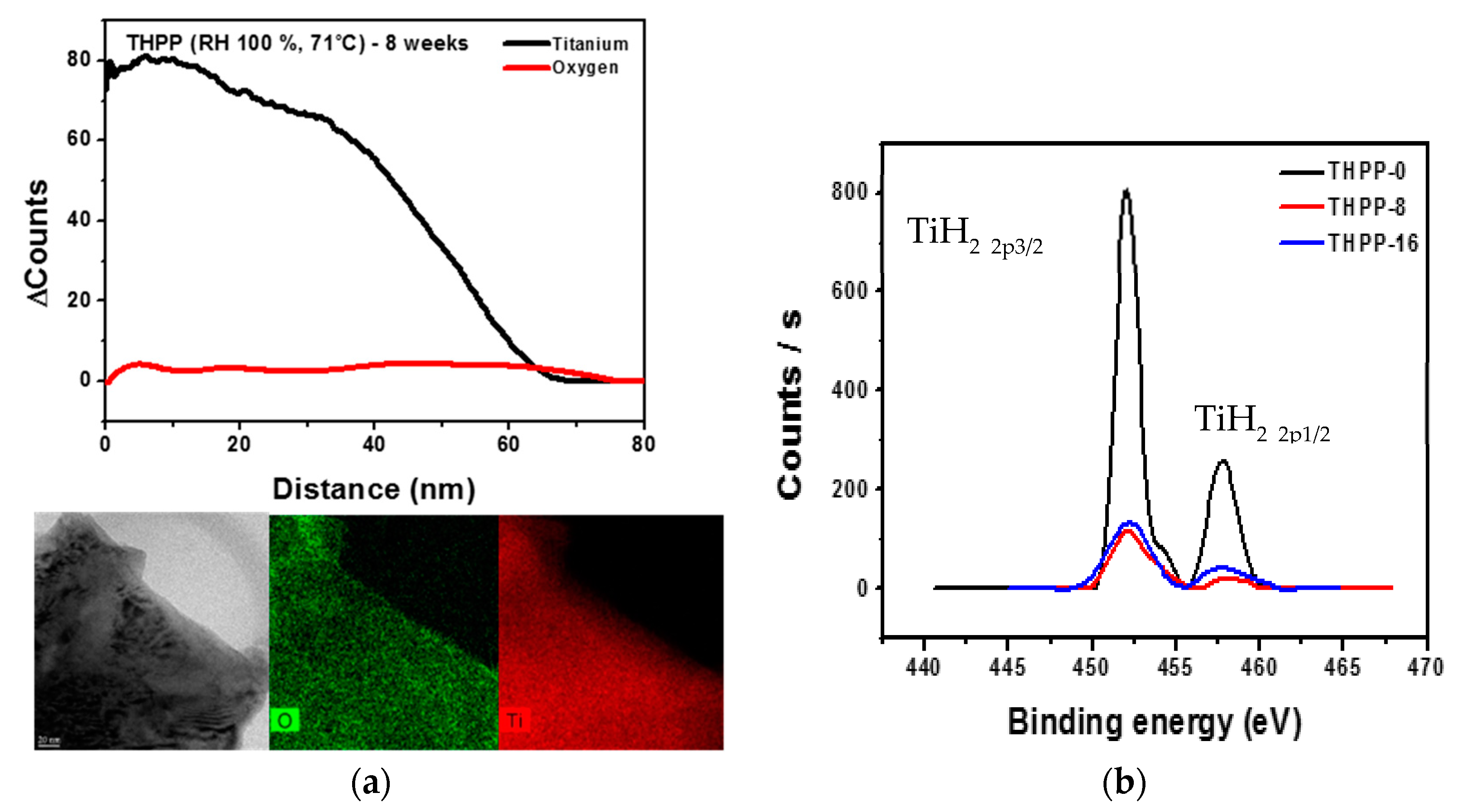
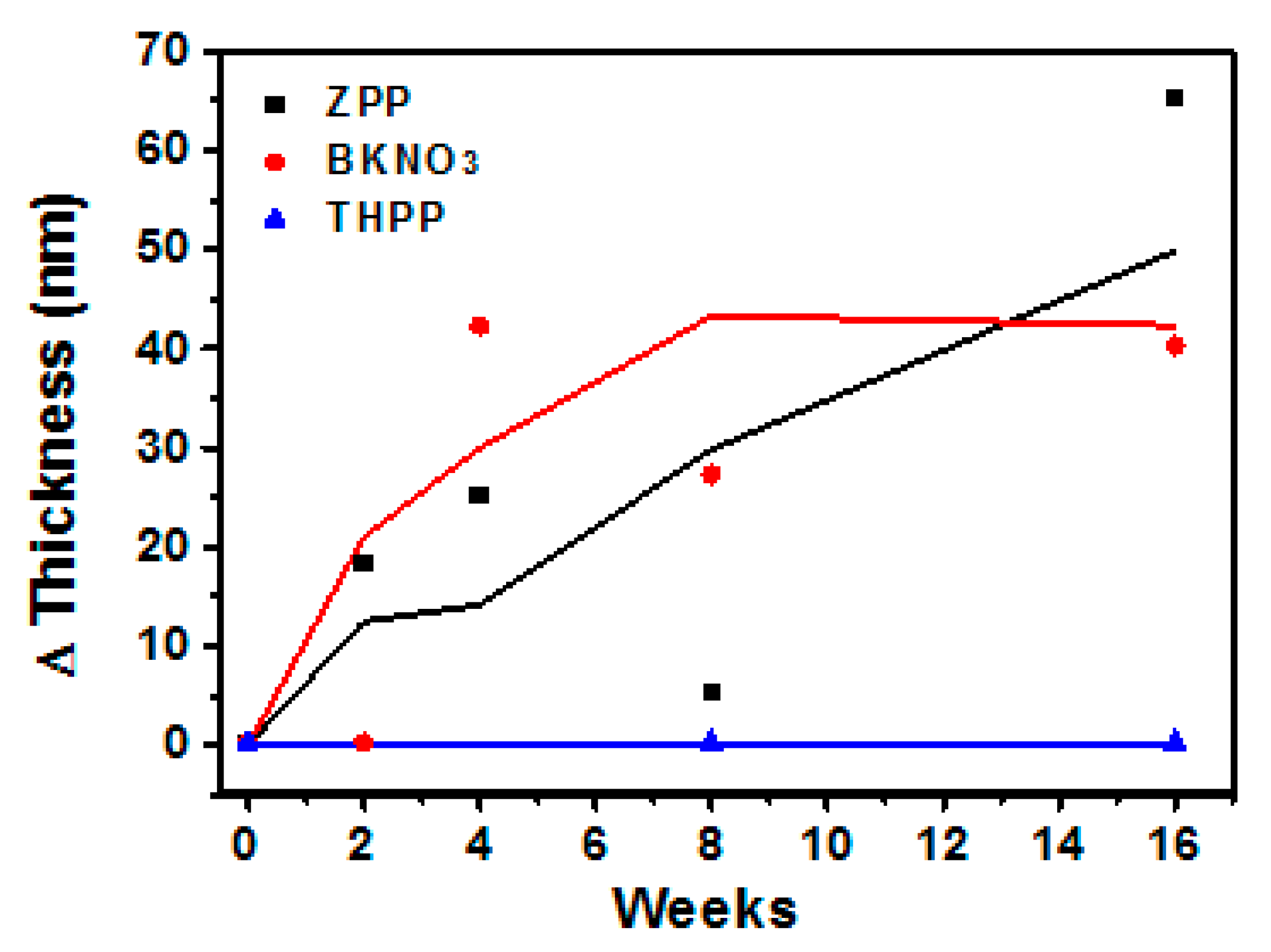
3.1. Oxide Shell Formation
3.2. Computational Interpretation of Oxygen Diffusion
4. Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bement, L.J.; Multhaup, H.A. Determining Functional Reliability of Pyrotechnic Mechanical Devices. AIAA J. 1999, 37, 357–363. [Google Scholar] [CrossRef]
- Lee, J.; Lin, L.; Lin, C.; Ch’en, P.; Huang, C.; Chang, S. A Study of Zirconium/Potassium Perchlorate Primer Mixtures. Thermochim. Acta 1990, 173, 211–218. [Google Scholar] [CrossRef]
- Ulas, A.; Risha, G.A.; Kuo, K.K. An Investigation of the Performance of a Boron/Potassium-nitrate Based Pyrotechnic Igniter. Propellants Explos. Pyrotech. 2006, 31, 311–317. [Google Scholar] [CrossRef]
- Yan, N.; Bao, B.; Zheng, F.; Li, C. Ignition Characteristics of Micro-energy Semiconductor Bridges with Different Ignition Compositions. Propellants Explos. Pyrotech. 2016, 41, 223–227. [Google Scholar] [CrossRef]
- Olmos, R.P.; Rios, A.; Martín, M.P.; Lapa, R.A.S.; Lima, J.C.F.C. Construction and Evaluation of Ion Selective Electrodes for Perchlorate with a Summing Operational Amplifier: Application to Pyrotechnics Mixtures Analysis. Analyst 1999, 124, 97–100. [Google Scholar] [CrossRef]
- Lai, K.S. Boron Potassium Nitrate (BKNO3) Aging Study. In Proceedings of the 34th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Cleveland, OH, USA, 13–15 July 1998. [Google Scholar]
- Lee, J.; Kim, T.; Ryu, S.U.; Choi, K.; Ahn, G.H.; Paik, J.G.; Ryu, B.; Park, T.; Won, Y.S. Study on the Aging Mechanism of Boron Potassium Nitrate (BKNO3) for Sustainable Efficiency in Pyrotechnic Mechanical Devices. Sci. Rep. 2018, 8, 11745–11754. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, K.; Ryu, S.U.; Ahn, G.H.; Paik, J.G.; Ryu, B.; Won, Y.S. Aging Mechanism of Zirconium Potassium Perchlorate Propellant in Pyrotechnic Mechanical Devices. Nanosci. Nanotechnol. Lett. 2018, 10, 735–740. [Google Scholar] [CrossRef]
- Eom, K.H.; An, H.Y.; Kim, K.M.; Ahn, G.H.; Paik, J.G.; Ryu, B.T.; Im, D.J.; Won, Y.S. Equilibrium Analysis on the Aging of a BKNO3 Igniter. J. Nanosci. Nanotechnol. 2017, 17, 7685–7688. [Google Scholar] [CrossRef]
- Kong, T.K.; Won, Y.S.; Ryu, B.; Ahn, G.H.; Im, D.J. Mathematical Modeling of ZrKClO4 Nano Particle Energy Release. J. Nanosci. Nanotechnol. 2017, 17, 8372–8377. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.M.; Kim, H.; Kim, D.; Won, Y.S.; Kim, S. Electrodeposition-fabricated PtCu-alloy Cathode Catalysts for High-temperature Proton Exchange Membrane Fuel Cells. Korean J. Chem. Eng. 2018, 35, 1547–1555. [Google Scholar] [CrossRef]
- Savizi, I.S.P.; Janik, M.J. Acetate and Phosphate Anion Adsorption Linear Sweep Voltammograms Simulated Using Density Functional Theory. Electrochim. Acta 2011, 56, 3996–4006. [Google Scholar] [CrossRef]
- Decker, B.F.; Kasper, J.S. The Crystal Structure of a Simple Rhombohedral Form of Boron Locality: Synthetic. Acta Crystallogr. 1959, 12, 503–506. [Google Scholar] [CrossRef]
- Wyckoff, R.W.G. Crystal Structures; John Wiley: New York, NY, USA, 1963. [Google Scholar]
- Villars, P. Springer & Material Phases Data System (MPDS); Switzerland & National Institute for Materials Science (NIMS): Tsukuba, Japan, 2016. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles Simulation: Ideas, Illustrations and the CASTEP Code. J. Phys. Condens. Matter 2002, 14, 2717–2744. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Carlos, F. Atoms, Molecules, Solids, and Surfaces: Applications of the Generalized Gradient Approximation for Exchange and Correlation. Phys. Rev. B 1992, 46, 6671–6687. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft Self-Consistent Pseudopotentials in a Generalized Eigenvalue Formalism. Phys. Rev. B 1990, 41, 7982. [Google Scholar] [CrossRef]
- Denise, B.; Debeau, M.; Depondt, P.; Heger, G. Orientational Disorder in the High Temperature Phase of KCIO4. J. Phys. France 1988, 49, 1203–1210. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Lee, J.; Choi, S.I.; Ahn, G.H.; Paik, J.G.; Ryu, B.T.; Kim, Y.H.; Won, Y.S. A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions. Energies 2019, 12, 151. https://doi.org/10.3390/en12010151
Kim KM, Lee J, Choi SI, Ahn GH, Paik JG, Ryu BT, Kim YH, Won YS. A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions. Energies. 2019; 12(1):151. https://doi.org/10.3390/en12010151
Chicago/Turabian StyleKim, Kyung Min, Junwoo Lee, Sung Il Choi, Gil Hwan Ahn, Jong Gyu Paik, Byung Tae Ryu, Yong Ha Kim, and Yong Sun Won. 2019. "A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions" Energies 12, no. 1: 151. https://doi.org/10.3390/en12010151
APA StyleKim, K. M., Lee, J., Choi, S. I., Ahn, G. H., Paik, J. G., Ryu, B. T., Kim, Y. H., & Won, Y. S. (2019). A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions. Energies, 12(1), 151. https://doi.org/10.3390/en12010151



