Thermodynamic Analysis on the Aging of THPP, ZPP and BKNO3 Explosive Charges in PMDs
Abstract
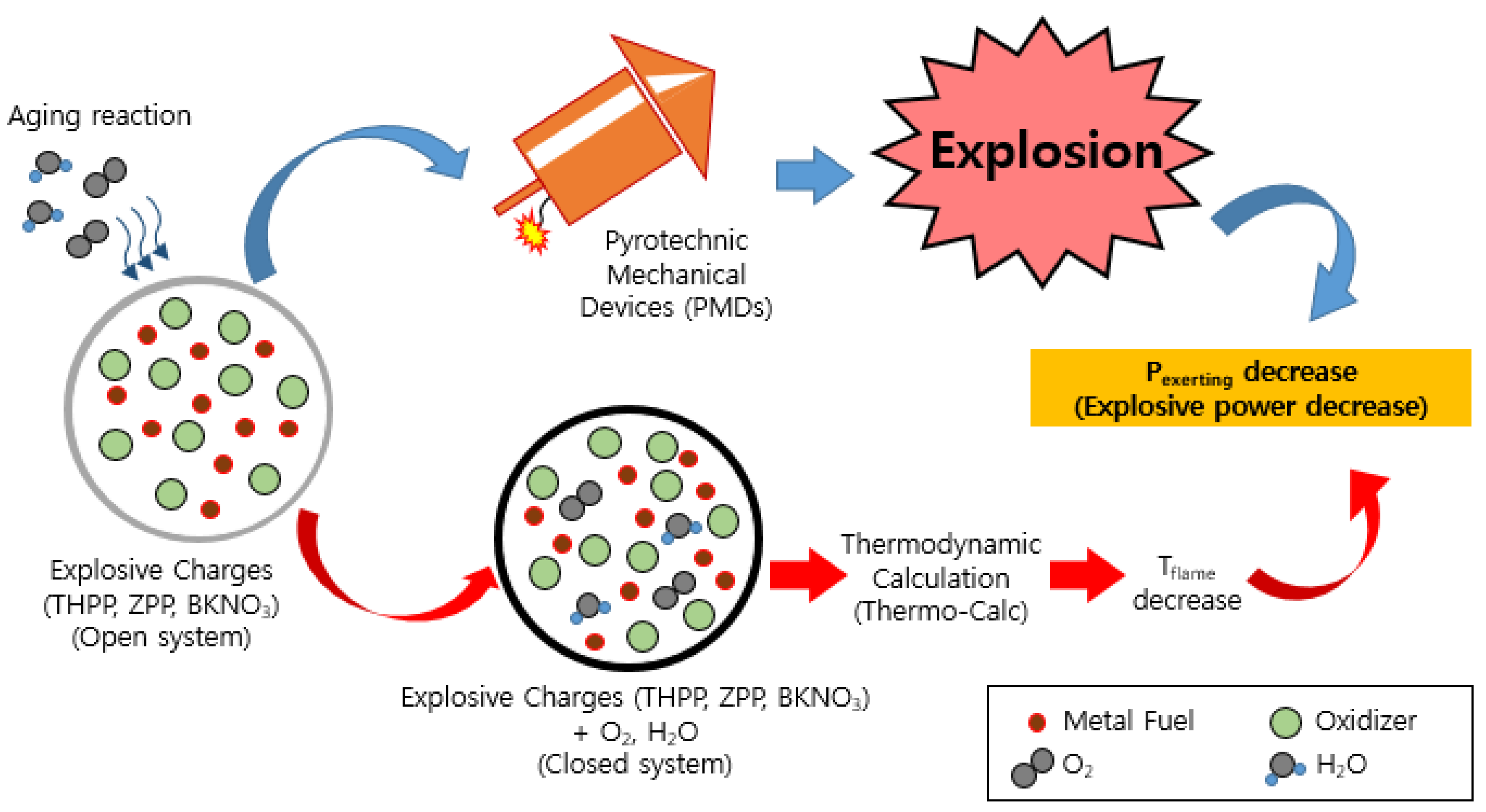
:1. Introduction
2. Materials and Methods
3. Results and Discussion
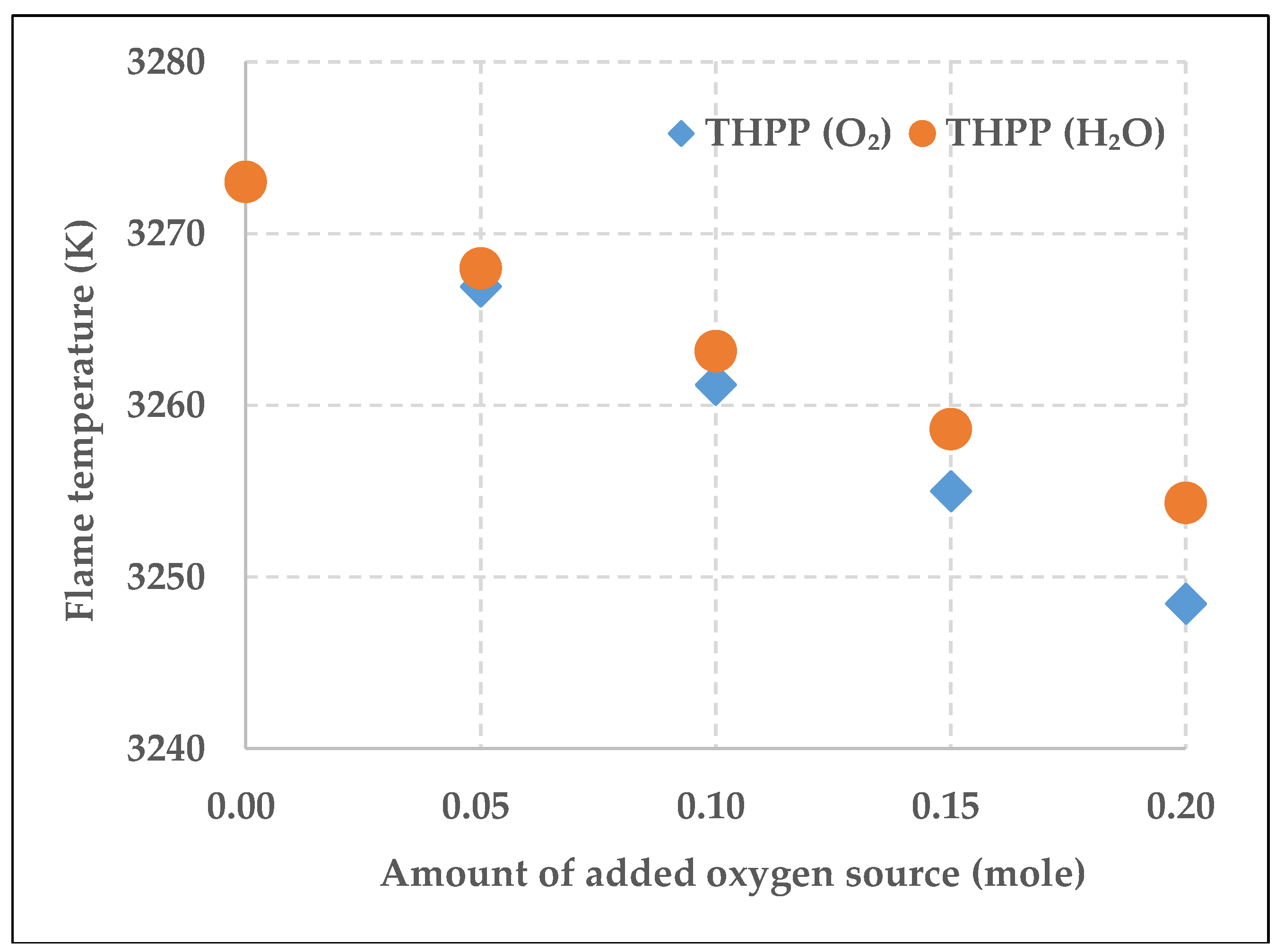
3.1. Aging of THPP Explosive Charge by Oxygen Sources
3.2. Aging of ZPP Explosive Charge by Oxygen Sources
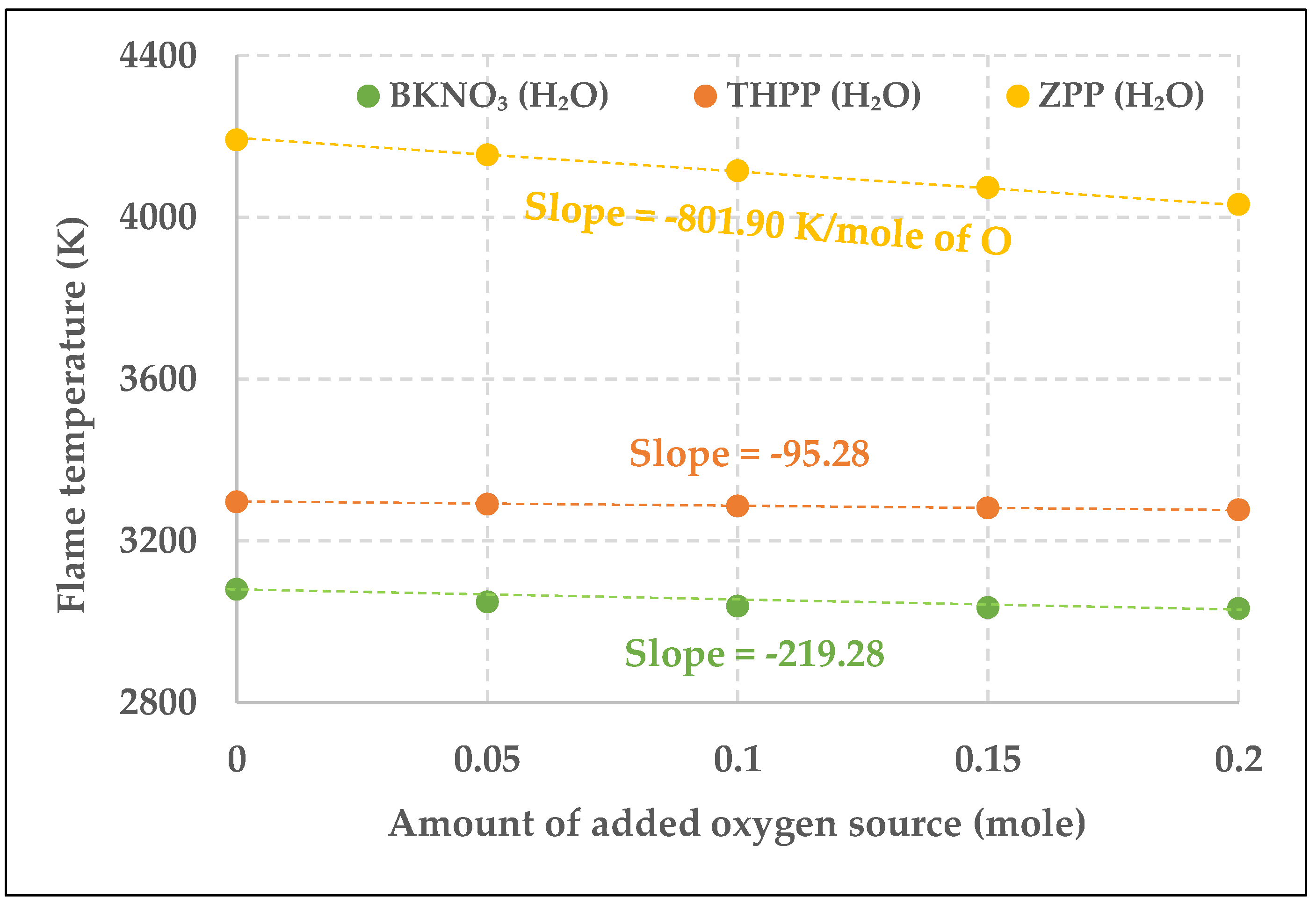
3.3. Comparison of the Aging Effect Among THPP, ZPP, and BKNO3
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paik, J.G.; Ryu, B.T.; Kwon, M. A Study on Acceleration Aging Characteristics of B-KNO3 Igniter. Korean Chem. Eng. Res. 2014, 52, 166–174. [Google Scholar] [CrossRef]
- Le, A.Q.; Sun, L.Z.; Miller, T.C. Detectability of Delaminations in Solid Rocket Motors with Embedded Stress Sensors. J. Propul. Power 2013, 29, 299–304. [Google Scholar] [CrossRef]
- Hosseini, S.G.; Pourmortazavi, S.M.; Hajimirsadeghi, S.S. Thermal Decomposition of Pyrotechnic Mixtures Containing Sucrose with either Potassium Chlorate or Potassium Perchlorate. Combust. Flame 2005, 141, 322–326. [Google Scholar] [CrossRef]
- Risha, G.A.; Kuo, K.K. An Investigation of the Performance of a Boron/Potassium-Nitrate Based Pyrotechnic Igniter. Propellants Explos. Pyrotech. 2006, 31, 311–317. [Google Scholar]
- Sorensen, D.N.; Quebral, A.P.; Baroody, E.E.; Sanborn, W.B. Investigation of the Thermal Degradation of the Aged Pyrotechnic Titanium Hydride/Potassium Perchlorate. J. Therm. Anal. Calorim. 2006, 85, 151–156. [Google Scholar] [CrossRef]
- Lee, J.S.; Lin, L.K.; Lin, C.H.; Chen, P.J.; Huang, C.W.; Chang, S.S. A Study of Zirconium/Potassium Perchlorate Primer Mixture. Thermochim. Acta 1990, 173, 211–218. [Google Scholar] [CrossRef]
- Moore, J.E.; Son, S.F.; Sivathanu, Y.R.; Lim, J. Experimental Characteristics of Particle Dynamics within Solid Rocket Motors Environments; Technical Paper (PA #09154); Air Force Research Laboratory: Dayton, OH, USA, 2009. [Google Scholar]
- Danali, S.M.; Palaiah, R.S.; Raha, K.C. Developments in Pyrotechnics (Review Paper). Defence Sci. J. 2010, 60, 152–158. [Google Scholar] [CrossRef]
- Conkling, J.A.; Mocella, C. Chemistry of Pyrotechnics: Basic Principles and Theory; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Lee, J.R.; Chia, C.C.; Kong, C.W. Review of Pyroshock Wave Measurement and Simulation for Space Systems. Measurement 2012, 45, 631–642. [Google Scholar] [CrossRef]
- Babar, Z.; Malik, A.Q. Synthesis of Micro Porous Barium Nitrate with Improved Ignition Reliability as a Reliable Pyrotechnic Oxidant. J. Saudi Chem. Soc. 2014, 18, 707–711. [Google Scholar] [CrossRef]
- Kuwahara, T.; Kubota, N. Role of Boron in Burning Rate Augmentation of AP composite propellants. Propellants Explos. Pyrotech. 1989, 14, 43–46. [Google Scholar] [CrossRef]
- De Klerk, W.P.C.; Colpa, W.; van Ekeren, P.J. Ageing Studies of Magnesium–Sodium Nitrate Pyrotechnic Compositions. J. Therm. Anal. Calorim. 2006, 85, 203–207. [Google Scholar] [CrossRef]
- Eom, K.H.; An, H.Y.; Kim, K.M.; Ahn, G.H.; Paik, J.G.; Ryu, B.T.; Won, Y.S. Equilibrium Analysis on the Aging of a BKNO3 Igniter. J. Nanosci. Nanotechnol. 2017, 17, 7685–7688. [Google Scholar] [CrossRef]
- Yang, L.C. Reaction Rate Analysis for Selected Solid-to-Solid-Reaction Pyrotechnic Compositions. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar]
- Kong, T.K.; Won, Y.S.; Ryu, B.T.; Ahn, G.H.; Im, D.J. Mathematical Modeling of ZrKClO4 Nano Particle Energy Release. J. Nanosci. Nanotechnol. 2017, 17, 8372–8377. [Google Scholar] [CrossRef]
- Lee, J.; Choi, K.; Ryu, S.U.; Ahn, G.H.; Ryu, B.T.; Won, Y.S. Aging Mechanism of Zirconium Potassium Perchlorate Propellant in Pyrotechnic Mechanical Devices. Nanosci. Nanotechnol. Lett. 2018, 10, 735–740. [Google Scholar] [CrossRef]
- Lee, J.; Kim, T.; Ryu, S.U.; Choi, K.; Ahn, G.H.; Paik, J.G.; Ryu, B.; Park, T.H.; Won, Y.S. Study on the Aging Mechanism of Boron Potassium Nitrate (BKNO3) for Sustainable Efficiency in Pyrotechnic Mechanical Devices. Sci. Rep. 2018, 8, 11745–11754. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lee, J.; Choi, S.I.; Ahn, G.H.; Paik, J.G.; Ryu, B.T.; Kim, Y.H.; Won, Y.S. A Combined Study of TEM-EDS/XPS and Molecular Modeling on the Aging of THPP, ZPP, and BKNO3 Explosive Charges in PMDs under Accelerated Aging Conditions. Energies 2018, 12, 151. [Google Scholar] [CrossRef]
- Thermo-Calc Software SSUB5 SGTE Substances Database Version 5.1. Available online: https://www.thermocalc.com/academia/researchers/how-to-cite-thermo-calc-products/ (accessed on 20 March 2016).

| Added O Amount (mole) | Pure THPP | 0.05 | 0.10 | 0.15 | 0.20 | |
|---|---|---|---|---|---|---|
| H2O addition | TiO2 (s) | 0.1721 mole | 0.1716 | 0.1712 | 0.1709 | 0.1707 |
| TiO (s) | 0.0140 | 0.0137 | 0.0133 | 0.0130 | 0.0127 | |
| TiO2 (l) | 0.8214 | 0.8134 | 0.8151 | 0.8158 | 0.8163 | |
| O2 addition | TiO2 (s) | 0.1721 | 0.1729 | 0.1734 | 0.1737 | 0.1738 |
| TiO (s) | 0.0140 | 0.0134 | 0.0128 | 0.0122 | 0.0116 | |
| TiO2 (l) | 0.8214 | 0.8134 | 0.8135 | 0.8138 | 0.8143 | |
| Added O Amount (Mole) | Pure ZPP | 0.05 | 0.10 | 0.15 | 0.20 | |
|---|---|---|---|---|---|---|
| H2O addition | ZrO2 (s) | 0.1080 mole | 0.1000 | 0.0908 | 0.0812 | 0.0720 |
| ZrO (s) | 0.0696 | 0.0525 | 0.0393 | 0.0292 | 0.0217 | |
| ZrO2 (l) | 0.8222 | 0.8437 | 0.8698 | 0.8895 | 0.9036 | |
| KO (rad) | 0.0078 | 0.0099 | 0.0117 | 0.0133 | 0.0146 | |
| ClO (rad) | 0.0004 | 0.0005 | 0.0005 | 0.0006 | 0.0007 | |
| O2 addition | ZrO2 (s) | 0.1080 | 0.1137 | 0.1171 | 0.1189 | 0.1198 |
| ZrO (s) | 0.0696 | 0.0597 | 0.0526 | 0.0471 | 0.0457 | |
| ZrO2 (l) | 0.8222 | 0.8264 | 0.8302 | 0.8339 | 0.8374 | |
| H (rad) | 0.0763 | 0.1446 | 0.2050 | 0.2580 | ||
| OH (rad) | 0.0123 | 0.0267 | 0.0427 | 0.0600 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.M.; Choi, S.I.; Eom, K.H.; Ahn, G.H.; Paik, J.G.; Ryu, B.T.; Kim, Y.H.; Won, Y.S. Thermodynamic Analysis on the Aging of THPP, ZPP and BKNO3 Explosive Charges in PMDs. Energies 2019, 12, 209. https://doi.org/10.3390/en12020209
Kim KM, Choi SI, Eom KH, Ahn GH, Paik JG, Ryu BT, Kim YH, Won YS. Thermodynamic Analysis on the Aging of THPP, ZPP and BKNO3 Explosive Charges in PMDs. Energies. 2019; 12(2):209. https://doi.org/10.3390/en12020209
Chicago/Turabian StyleKim, Kyung Min, Sung Il Choi, Ki Heon Eom, Gil Hwan Ahn, Jong Gyu Paik, Byung Tae Ryu, Yong Ha Kim, and Yong Sun Won. 2019. "Thermodynamic Analysis on the Aging of THPP, ZPP and BKNO3 Explosive Charges in PMDs" Energies 12, no. 2: 209. https://doi.org/10.3390/en12020209
APA StyleKim, K. M., Choi, S. I., Eom, K. H., Ahn, G. H., Paik, J. G., Ryu, B. T., Kim, Y. H., & Won, Y. S. (2019). Thermodynamic Analysis on the Aging of THPP, ZPP and BKNO3 Explosive Charges in PMDs. Energies, 12(2), 209. https://doi.org/10.3390/en12020209




