Li-Ion Battery Fire Hazards and Safety Strategies
Abstract
1. Introduction
2. The Thermal Runaway Process
3. Preventing Thermal Runaway Using Separators
4. Fire Prevention Using Flame Retardants
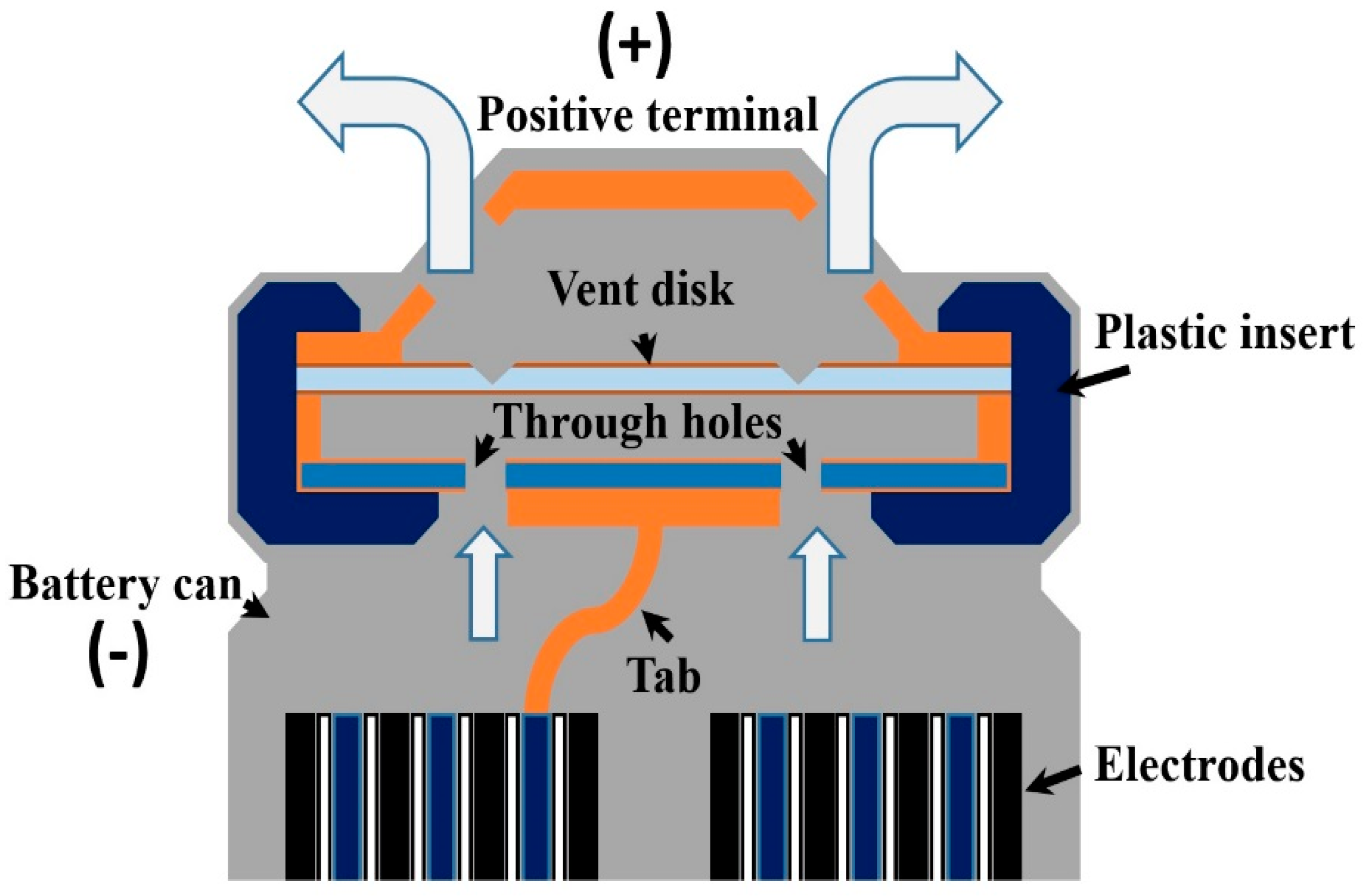
5. Fire and Explosion Prevention Using Cell Venting
6. Extinguishing Li-ion Battery Fires
7. Conclusions and Recommendations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652. [Google Scholar] [CrossRef] [PubMed]
- FAA Office of Security and Hazardous Materials Safety. Available online: https://www.faa.gov/about/office_org/headquarters_offices/ash/ash_programs/hazmat/aircarrier_info/media/battery_incident_chart.pdf (accessed on 9 July 2018).
- Could Chevy Volt Lithium-Ion Battery Fires Burn out Interest in EVs and Hybrids? Available online: https://blogs.scientificamerican.com/observations/could-chevy-volt-lithium-ion-battery-fires-burn-out-interest-in-evs-and-hybrids/ (accessed on 31 July 2018).
- Can You Trust the Lithium-Ion Battery in Your Pocket? Available online: https://www.washingtonpost.com/news/the-switch/wp/2016/09/16/can-you-trust-the-lithium-ion-battery-in-your-pocket/?utm_term=.f7ae4130ed74 (accessed on 6 August 2017).
- Why Lithium-Ion Smartphone Batteries Keep Exploding. Available online: http://globalnews.ca/news/1714748/why-lithium-ion-smartphone-batteries-keep-exploding/ (accessed on 6 August 2017).
- Dramatic CCTV Footage Shows e-Cigarette Battery Exploding in Man’s Pocket-Prompting Storage Warning from Fire Services. Available online: https://www.telegraph.co.uk/news/2016/12/22/dramatic-cctv-footage-shows-e-cigarette-battery-exploding-mans/ (accessed on 31 July 2018).
- Man Says e-Cigarette Battery Exploded in His Pocket. Available online: https://www.cnn.com/2016/02/25/health/e-cigarette-explodes-in-mans-pocket/index.html (accessed on 6 August 2017).
- Saxena, S.; Kong, L.; Pecht, M.G. Exploding E-cigarettes: A battery safety issue. IEEE Access 2018, 6, 21442–21466. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Ramesh, R.; Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Thermal Behavior of Lithiated Graphite with Electrolyte in Lithium-Ion Batteries. J. Electrochem. Soc. 2006, 153, A329–A333. [Google Scholar] [CrossRef]
- Spotnitz, R.; Franklin, J. Abuse behavior of high-power, lithium-ion cells. J. Power Sources 2003, 113, 81–100. [Google Scholar] [CrossRef]
- Al Hallaj, S.; Maleki, H.; Hong, J.; Selman, J. Thermal modeling and design considerations of lithium-ion batteries. J. Power Sources 1999, 83, 1–8. [Google Scholar] [CrossRef]
- Feng, X.; Fang, M.; He, X.; Ouyang, M.; Lu, L.; Wang, H.; Zhang, M. Thermal runaway features of large format prismatic lithium ion battery using extended volume accelerating rate calorimetry. J. Power Sources 2014, 255, 294–301. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Huang, X.; Chen, L. Gas evolution behaviors for several cathode materials in lithium-ion batteries. J. Power Sources 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Guo, G.; Long, B.; Cheng, B.; Zhou, S.; Xu, P.; Cao, B. Three-dimensional thermal finite element modeling of lithium-ion battery in thermal abuse application. J. Power Sources 2010, 195, 2393–2398. [Google Scholar] [CrossRef]
- Forgez, C.; Do, D.V.; Friedrich, G.; Morcrette, M.; Delacourt, C. Thermal modeling of a cylindrical LiFePO4/graphite lithium-ion battery. J. Power Sources 2010, 195, 2961–2968. [Google Scholar] [CrossRef]
- Shin, J.S.; Han, C.H.; Jung, U.H.; Lee, S.I.; Kim, H.J.; Kim, K. Effect of Li2CO3 additive on gas generation in lithium-ion batteries. J. Power Sources 2002, 109, 47–52. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef]
- Spinner, N.S.; Field, C.R.; Hammond, M.H.; Williams, B.A.; Myers, K.M.; Lubrano, A.L.; Rose-Pehrsson, S.L.; Tuttle, S.G. Physical and chemical analysis of lithium-ion battery cell-to-cell failure events inside custom fire chamber. J. Power Sources 2015, 279, 713–721. [Google Scholar] [CrossRef]
- Hammami, A.; Raymond, N.; Armand, M. Lithium-ion batteries: Runaway risk of forming toxic compounds. Nature 2003, 424, 635–636. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.P.; Crafts, C.C.; Doughty, D.H.; McBreen, J. Advanced Technology Development Program for Lithium-Ion Batteries: Thermal Abuse Performance of 18650 Li-Ion Cells; Sandia Report; Sandia National Laboratories: Albuquerque, NM, USA, 2004.
- Feng, X.; Sun, J.; Ouyang, M.; Wang, F.; He, X.; Lu, L.; Peng, H. Characterization of penetration induced thermal runaway propagation process within a large format lithium ion battery module. J. Power Sources 2015, 275, 261–273. [Google Scholar] [CrossRef]
- Lopez, C.F.; Jeevarajan, J.A.; Mukherjee, P.P. Experimental analysis of thermal runaway and propagation in lithium-ion battery modules. J. Electrochem. Soc. 2015, 162, A1905–A1915. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Steele, L.A.M.; Spangler, S.W. Failure propagation in multi-cell lithium ion battereis. J. Power Sources 2015, 283, 517–523. [Google Scholar] [CrossRef]
- Venugopal, G.; Moore, J.; Howard, J.; Pendalwar, S. Characterization of microporous separators for lithium-ion batteries. J. Power Sources 1999, 77, 34–41. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Michiel, M.D.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef] [PubMed]
- Orendorff, C.J. The role of separators in lithium-ion cell safety. Electrochem. Soc. Interf. 2012, 21, 61–65. [Google Scholar] [CrossRef]
- Yuan, X.; Liu, H.; Zhang, J. Lithium-Ion Batteries: Advanced Materials and Technologies; CRC Press: Boca Raton, FL, USA, 2011; ISBN 9781439841280. [Google Scholar]
- Xu, K.; Zhang, S.; Allen, J.L.; Jow, T.R. Nonflammable electrolytes for Li-ion batteries based on a fluorinated phosphate. J. Electrochem. Soc. 2002, 149, A1079–A1082. [Google Scholar] [CrossRef]
- Xu, K.; Ding, M.S.; Zhang, S.; Allen, J.L.; Jow, T.R. Evaluation of fluorinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries: I. Physical and electrochemical properties. J. Electrochem. Soc. 2003, 150, A161–A169. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Choi, J.A.; Sun, Y.K.; Shim, E.G.; Scrosati, B.; Kim, D.W. Effect of 1-butyl-1-methylpyrrolidinium hexafluorophosphate as a flame-retarding additive on the cycling performance and thermal properties of lithium-ion batteries. Electrochim. Acta 2011, 56, 10179–10184. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Sun, J.; Chen, C. Improved thermal stability of lithium ion battery by using cresyl diphenyl phosphate as an electrolyte additive. J. Power Sources 2010, 195, 7457–7461. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Sun, J.; Chen, C. Cresyl diphenyl phosphate effect on the thermal stabilities and electrochemical performances of electrodes in lithium ion battery. J. Power Sources 2011, 196, 5960–5965. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. 4-Isopropyl phenyl diphenyl phosphate as flame-retardant additive for lithium-ion battery electrolyte. Electrochem. Solid-State Letters 2005, 8, A467–A470. [Google Scholar] [CrossRef]
- Liu, K.; Liu, W.; Qiu, Y.; Kong, B.; Sun, Y.; Chen, Z.; Zhuo, D.; Lin, D.; Cui, Y. Electrospun core-shell microfiber separator with thermal-triggered flame-retardant properties for lithium-ion batteries. Sci. Adv. 2017, 3, e1601978. [Google Scholar] [CrossRef] [PubMed]
- Arbizzani, C.; Gabrielli, G.; Mastragostino, M. Thermal stability and flammability of electrolytes for lithium-ion batteries. J. Power Sources 2011, 196, 4801–4805. [Google Scholar] [CrossRef]
- Wang, J.; Yamada, Y.; Sodeyama, K.; Watanabe, E.; Takada, K.; Tateyama, Y.; Yamada, A. Fire-extinguishing organic electrolytes for safe batteries. Nat. Energy 2018, 3, 22–29. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Yu, L.; Ren, X.; Engelhard, M.H.; Niu, C.; Lee, H.; Xu, W.; Xiao, J.; Liu, J.; Zhang, J.G. High-efficiency lithium metal batteries with fire-retardant electrolytes. Joule 2018, 2, 1–11. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, H.; Wang, Z.; Chen, C. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Shim, E.G.; Nam, T.H.; Kim, J.G.; Kim, H.S.; Moon, S.I. Diphenyloctyl phosphate as a flame-retardant additive in electrolyte for Li-ion batteries. J. Power Sources 2008, 175, 533–539. [Google Scholar] [CrossRef]
- Lee, C.W.; Venkatachalapathy, R.; Prakash, J.A. novel flame-retardant additive for lithium batteries. Electrochem. Solid-State Lett. 2000, 3, 63–65. [Google Scholar] [CrossRef]
- Yao, X.; Xie, S.; Chen, C.; Wang, Q.; Sun, J.; Li, Y.; Lu, S. Comparative study of trimethyl phosphite and trimethyl phosphate as electrolyte additives in lithium ion batteries. J. Power Sources 2005, 144, 170–175. [Google Scholar] [CrossRef]
- Shim, E.G.; Nam, T.H.; Kim, J.G.; Kim, H.S.; Moon, S.I. Electrochemical performance of lithium-ion batteries with triphenylphosphate as a flame-retardant additive. J. Power Sources 2007, 172, 919–924. [Google Scholar] [CrossRef]
- Kim, K.; Ahn, S.; Kim, H.S.; Liu, H.K. Electrochemical and thermal properties of 2, 4, 6-tris (trifluoromethyl)-1, 3, 5-triazine as a flame retardant additive in Li-ion batteries. Electrochim. Acta 2009, 54, 2259–2265. [Google Scholar] [CrossRef]
- Nam, T.H.; Shim, E.G.; Kim, J.G.; Kim, H.S.; Moon, S.I. Diphenyloctyl phosphate and tris (2,2,2-trifluoroethyl) phosphite as flame-retardant additives for Li-ion cell electrolytes at elevated temperature. J. Power Sources 2008, 180, 561–567. [Google Scholar] [CrossRef]
- Rechargeable Batteries for Cellular Telephones; IEEE Standard 1725; IEEE Power Engineering Society Press: New York, NY, USA, 2011.
- Mier, F.A.; Morales, R.; Coultas-McKenney, C.A.; Hargather, M.J.; Ostanek, J. Overcharge and thermal destructive testing of lithium metal oxide and lithium metal phosphate batteries incorporating optical diagnostics. J. Energy Storage 2017, 13, 378–386. [Google Scholar] [CrossRef]
- Chu, A.C.; Gozdz, A.S.; Riley, G.N., Jr.; Hoff, C.M. Battery Tab Location Design and Method of Construction. U.S. Patent US8084158B2, 27 December 2011. [Google Scholar]
- Kaschmitter, J.L.; Martucci, F.L.; Mayer, S.T.; Souh, J.H.; Thompson, S. Cell Cap Assembly Having Frangible Tab Disconnect Mechanism. U.S. Patent 5,609,972, 11 March 1997. [Google Scholar]
- Hua, J.; Yang, J. Mesoporous Lithium-Ion Battery Vented at Both Ends, and Method for Manufacturing Same. W.O. Patent 2,016,008,137, 21 January 2016. [Google Scholar]
- Finegan, D.P.; Darcy, E.; Keyser, M.; Tjaden, B.; Heenan, T.M.M.; Jervis, R.; Bailey, J.J.; Vo, N.T.; Magdysyuk, O.V.; Drakopoulos, M.; et al. Identifying the cause of rupture of Li-ion batteries during thermal runaway. Adv. Sci. 2018, 5, 1700369. [Google Scholar] [CrossRef] [PubMed]
- IEC Standard 62133. Secondary Cells and Batteries Containing Alkaline or Other Non-acid Electrolytes—Safety Requirements for Portable Sealed Secondary Cells, and for Batteries Made from Them, for Use in Portable Applications; IEC Standard 62133: Geneva, Switzerland, 2012. [Google Scholar]
- Josefowitz, W.; Kranz, H.; Macerata, D.; Soczka-Guth, T.; Mettlach, H.; Porcellato, D.; Orsini, F.; Hansson, J. Assessment and Testing of Advanced Energy Storage Systems for Propulsion–European Testing Report. In Proceedings of the 21st Worldwide Battery, Hybrid and Fuel Cell Electric Vehicle Symposium & Exhibition, Monte Carlo, Monaco, 2–6 April 2005. [Google Scholar]
- National Fire Protection Association. Portable Fire Extinguishers; National Fire Protection Association: Quincy, MA, USA, 2013. [Google Scholar]
- Mikolajczak, C.; Kahn, M.; White, K.; Long, R.T. Lithium-ion Batteries Hazard and Use Assessment; Springer Science & Business Media Press: New York, NY, USA, 2012; ISBN 978-1-4614-3485-6. [Google Scholar]
- Long, R.T.; Blum, A.F.; Bress, T.J.; Cotts, B.R. Best Practices for Emergency Response to Incidents Involving Electric Vehicles Battery Hazards: A Report on Full-Scale Testing Results; National Fire Protection Research Foundation: Quincy, MA, USA, 2013. [Google Scholar]
- Lithium-Ion Battery Car Fires Pose A New Challenge for Firefighters. Available online: http://www.rightinginjustice.com/news/2017/02/06/lithium-ion-battery-car-fires-pose-a-new-challenge-for-firefighters/ (accessed on 6 August 2017).
| Abbrev. | Full Name | Ref. |
|---|---|---|
| BMP-PF6 | 1-Butyl-1-methylpyrrolidinium hexafluorophosphate | [32] |
| CDP | Cresyl diphenyl phosphate | [33] |
| DMMP | Dimethyl methyl phosphonate | [40] |
| DPOF | Diphenyloctyl phosphate | [41] |
| HMPN | Hexamethylcyclophosphazene | [29] |
| IPPP | 4-Isopropyl phenyl diphenyl phosphate | [35] |
| [NP(OCH3)2]3 | Hexamethoxycyclotriphosphazene | [42] |
| TEP | Triethyl phosphate | [29] |
| TMP | Trimethyl phosphate | [29] |
| TMP(a) | Trimethyl phosphate | [43] |
| TMP(i) | Trimethyl phosphite | [43] |
| TPP | Triphenyl phosphate | [44] |
| TTFMT | 2,4,6-Tris(trifluoromethyl)-1,3,5-triazine (TTFMT) | [45] |
| TTFP | Tris(2,2,2-trifluoroethyl) phosphite | [46] |
| Hazard Level | Description | Classification Criteria and Effect |
|---|---|---|
| 0 | No effect | No effect. No loss of functionality. |
| 1 | Passive protection activated | No defect; no leakage; no venting, fire, or flame; no rupture; no explosion; no exothermic reaction or thermal runaway. Cell reversibly damaged. Repair of protection device needed. |
| 2 | Defect/damage | No leakage; no venting, fire, or flame; no rupture; no explosion; no exothermic reaction or thermal runaway. Cell irreversibly damaged. Repair needed. |
| 3 | Leakage Δmass < 50% | No venting, fire, or flame* no rupture; no explosion. Weight loss <50% of electrolyte weight (electrolyte = solvent + salt). |
| 4 | Venting Δmass ≥ 50% | No fire or flame*; no rupture; no explosion. Weight loss ≥50% of electrolyte weight (electrolyte = solvent + salt). |
| 5 | Fire or flame | No rupture; no explosion (i.e., no flying parts). |
| 6 | Rupture | No explosion, but flying parts of the active mass. |
| 7 | Explosion | Explosion (i.e., disintegration of the cell). |
| Class | Description |
|---|---|
| A | Fires in ordinary combustible materials, such as wood, cloth, paper, rubber, and many plastics. |
| B | Fires in flammable liquids, combustible liquids, petroleum greases, tars, oils, oil-based paints, solvents, lacquers, alcohols, and flammable gases. |
| C | Fires that involve energized electrical equipment. |
| D | Fires in combustible metals, such as magnesium, titanium, zirconium, sodium, lithium, and potassium. |
| K | Fires in cooking appliances that involve combustible cooking media (vegetable or animal oils and fats). |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, L.; Li, C.; Jiang, J.; Pecht, M.G. Li-Ion Battery Fire Hazards and Safety Strategies. Energies 2018, 11, 2191. https://doi.org/10.3390/en11092191
Kong L, Li C, Jiang J, Pecht MG. Li-Ion Battery Fire Hazards and Safety Strategies. Energies. 2018; 11(9):2191. https://doi.org/10.3390/en11092191
Chicago/Turabian StyleKong, Lingxi, Chuan Li, Jiuchun Jiang, and Michael G. Pecht. 2018. "Li-Ion Battery Fire Hazards and Safety Strategies" Energies 11, no. 9: 2191. https://doi.org/10.3390/en11092191
APA StyleKong, L., Li, C., Jiang, J., & Pecht, M. G. (2018). Li-Ion Battery Fire Hazards and Safety Strategies. Energies, 11(9), 2191. https://doi.org/10.3390/en11092191







