Physical Activation of Waste-Derived Materials for Biogas Cleaning
Abstract
:1. Introduction
2. Experimental Methods
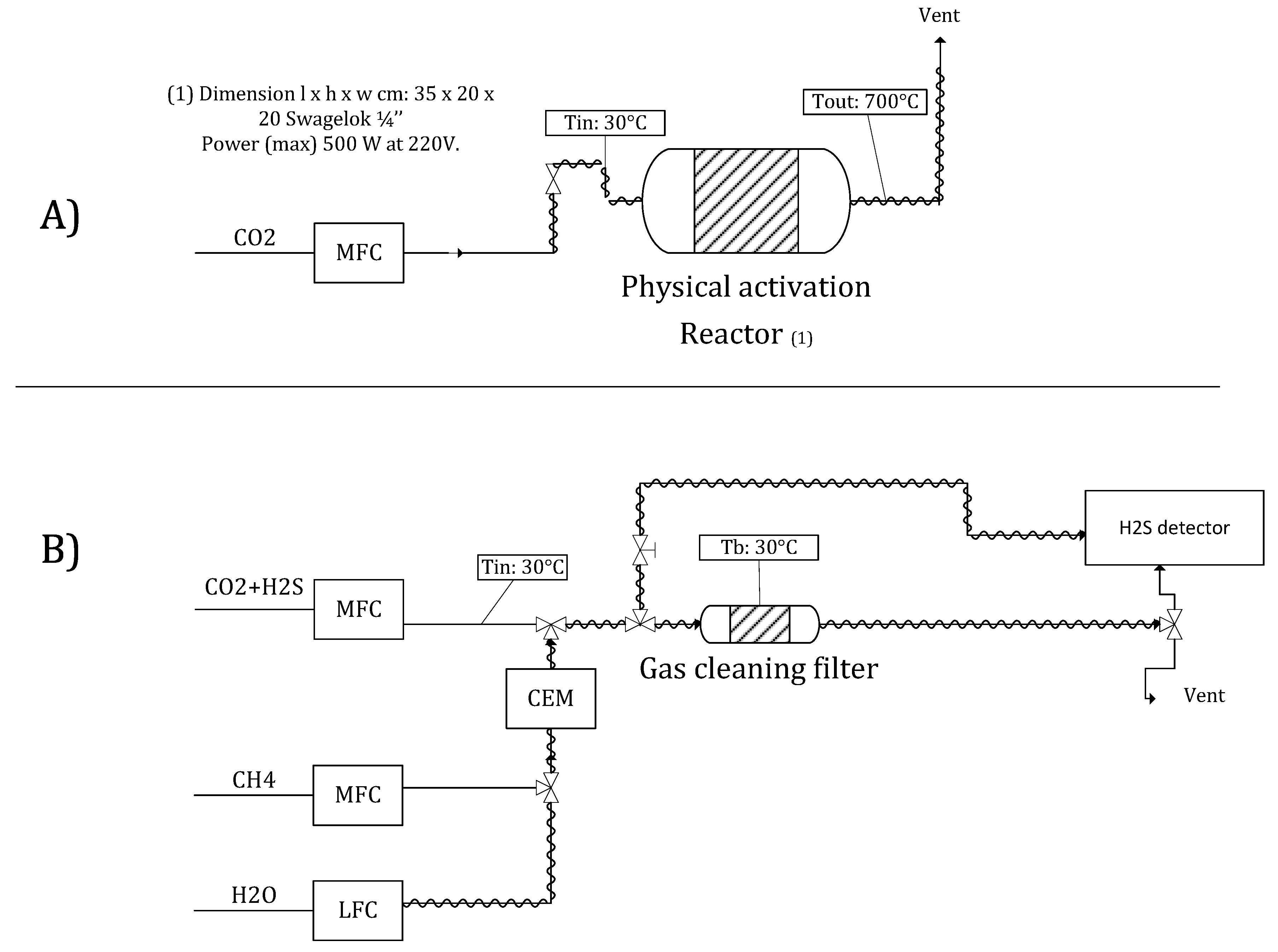
2.1. Description of the Experimental Setup
2.2. Methodology
- t0, is the cleaning service time;
- t1, is the last detection interval, corresponding to initial concentration for H2S of 1%, 10% and 100%;
- Qtot = total gas flow rate (NL/h);
- MW = molecular weight of the trace compound removed (g/mol);
- Cin = inlet trace compound concentration (ppm(v));
- Vm = molar volume (22.414 NL/mol);
- m = mass of sorbent (g).
3. Results and Discussion
3.1. Sorbent Characterisation—EDS/SEM
3.2. Adsorption Capacity
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torrijos, M. State of Development of Biogas Production in Europe. Procedia Environ. Sci. 2016, 35, 881–889. [Google Scholar] [CrossRef]
- Papurello, D.; Soukoulis, C.; Schuhfried, E.; Cappellin, L.; Gasperi, F.; Silvestri, S.; Santarelli, M.; Biasioli, F. Monitoring of volatile compound emissions during dry anaerobic digestion of the Organic Fraction of Municipal Solid Waste by Proton Transfer Reaction Time-of-Flight Mass Spectrometry. Bioresour. Technol. 2012, 126. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Lanzini, A. SOFC single cells fed by biogas: Experimental tests with trace contaminants. Waste Manag. 2017. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Silvestri, S.; Tomasi, L.; Belcari, I.; Biasioli, F.; Santarelli, M. Biowaste for SOFCs. Energy Procedia 2016, 101, 424–431. [Google Scholar] [CrossRef]
- Rasi, S.; Veijanen, A.; Rintala, J. Trace compounds of biogas from different biogas production plants. Energy 2007, 32, 1375–1380. [Google Scholar] [CrossRef]
- Rasi, S.; Läntelä, J.; Rintala, J. Trace compounds affecting biogas energy utilisation—A review. Energy Convers. Manag. 2011, 52, 3369–3375. [Google Scholar] [CrossRef]
- Van Foreest, F. Perspectives for Biogas in Europe; Oxford Institute for Energy Studies: Oxford, UK, 2012; ISBN 9781907555633. [Google Scholar]
- Papurello, D.; Lanzini, A.; Tognana, L.; Silvestri, S.; Santarelli, M. Waste to energy: Exploitation of biogas from organic waste in a 500 Wel solid oxide fuel cell (SOFC) stack. Energy 2015, 85. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Generation of heat and power from biogas for stationary applications: Boilers, gas engines and turbines, combined heat and power (CHP) plants and fuel cells. In The Biogas Handbook, Science, Production and Applications; Elsevier: New York, NY, USA, 2013; pp. 404–427. [Google Scholar]
- Patrizio, P.; Leduc, S.; Chinese, D.; Dotzauer, E.; Kraxner, F. Biomethane as transport fuel—A comparison with other biogas utilization pathways in northern Italy. Appl. Energy 2015, 157, 25–34. [Google Scholar] [CrossRef]
- Saldivia, A.; Mainero, D. Biomethane from OFMSW. In Proceedings of the Ecomondo, Rimini, Italy, 5–8 November 2014. [Google Scholar]
- Facci, A.L.; Cigolotti, V.; Jannelli, E.; Ubertini, S. Technical and economic assessment of a SOFC-based energy system for combined cooling, heating and power. Appl. Energy 2016. [Google Scholar] [CrossRef]
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sustain. Energy Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Papurello, D.; Borchiellini, R.; Bareschino, P.; Chiodo, V.; Freni, S.; Lanzini, A.; Pepe, F.; Ortigoza, G.A.; Santarelli, M. Performance of a Solid Oxide Fuel Cell short-stack with biogas feeding. Appl. Energy 2014, 125. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A.; Leone, P.; Santarelli, M. The effect of heavy tars (toluene and naphthalene) on the electrochemical performance of an anode-supported SOFC running on bio-syngas. Renew. Energy 2016, 99. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A.; Drago, D.; Leone, P.; Santarelli, M. Limiting factors for planar solid oxide fuel cells under different trace compound concentrations. Energy 2016, 95. [Google Scholar] [CrossRef]
- Lanzini, A.; Madi, H.; Chiodo, V.; Papurello, D.; Maisano, S.; Santarelli, M.; Van herle, J. Dealing with fuel contaminants in biogas-fed solid oxide fuel cell (SOFC) and molten carbonate fuel cell (MCFC) plants: Degradation of catalytic and electro-catalytic active surfaces and related gas puri fi cation methods. Prog. Energy Combust. Sci. 2017, 61, 150–188. [Google Scholar] [CrossRef]
- Madi, H.; Lanzini, A.; Papurello, D.; Diethelm, S.; Ludwig, C.; Santarelli, M.; Van herle, J. Solid oxide fuel cell anode degradation by the effect of hydrogen chloride in stack and single cell environments. J. Power Sources 2016, 326, 349–356. [Google Scholar] [CrossRef]
- Xu, C.; Gong, M.; Zondlo, J.W.; Liu, X.; Finklea, H.O. The effect of HCl in syngas on Ni-YSZ anode-supported solid oxide fuel cells. J. Power Sources 2010, 195, 2149–2158. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Wang, W.; Chen, D.; Li, S.D.; Shao, Z. H2S poisoning effect and ways to improve sulfur tolerance of nickel cermet anodes operating on carbonaceous fuels. Appl. Energy 2016, 179, 765–777. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A.; Fiorilli, S.; Smeacetto, F.; Singh, R.; Santarelli, M. Sulfur poisoning in Ni-anode solid oxide fuel cells (SOFCs): Deactivation in single cells and a stack. Chem. Eng. J. 2016, 283. [Google Scholar] [CrossRef]
- Papurello, D.; Lanzini, A.; Leone, P.; Santarelli, M.; Silvestri, S. Biogas from the organic fraction of municipal solid waste: Dealing with contaminants for a solid oxide fuel cell energy generator. Waste Manag. 2014, 34, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Chiang, P.-C.; Huang, C.-P. Effects of pore structure and temperature on VOC adsorption on activated carbon. Carbon 2001, 39, 523–534. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Santarelli, M.; Biasioli, F. Proton transfer reaction-mass spectrometry as a rapid inline tool for filter efficiency of activated charcoal in support of the development of Solid Oxide Fuel Cells fueled with biogas. Fuel Process. Technol. 2015, 130. [Google Scholar] [CrossRef]
- Papurello, D.; Tognana, L.; Lanzini, A.; Smeacetto, F.; Santarelli, M.; Belcari, I.; Silvestri, S.; Biasioli, F. Proton transfer reaction mass spectrometry technique for the monitoring of volatile sulfur compounds in a fuel cell quality clean-up system. Fuel Process. Technol. 2015, 130. [Google Scholar] [CrossRef]
- Papurello, D.; Schuhfried, E.; Lanzini, A.; Romano, A.; Cappellin, L.; Märk, T.D.; Silvestri, S.; Biasioli, F. Influence of co-vapors on biogas filtration for fuel cells monitored with PTR-MS (Proton Transfer Reaction-Mass Spectrometry). Fuel Process. Technol. 2014, 118. [Google Scholar] [CrossRef]
- Aksoylu, A.E.; Madalena, M.; Freitas, A.; Pereira, M.F.R.; Figueiredo, J.L. Effects of different activated carbon supports and support modifications on the properties of Pt/AC catalysts. Carbon 2001, 39, 175–185. [Google Scholar] [CrossRef]
- Monteleone, G.; De Francesco, M.; Galli, S.; Marchetti, M.; Naticchioni, V. Deep H2S removal from biogas for molten carbonate fuel cell (MCFC) systems. Chem. Eng. J. 2011, 173, 407–414. [Google Scholar] [CrossRef]
- Arnold, M. Reduction and Monitoring of Biogas Trace Compounds. Available online: https://www.vtt.fi/inf/pdf/tiedotteet/2009/T2496.pdf (accessed on 30 August 2018).
- Barelli, L.; Bidini, G.; De Arespacochaga, N.; Laura, P.; Sisani, E. Biogas use in high temperature fuel cells: Enhancement of KOH-KI activated carbon performance toward H2S removal. Int. J. Hydrogen Energy 2017, 42, 10341–10353. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Santarelli, M. Evaluation of the Wheeler-Jonas parameters for biogas trace compounds removal with activated carbons. Fuel Process. Technol. 2016, 152. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S. Proton transfer reaction mass spectrometry for the gas cleaning using commercial and waste-derived materials: Focus on the siloxane removal for SOFC applications. Int. J. Mass Spectrom. 2018, 430. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Karatza, D.; Lancia, A.; Musmarra, D.; Pepe, F.; Volpicelli, G. Removal of mercuric chloride from flue gas by sulfur impregnated activated carbon. Hazard. Waste Hazard. Mater. 1996, 13, 95–105. [Google Scholar] [CrossRef]
- Papurello, D.; Silvestri, S.; Lanzini, A. Biogas cleaning: Trace compounds removal with model validation. Sep. Purif. Technol. 2018, 210, 80–92. [Google Scholar] [CrossRef]
- Ayse, E.; Nurgul, O.; Eylem, P.O.; Ersan, P. Fixed-bed pyrolysis of cotton stalk for liquid and solid products. Fuel Process. Technol. 2005, 86, 1207–1219. [Google Scholar] [CrossRef]
- Shang, G.; Li, Q.; Liu, L.; Chen, P.; Huang, X. Adsorption of hydrogen sulfide by biochars derived from pyrolysis of different agricultural/forestry wastes. J. Air Waste Manag. Assoc. 2016, 66, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez Ortiz, F.J.; Aguilera, P.G.; Ollero, P. Biogas desulfurization by adsorption on thermally treated sewage-sludge. Sep. Purif. Technol. 2014, 123, 200–213. [Google Scholar] [CrossRef]
- Papurello, D.; Tomasi, L.; Silvestri, S.; Belcari, I.; Santarelli, M.; Smeacetto, F.; Biasioli, F. Biogas trace compound removal with ashes using proton transfer reaction time-of-flight mass spectrometry as innovative detection tool. Fuel Process. Technol. 2016, 145. [Google Scholar] [CrossRef]
- Ducom, G.; Radu-Tirnoveanu, D.; Pascual, C.; Benadda, B.; Germain, P.; Kwong, C.W.; Chao, C.Y.H.; Subramanian, S.; Pande, G.; De Weireld, G.; et al. Sugarcane bagasse fly ash as an attractive agro-industry source for VOC removal on porous carbon. J. Hazard. Mater. 2013, 49, 683–690. [Google Scholar] [CrossRef]
- Sigot, L.; Ducom, G.; Benadda, B.; Labouré, C. Comparison of adsorbents for H2S and D4 removal for biogas conversion in a solid oxide fuel cell. Environ. Technol. 2016, 37, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Ducom, G.; Radu-Tirnoveanu, D.; Pascual, C.; Benadda, B.; Germain, P. Biogas—Municipal solid waste incinerator bottom ash interactions: Sulphur compounds removal. J. Hazard. Mater. 2009, 166, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Kastner, J.R.; Das, K.C.; Buquoi, Q.; Melear, N.D. Low temperature catalytic oxidation of hydrogen sulfide and methanethiol using wood and coal fly ash. Environ. Sci. Technol. 2003, 37, 2568–2574. [Google Scholar] [CrossRef] [PubMed]
- Papurello, D.; Boschetti, A.; Silvestri, S.; Khomenko, I.; Biasioli, F. Real-time monitoring of removal of trace compounds with PTR-MS: Biochar experimental investigation. Renew. Energy 2018, 125. [Google Scholar] [CrossRef]
- De Arespacochaga, N.; Valderrama, C.; Mesa, C.; Bouchy, L.; Cortina, J.L. Biogas deep clean-up based on adsorption technologies for Solid Oxide Fuel Cell applications. Chem. Eng. J. 2014, 255, 593–603. [Google Scholar] [CrossRef]
- Beeckman, J.W.L.; Fassbender, N.A.; Datz, T.E. Length to Diameter Ratio of Extrudates in Catalyst Technology I. Modeling Catalyst Breakage by Impulsive Forces. AIChE J. 2016, 62, 639–647. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Hern, E.; Sisani, E.; Alvarez, J.M. Performance characterization of a novel Fe-based sorbent for anaerobic gas desulfurization finalized to high temperature fuel cell applications. Int. J. Hydrogen Energy 2017, 42, 1859–1874. [Google Scholar] [CrossRef]
- Tepper, F.; King, J.; Greer, J. The Alkali metal. In Proceedings of the Chemical Society, London, UK, 19–22 July 1967. [Google Scholar]
- Richardson, J.; Bjorheden, R.; Hakkala, P.; Lowe, A.T.; Smith, C.T. Bioenergy from Sustainable Forestry—Guiding Principles and Practice; Kluwer Academic Publisher: New York, NY, USA; Boston, MA, USA; Dordrecht, The Netherlands; London, UK; Moscow, Russia, 2002; ISBN 0306475197. [Google Scholar]
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar] [CrossRef]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.W.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Pre-formed activated carbon matting derived from the pyrolysis of biomass natural fibre textile waste. J. Anal. Appl. Pyrolysis 2003, 70, 563–577. [Google Scholar] [CrossRef]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y. Preparing Desirable Activated Carbons from Agricultural Residues for Potential Uses in Water Treatment. Waste Biomass Valoriz. 2015, 6, 1029–1036. [Google Scholar] [CrossRef]
- Nabais, V.J.M.; Nunes, P.; Carrott, P.J.M.; Ribeiro, M.M.L.; García, A.M.; Díaz-díez, M.A. Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Process. Technol. 2007, 9. [Google Scholar] [CrossRef]
- Zhang, T.; Walawender, W.P.; Fan, L.T.; Fan, M.; Daugaard, D.; Brown, R.C. Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem. Eng. J. 2004, 105, 53–59. [Google Scholar] [CrossRef]
- Guo, S.; Peng, J.; Li, W.; Yang, K.; Zhang, L.; Zhang, S.; Xia, H. Effects of CO2 activation on porous structures of coconut shell-based activated carbons. Appl. Surface Sci. 2009, 255, 8443–8449. [Google Scholar] [CrossRef]
- Cha, J.S.; Park, S.H.; Jung, S.; Ryu, C.; Jeon, J.; Shin, M.; Park, Y. Journal of Industrial and Engineering Chemistry Production and utilization of biochar: A review. J. Ind. Eng. Chem. 2016, 40, 1–15. [Google Scholar] [CrossRef]
- Adib, F.; Bagreev, A.; Bandosz, T.J. Analysis of the Relationship between H2S Removal Capacity and Surface Properties of Unimpregnated Activated Carbons. Environ. Sci. Technol. 2000, 34, 686–692. [Google Scholar] [CrossRef]
- Aravind, P.V.; De Jong, W. Evaluation of high temperature gas cleaning options for biomass gasification product gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Sethupathi, S.; Zhang, M.; Rajapaksha, A.U.; Lee, S.R.; Nor, N.M.; Mohamed, A.R.; Al-Wabel, M.; Lee, S.S.; Ok, Y.S. Biochars as potential adsorbers of CH4, CO2 and H2S. Sustainability 2017, 9, 121. [Google Scholar] [CrossRef]
- Xue, M.; Chitrakar, R.; Sakane, K.; Ooi, K. Screening of adsorbents for removal of H2S at room temperature. Green Chem. 2003, 5, 529–534. [Google Scholar] [CrossRef]
- Wood, G.O. Activated carbon adsorption capacities for vapors. Carbon 1992, 30, 593–599. [Google Scholar] [CrossRef]
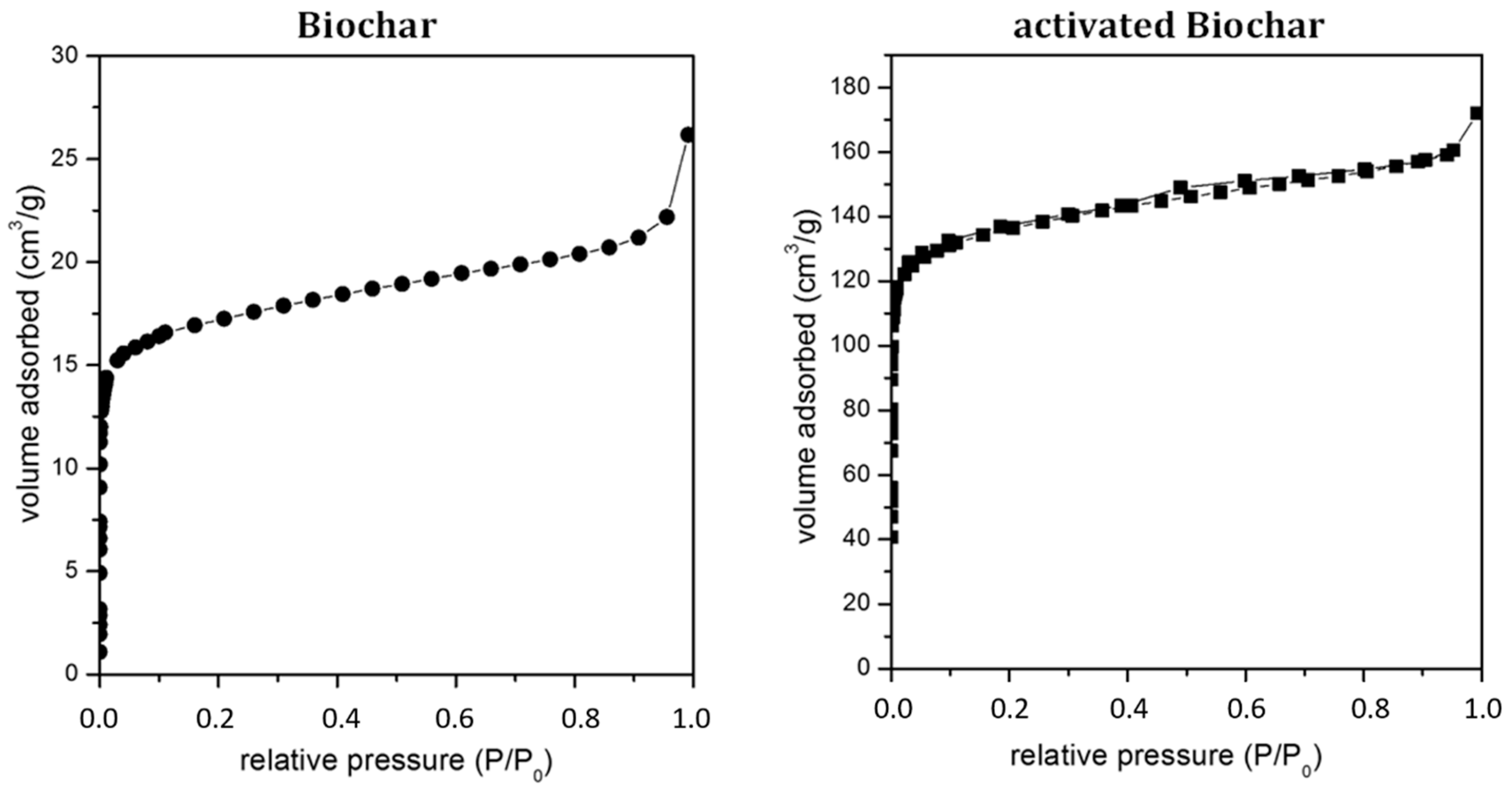


| Biochar | Activated Biochar | Ash | Activated Ash | Commercial Carbon (RST3) | Commercial Carbon (Carbox) | ZnO | |
|---|---|---|---|---|---|---|---|
| C | 77.5 | 81.8 | 14.26 | 14.8 | 91.1 | 80.9 | |
| O | 13.98 | 16.65 | 49.29 | 49.4 | 5.3 | 9.01 | 24.79 |
| Si | 23.73 | 22.2 | 1.4 | 0.55 | |||
| Al | 0.60 | 0.59 | 1.98 | 0.96 | |||
| K | 0.93 | 0.33 | 9.99 | 10.75 | 0.79 | 0.78 | |
| Ca | 0.90 | 1.63 | 1.3 | 1.4 | 0.75 | 1.65 | 0.75 |
| Mg | 0.24 | 0.19 | 0.62 | 0.64 | 0.31 | 1.19 | |
| Cl | 0.21 | 0.22 | 0.33 | ||||
| P | 0.81 | ||||||
| S | 0.59 | 0.11 | |||||
| Mn | 0.41 | ||||||
| Fe | 1.43 | ||||||
| Zn | 1.16 | 72.95 |
| Biochar | Activated Biochar | Ash | Activated Ash | Commercial Carbon (RST3) | Commercial Carbon (Carbox) | ZnO | ||
|---|---|---|---|---|---|---|---|---|
| Specific surface area | (m2/g) | 75 | 593 | 0.9 | 6 | 1117 | 1237 | 35.8 |
| Microporous volume | (cm3/g) | 0.04 | 0.26 | 0.01 | 0.07 | 0.3 | 0.328 | |
| Total pore volume | (cm3/g) | 0.06 | 0.37 | 0.015 | 0.1 | 0.447 | 0.407 | 0.196 |
| Sorbent | Load (g) | Cads. (mg/g)-1% | Std. Dev | Cads. (mg/g)-10% | Std. Dev | Cads. (mg/g)-100% | Std. Dev | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial sorbents | |||||||||||||
| Airdep CarbOx | 0.53 | 1.88 | 1.88 | 1.94 | 0.03 | 6.75 | 6.74 | 6.77 | 0.01 | 12.45 | 12.54 | 12.65 | 0.08 |
| Norit RST3 | 0.3 | 1.95 | 2.04 | 1.98 | 0.04 | 2.76 | 2.81 | 2.83 | 0.03 | 12.10 | 12.11 | 12.17 | 0.03 |
| Zinc oxides | 0.33 | 0.92 | 0.98 | 1.02 | 0.04 | 24.74 | 24.75 | 24.81 | 0.03 | 47.07 | 47.17 | 47.21 | 0.06 |
| Waste derived materials | |||||||||||||
| Biochar | 0.25 | 0.12 | 0.19 | 0.23 | 0.05 | 0.27 | 0.31 | 0.40 | 0.05 | 4.76 | 4.85 | 4.92 | 0.07 |
| Biochar act | 0.16 | 1.30 | 1.38 | 1.45 | 0.06 | 1.95 | 2.01 | 2.11 | 0.07 | 9.85 | 9.89 | 10.01 | 0.07 |
| Ash | 0.95 | 0.02 | 0.06 | 0.08 | 0.02 | 0.16 | 0.10 | 0.17 | 0.03 | 0.23 | 0.25 | 0.27 | 0.02 |
| Ash act | 1.03 | 0.29 | 0.37 | 0.48 | 0.08 | 0.65 | 0.66 | 0.69 | 0.02 | 1.77 | 1.79 | 1.75 | 0.02 |
| Sorbent | Load (g) | Cads. (mg/g)-1% + H2O (30%) | Std. Dev | Cads. (mg/g)-10% + H2O (30%) | Std. Dev | Cads. (mg/g)-100% + H2O (30%) | Std. Dev | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Commercial sorbents | |||||||||||||
| Airdep CarbOx | 0.17 | 1.30 | 1.37 | 1.49 | 0.08 | 5.19 | 5.27 | 5.39 | 0.08 | 8.65 | 8.74 | 8.78 | 0.05 |
| Zinc oxides | 0.30 | 0.34 | 0.43 | 0.54 | 0.08 | 2.45 | 2.51 | 2.62 | 0.07 | 29.44 | 29.48 | 29.55 | 0.05 |
| Waste derived materials | |||||||||||||
| Biochar | 0.31 | 0.01 | 0.02 | 0.08 | 0.03 | 0.08 | 0.12 | 0.13 | 0.02 | 2.65 | 2.69 | 2.80 | 0.06 |
| Biochar act | 0.28 | 0.90 | 0.93 | 1.04 | 0.06 | 1.26 | 1.29 | 1.35 | 0.04 | 5.93 | 5.98 | 6.02 | 0.04 |
| Ash | 1 | 0.01 | 0.02 | 0.04 | 0.01 | 0.14 | 0.15 | 0.18 | 0.02 | 0.26 | 0.17 | 0.20 | 0.04 |
| Ash act | 1.04 | 0.17 | 0.19 | 0.25 | 0.03 | 0.42 | 0.47 | 0.50 | 0.03 | 1.10 | 1.17 | 1.19 | 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papurello, D.; Santarelli, M.; Fiorilli, S. Physical Activation of Waste-Derived Materials for Biogas Cleaning. Energies 2018, 11, 2338. https://doi.org/10.3390/en11092338
Papurello D, Santarelli M, Fiorilli S. Physical Activation of Waste-Derived Materials for Biogas Cleaning. Energies. 2018; 11(9):2338. https://doi.org/10.3390/en11092338
Chicago/Turabian StylePapurello, Davide, Massimo Santarelli, and Sonia Fiorilli. 2018. "Physical Activation of Waste-Derived Materials for Biogas Cleaning" Energies 11, no. 9: 2338. https://doi.org/10.3390/en11092338
APA StylePapurello, D., Santarelli, M., & Fiorilli, S. (2018). Physical Activation of Waste-Derived Materials for Biogas Cleaning. Energies, 11(9), 2338. https://doi.org/10.3390/en11092338







