Nanoscale Characteristics and Reactivity of Nascent Soot from n-Heptane/2,5-Dimethylfuran Inverse Diffusion Flames with/without Magnetic Fields
Abstract
1. Introduction
2. Experimental System and Analysis Methods
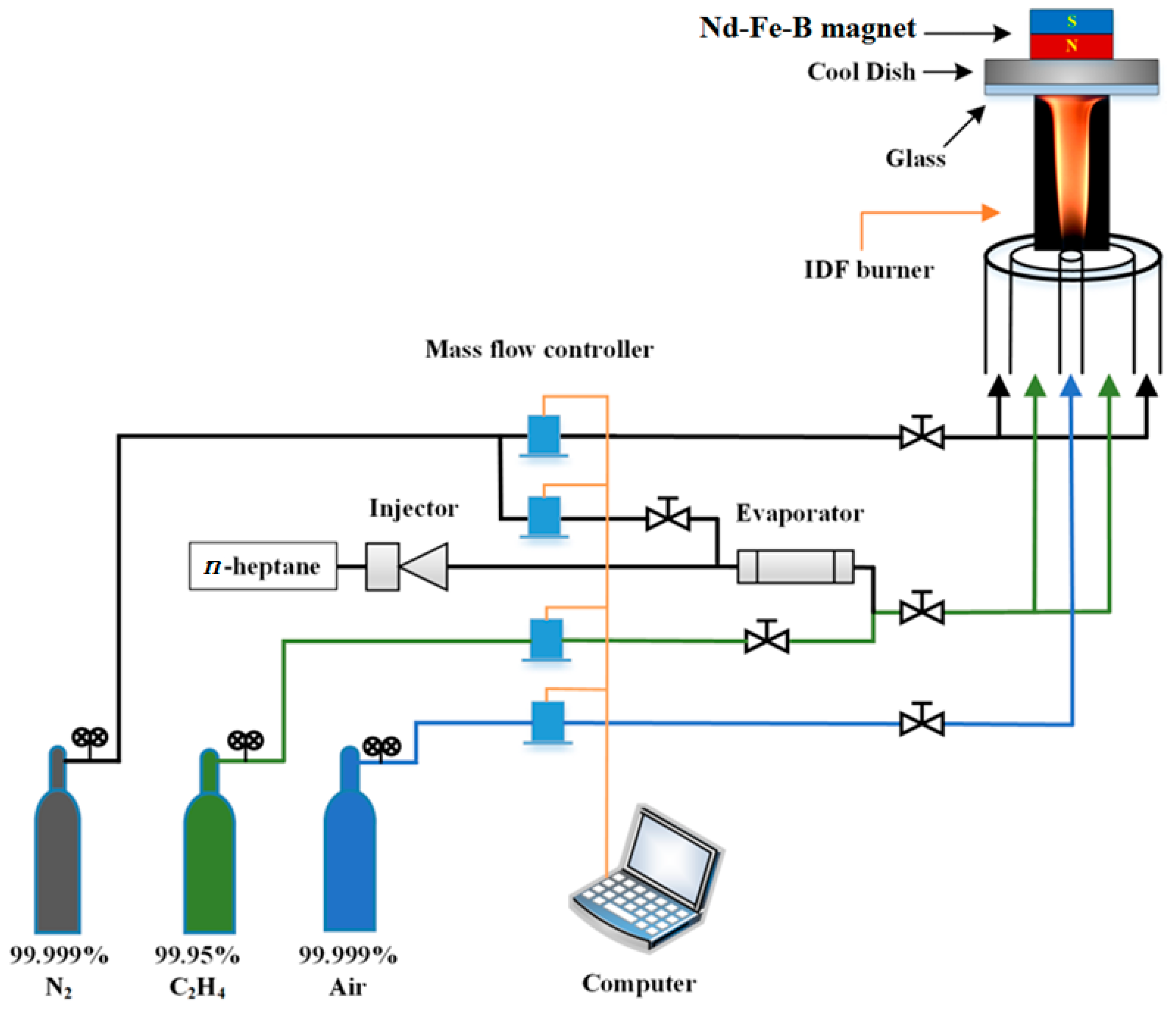
2.1. Experimental System
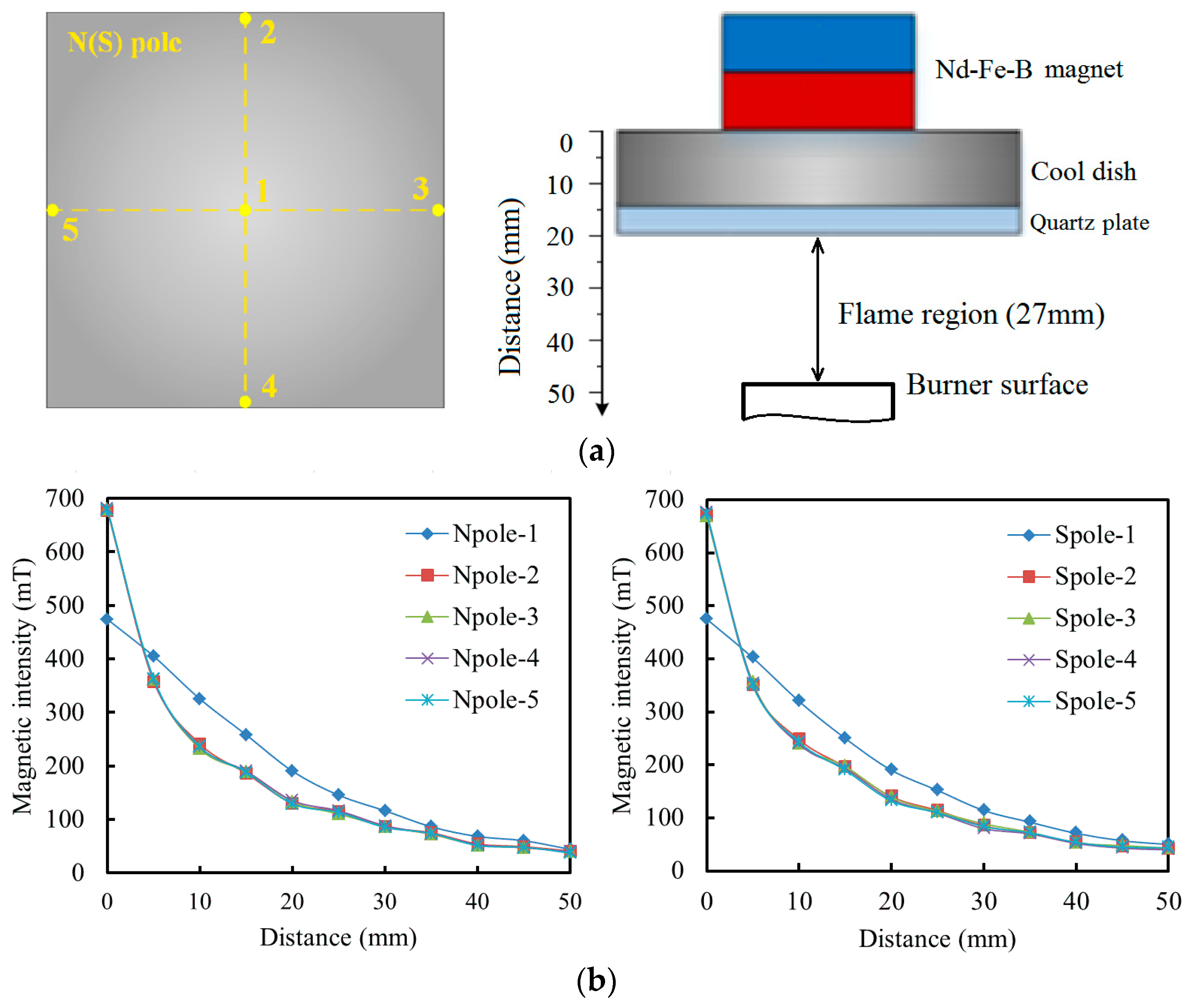
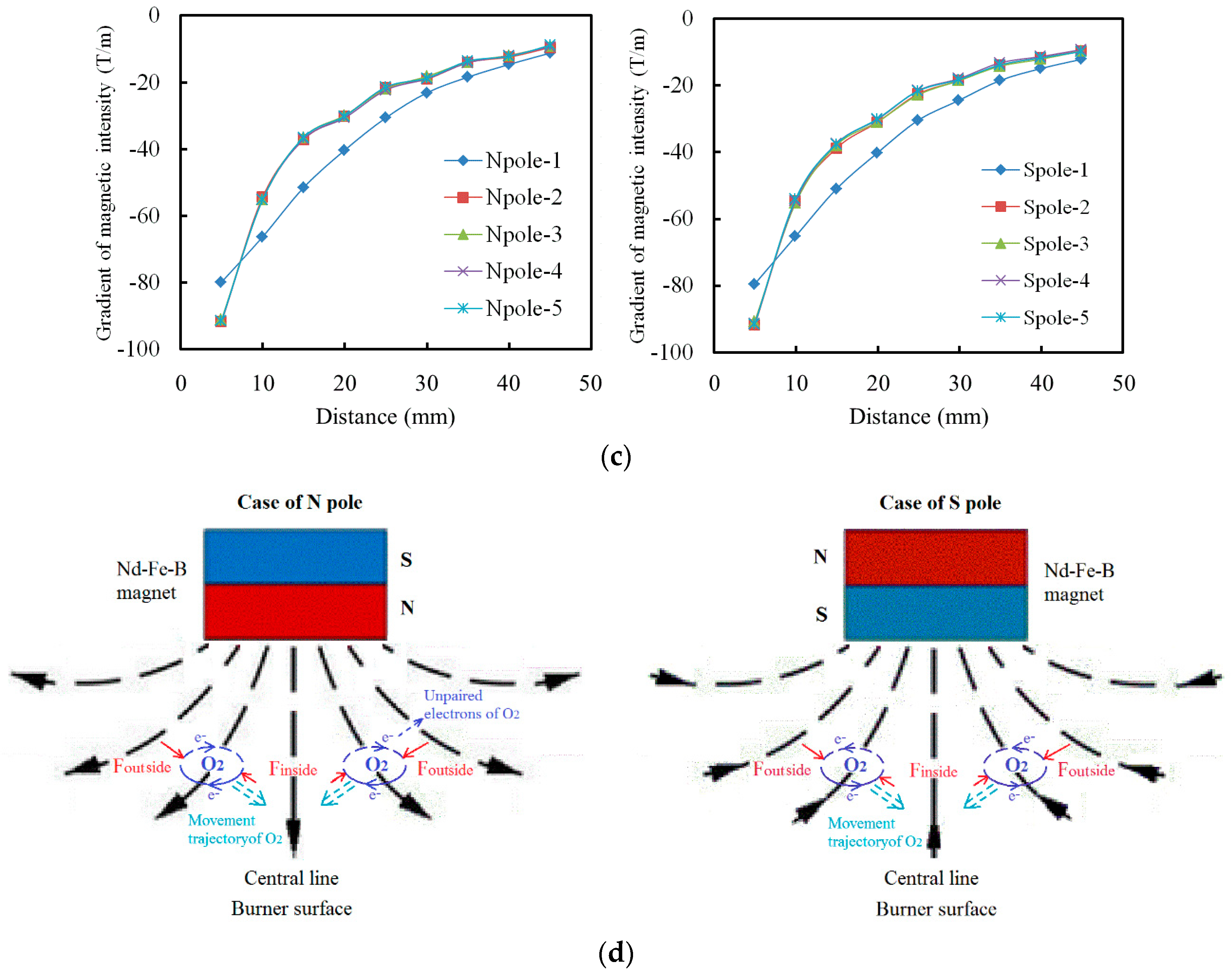
2.2. Analysis Methods
3. Results and Discussion
3.1. Soot Production from n-Heptane/DMF IDF with/without Magnetic Fields
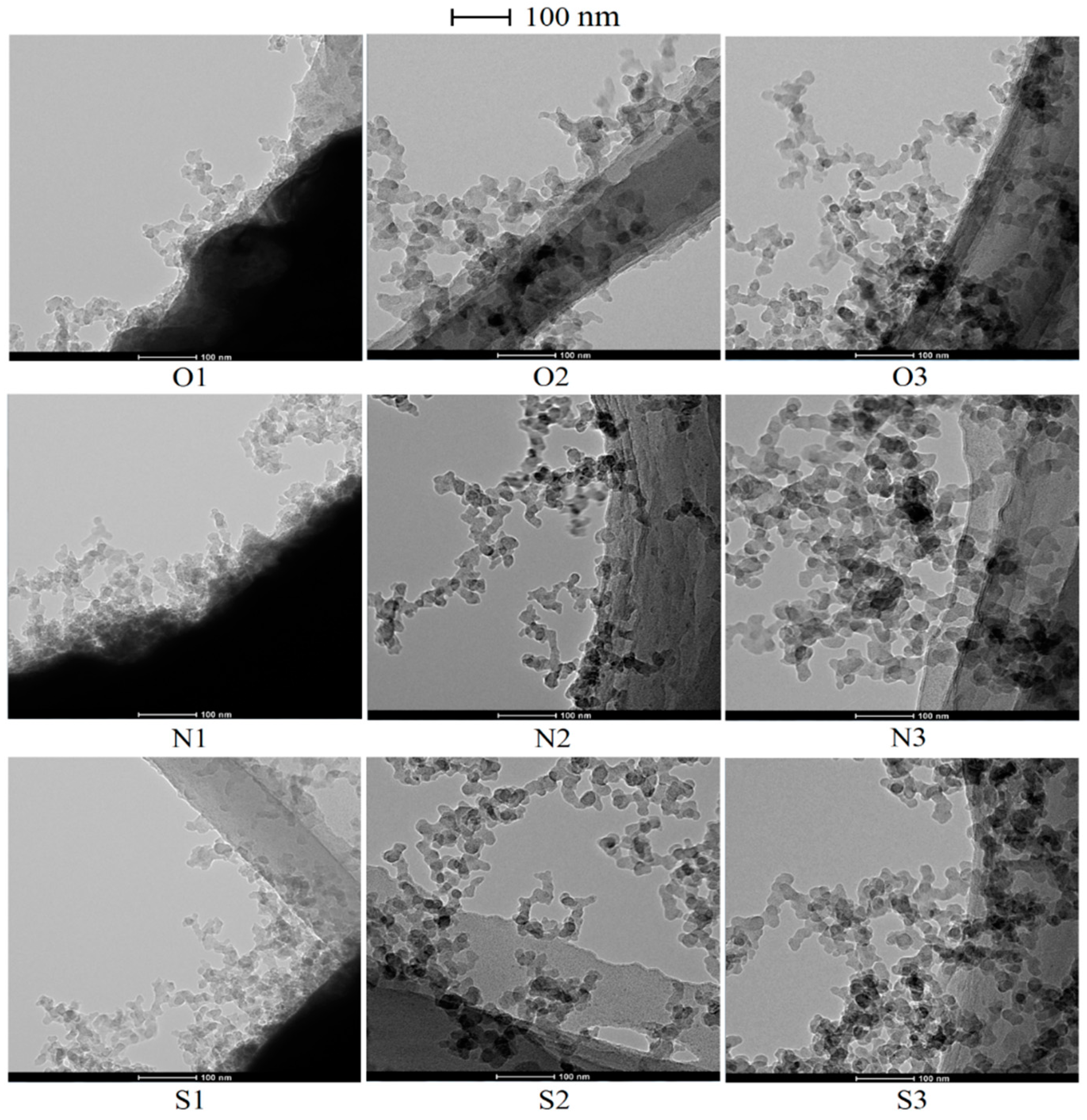
3.2. Soot Morphology Analysis by TEM
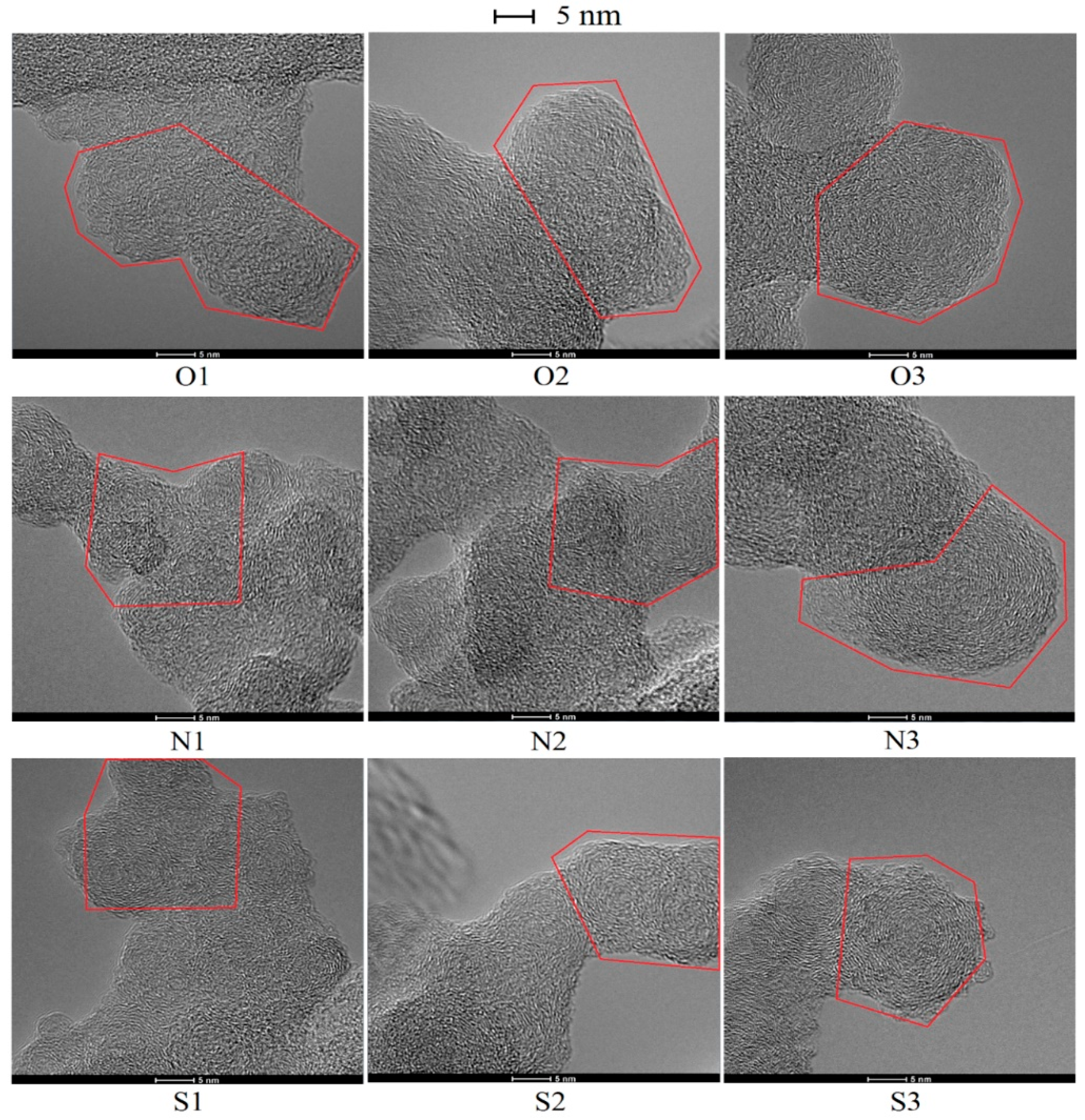
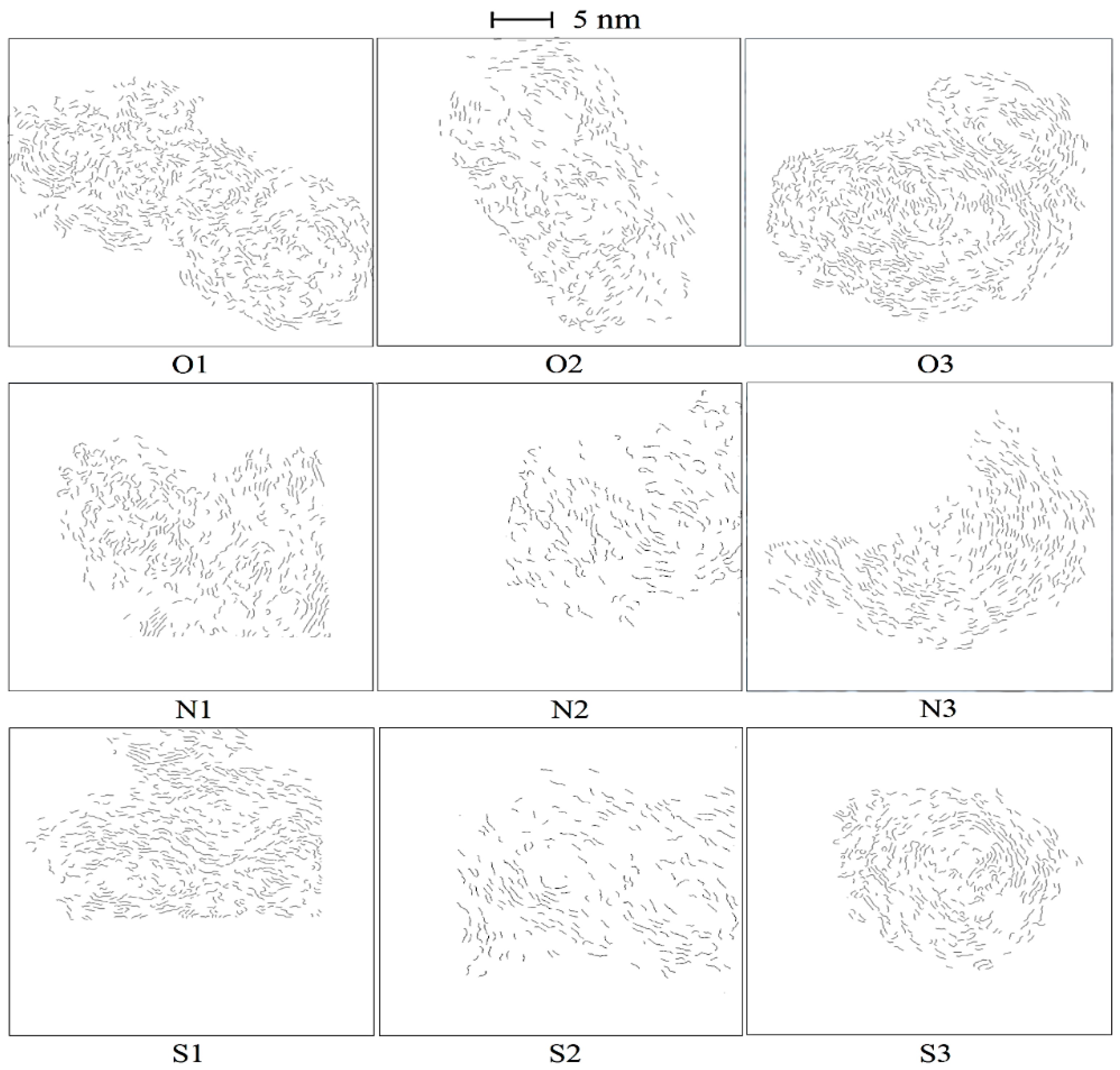
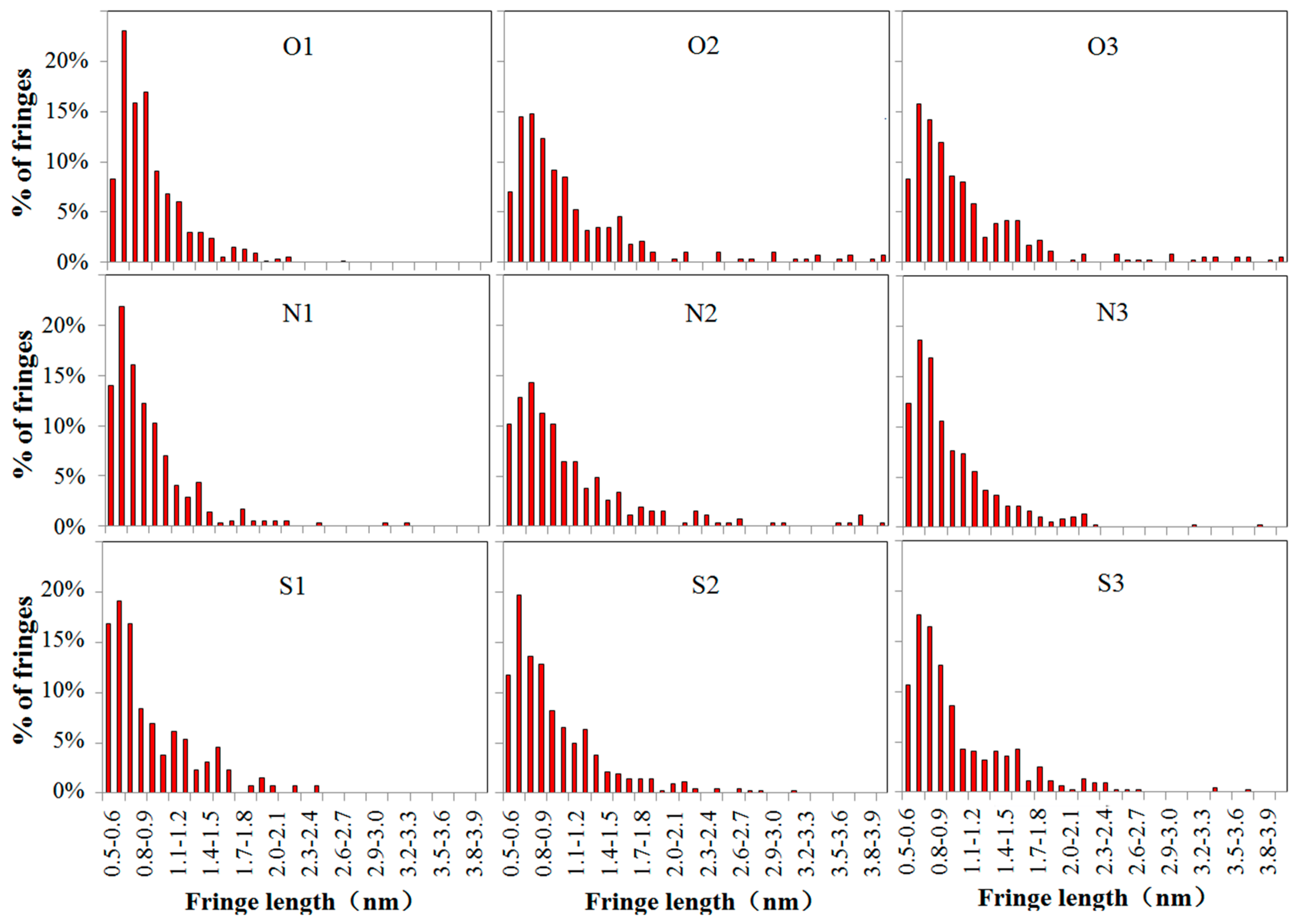
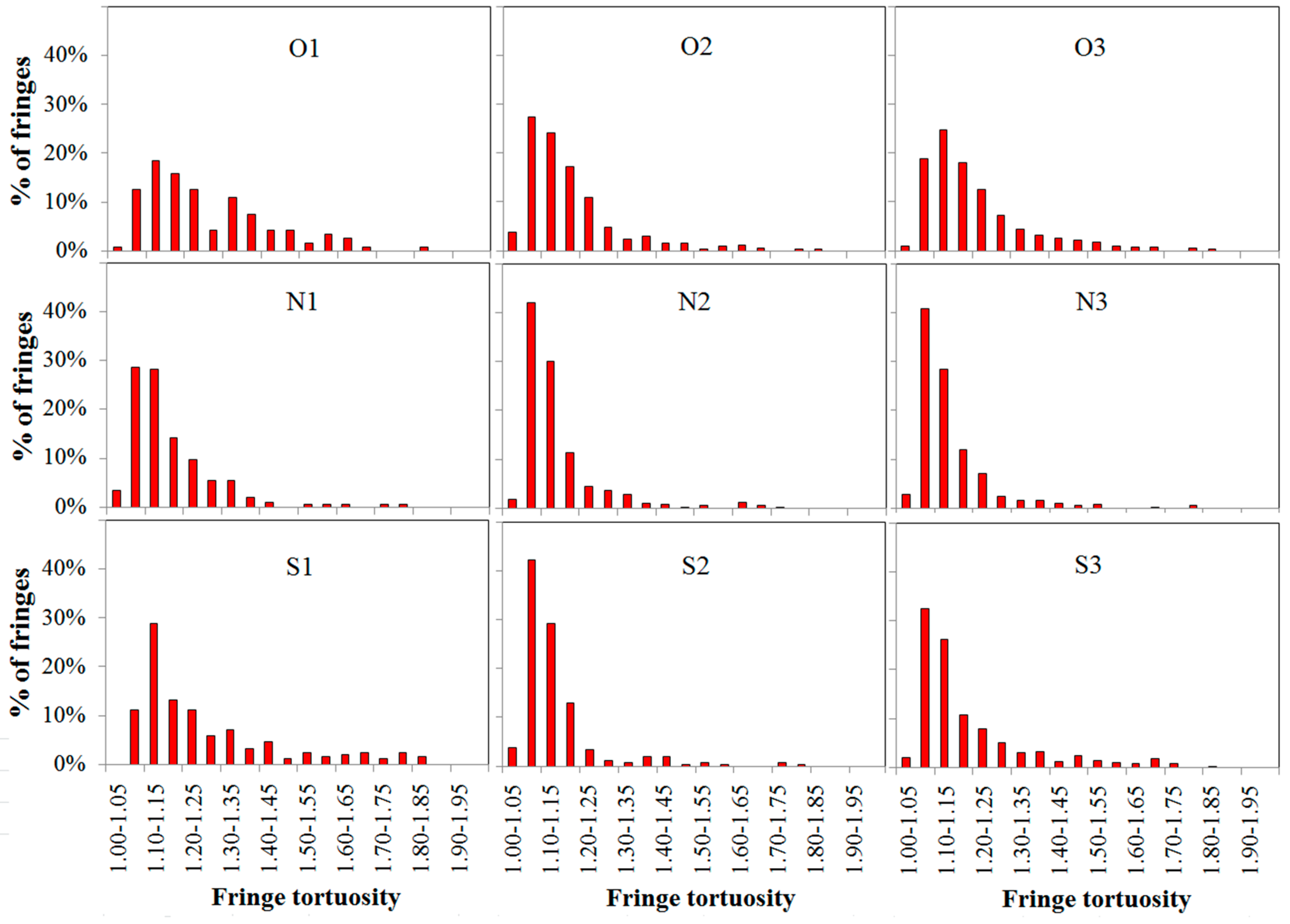
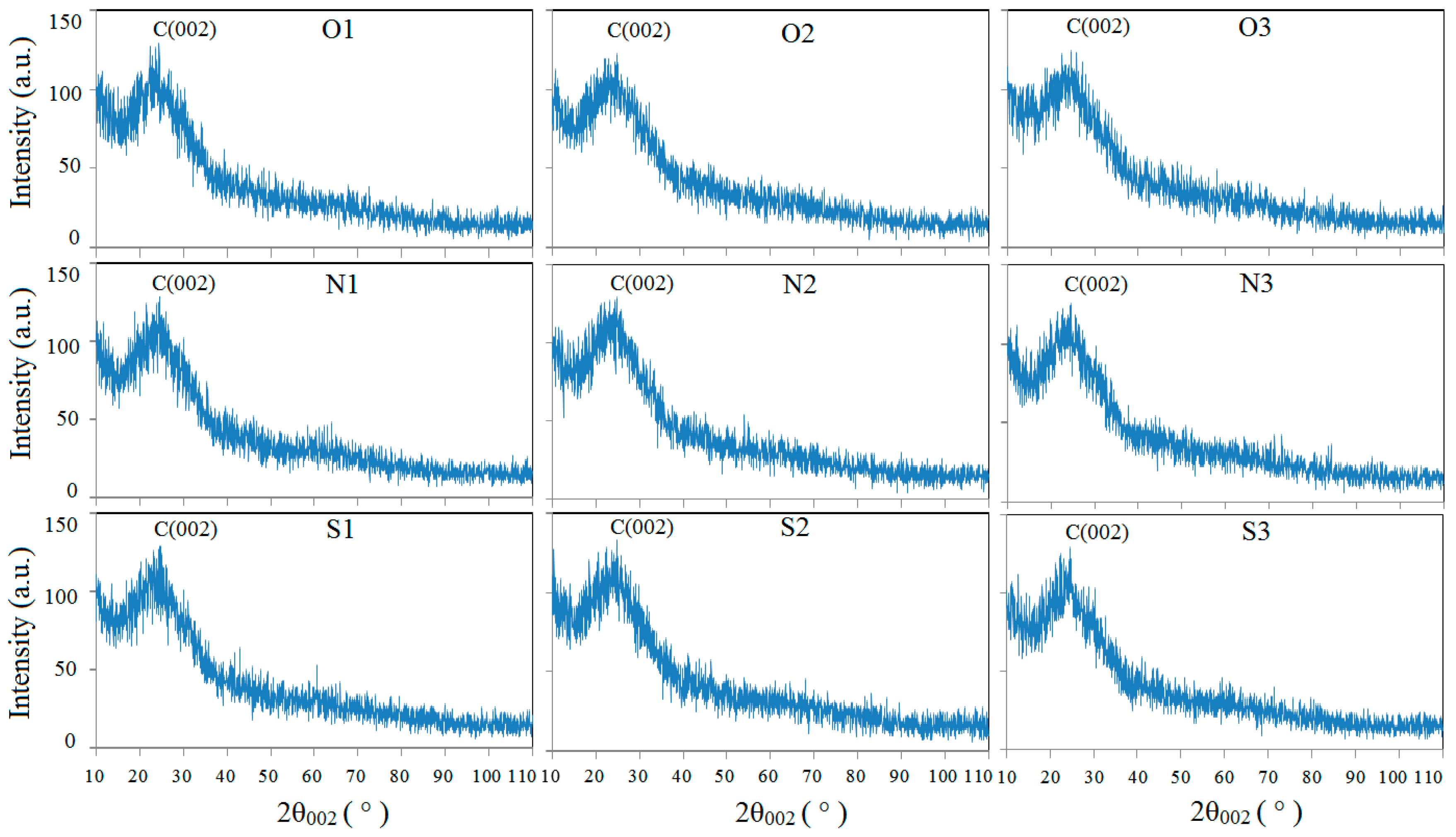
3.3. Soot Nanostructure Analysis by HRTEM and Fringe Analysis
3.4. Soot Graphitization Analysis
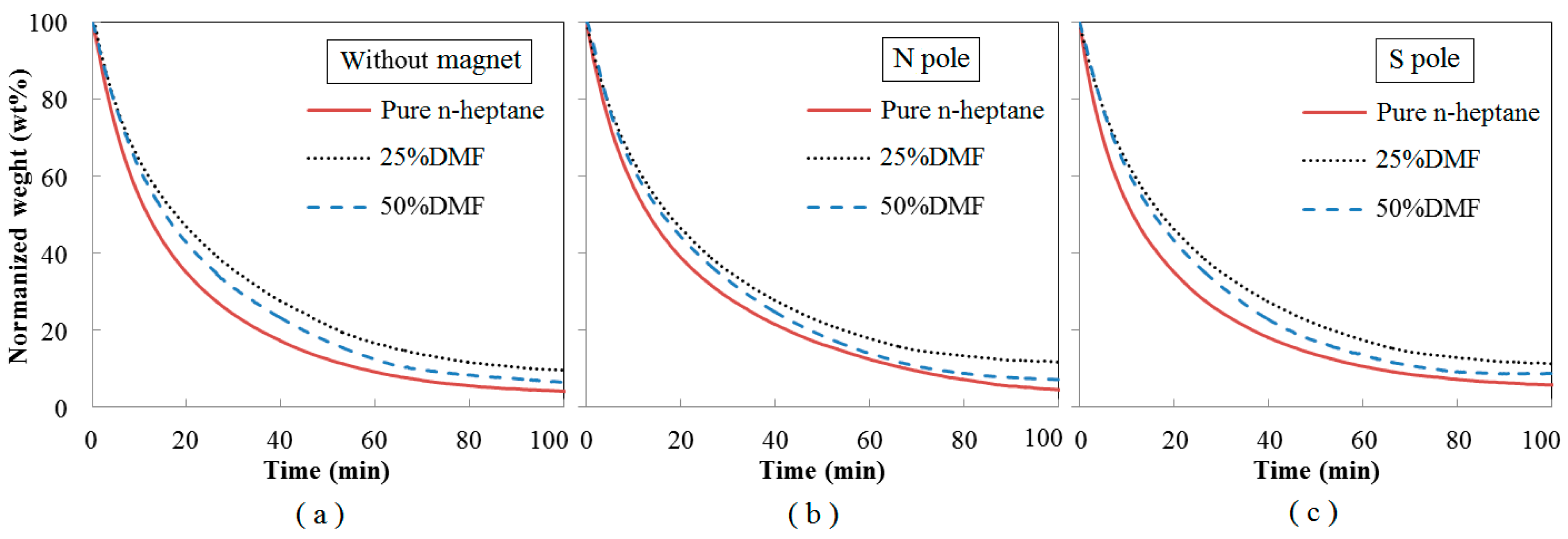
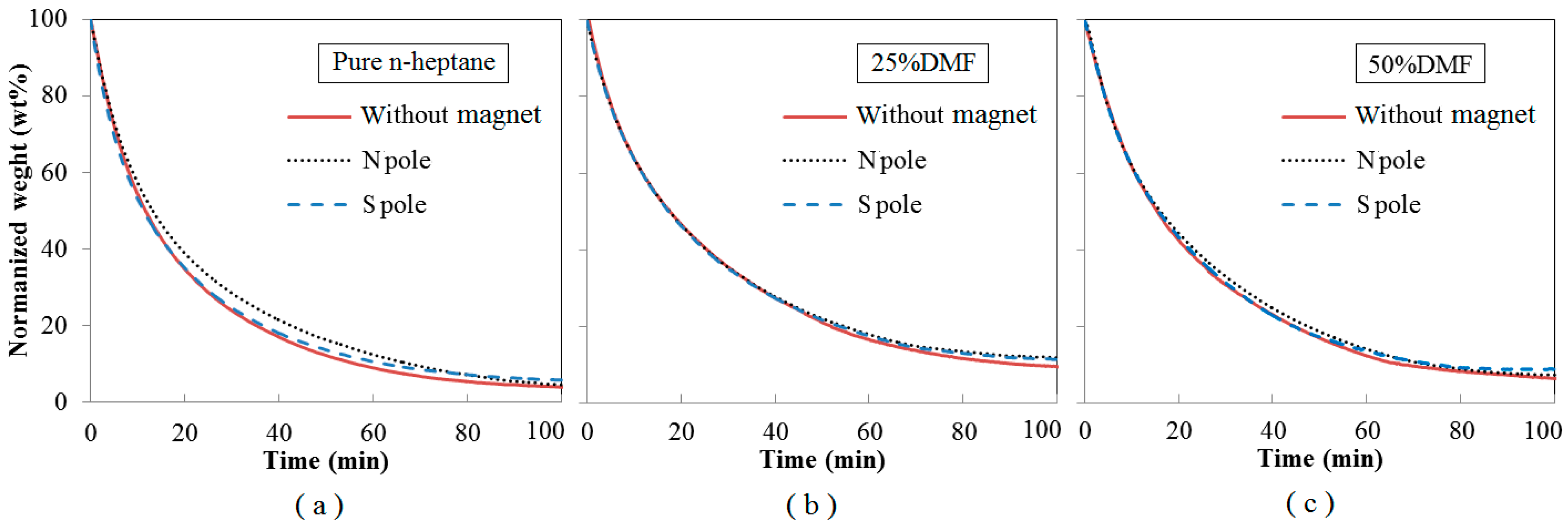
3.5. Oxidation Reactivity of the Soot
4. Conclusions
- (1)
- Dozens or hundreds of the soot particles clustered in chains, branched or tufted forms. The younger soot tufted as transparent liquid-like sticky material forms with a mass of amorphous structures and high oxidation reactivity from pure n-heptane IDF without magnetic fields.
- (2)
- With additions of DMF-doped, the soot production increased obviously. Soot particles with sticky characters and irregular shapes presented as over-layers or liquid-like materials embedded with each other. The core-shell structures of soot particles with well-organized fringes were exhibited. The diameters of soot particles increased, mean fringe lengths changed larger and mean fringe tortuosity switched smaller, the soot presented lager peak diffraction angles, higher graphitization degrees, and lower oxidation reactivity.
- (3)
- With effects of magnetic fields, no matter in N pole case or in S pole case, the soot precursors were promoted, and the soot production was enhanced. The enhancements of soot production in cases of S pole facing the flame were a little larger than that of N pole. Soot particles aggregated as approximate spherical particles with irregular shaped over-layers.
- (4)
- Under magnetic fields, the soot presented as a typical core-shell structure, thicker shell, longer fringe lengths, smaller fringe tortuosity, higher graphitization degree and lower oxidation reactivity. Oxygen was trapped to have an increased residence time to lead more soot to be yielded and oxidized. Soot particles were part oxidized and graphitized. They became relatively a little more mature in the cases of the N pole facing the flame than that of S pole cases.
Author Contributions
Funding
Conflicts of Interest
References
- Hayne, B.S.; Wagner, H.G. Soot formation. Prog. Energy Combust. Sci. 1981, 7, 229–273. [Google Scholar] [CrossRef]
- McEnally, C.S.; Pfefferle, L.D.; Atakan, B.; Kohse-Höinghaus, K. Studies of aromatic hydrocarbon formation mechanisms in flames: Progress towards closing the fuel gap. Prog. Energy Combust. 2006, 32, 247–294. [Google Scholar] [CrossRef]
- The Worldwatch Institute. State of the World into a Warming World; The Worldwatch Institute: Washington, DC, USA, 2009. [Google Scholar]
- Barfknecht, T.R. Toxicology of soot. Prog. Energy Combust. Sci. 1983, 9, 199–237. [Google Scholar] [CrossRef]
- Donaldson, K.; Li, X.Y.; MacNee, W. Ultrafine (nanometre) particle mediated lung injury. J. Aerosol Sci. 1998, 29, 553–560. [Google Scholar] [CrossRef]
- Neumann, H.-G. Health risk of combustion products: Toxicological considerations. Chemosphere 2001, 42, 473–479. [Google Scholar] [CrossRef]
- Glassman, I. Soot formation in combustion processes. Symp. Int. Combust. 1988, 22, 295–311. [Google Scholar] [CrossRef]
- Dobbins, R.A.; Fletcher, R.A.; Chang, H.-C. The evolution of soot precursor particles in a diffusion flame. Combust. Flame 1998, 115, 285–598. [Google Scholar] [CrossRef]
- Kennedy, I.M. Models of soot formation and oxidation. Prog. Energy Combust. Sci. 1997, 23, 95–132. [Google Scholar] [CrossRef]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Stanmore, B.R.; Brilhac, J.F.; Gilot, P. The oxidation of soot: A review of experiments, mechanisms and models. Carbon 2001, 39, 2247–2268. [Google Scholar] [CrossRef]
- Wang, H. Formation of nascent soot and other condensed-phase materials in flames. Proc. Combust. Inst. 2011, 33, 41–67. [Google Scholar] [CrossRef]
- Camacho, J.; Lieb, S.; Wang, H. Evolution of size distribution of nascent soot in n- and i-butanol flames. Proc. Combust. Inst. 2013, 34, 1853–1860. [Google Scholar] [CrossRef]
- Kang, K.T.; Hwnag, J.Y.; Chung, S.H.; Lee, W. Soot zone structure and sooting limit in diffusion flames: Comparison of counterflow and co-flow flames. Combust. Flame 1997, 109, 266–281. [Google Scholar] [CrossRef]
- Mikofski, M.A.; Williams, T.C.; Shaddix, C.R.; Fernandez-Pello, A.C.; Blevins, L.G. Structure of laminar sooting inverse diffusion flames. Combust. Flame 2007, 149, 463–478. [Google Scholar] [CrossRef]
- Blevins, L.G.; Fletcher, R.A.; Benner, J.B.; Steel, E.B.; Mulholland, G.W. The existence of young soot in the exhaust of inverse diffusion flames. Proc. Combust. Inst. 2002, 29, 2325–2333. [Google Scholar] [CrossRef]
- Katta, V.R.; Blevins, L.G.; Roquemore, W.M. Dynamics of an inverse diffusion flame and its role in polycyclic-aromatic-hydrocarbon and soot formation. Combust. Flame 2005, 142, 33–51. [Google Scholar] [CrossRef]
- Lee, E.J.; Oh, K.C.; Shin, H.D. Soot formation in inverse diffusion flames of diluted ethane. Fuel 2005, 84, 543–550. [Google Scholar] [CrossRef]
- Makel, D.B.; Kennedy, I.M. Soot formation in laminar inverse diffusion flames. Combust. Sci. Technol. 1994, 97, 303–314. [Google Scholar] [CrossRef]
- Oh, K.C.; Lee, U.D.; Shin, H.D.; Lee, E.J. The evolution of incipient soot particles in an inverse diffusion flame of ethene. Combust. Flame 2005, 140, 249–254. [Google Scholar] [CrossRef]
- Ying, Y.; Liu, D. Effects of butanol isomers additions on soot nanostructure and reactivity in normal and inverse ethylene diffusion flames. Fuel 2017, 205, 109–129. [Google Scholar] [CrossRef]
- Ying, Y.; Xu, C.; Liu, D.; Jiang, B.; Wang, P.; Wang, W. Nanostructure and oxidation reactivity of nascent soot particles in ethylene/pentanol flames. Energies 2017, 10, 122. [Google Scholar] [CrossRef]
- Escudero, F.; Fuentes, A.; Demarco, R.; Consalvi, J.-L.; Liu, F.; Elicer-Cortés, J.C.; Fernandez-Pelloe, C. Effects of oxygen index on soot production and temperature in an ethylene inverse diffusion flame. Exp. Therm. Fluid Sci. 2016, 73, 101–108. [Google Scholar] [CrossRef]
- Jung, Y.; Oh, K.C.; Bae, C.; Shin, H.D. The effect of oxygen enrichment on incipient soot particles in inverse diffusion flames. Fuel 2012, 102, 199–207. [Google Scholar] [CrossRef]
- Demain, A.L. Biosolutions to the energy problem. J. Ind. Microbiol. Biotechnol. 2009, 36, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, S.; Hanai, T.; Liao, J.C. Non-fermentative pathways for synthesis of branched-chain higher alcohols as biofuels. Nature 2008, 451, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Shulga, S.; Blum, Y.; Tkachenko, A. Biotechnology and effective strains-producers of biobutanol. New Biotechnol. 2009, 25. [Google Scholar] [CrossRef]
- Kohse-Höinghaus, K.; Oßwald, P.; Cool, T.A.; Kasper, T.; Hansen, N.; Qi, F.; Westbrook, C.K.; Westmoreland, P.R. Biofuel combustion chemistry: From ethanol to biodiesel. Angew. Chem. Int. Ed. 2010, 49, 3572–3597. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Alam, M.; Boehman, A.L.; Kim, U. Examination of the oxidation behavior of biodiesel soot. Combust. Flame 2006, 146, 589–604. [Google Scholar] [CrossRef]
- Knauer, M.; Carrara, M.; Rothe, D.; Niessner, R.; Ivleva, N.P. Changes in structure and reactivity of soot during oxidation and gasification by oxygen, studied by micro-Raman spectroscopy and temperature programmed oxidation. Aerosol Sci. Technol. 2009, 43, 1–8. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Mueller, C.J. Initial investigation of effects of fuel oxygenation on nanostructure of soot from a direct-injection diesel engine. Energy Fuels 2006, 20, 2364–2369. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Armas, O.; Boehman, A.L. Impact of fuel formulation on the nanostructure and reactivity of diesel soot. Combust. Flame 2012, 159, 3597–3606. [Google Scholar] [CrossRef]
- Gogoi, B.; Raj, A.; Alrefaai, M.M.; Stephen, S.; Anjana, T.; Pillai, V.; Bojanampati, S. Effects of 2,5-dimethylfuran addition to diesel on soot nanostructures and reactivity. Fuel 2015, 159, 766–775. [Google Scholar] [CrossRef]
- Santamaria, A.; Yang, N.; Eddings, E.; Mondragon, F. Chemical and morphological characterization of soot and soot precursors generated in an inverse diffusion flame with aromatic and aliphatic fuels. Combust. Flame 2010, 157, 33–42. [Google Scholar] [CrossRef]
- Velásquez, M.; Mondragón, F.; Santamaría, A. Chemical characterization of soot precursors and soot particles produced in hexane and diesel surrogates using an inverse diffusion flame burner. Fuel 2013, 104, 681–690. [Google Scholar] [CrossRef]
- Megaridis, C.M.; Dobbins, R.A. Soot aerosol dynamics in a laminar ethylene diffusion flame. Symp. Int. Combust. 1989, 22, 353–362. [Google Scholar] [CrossRef]
- Merchan-Merchan, W.; Abdihamzehkolaei, A.; Merchan-Breuer, D.A. Formation and evolution of carbon particles in co-flow diffusion air flames of vaporized biodiesel, diesel and biodiesel-diesel blends. Fuel 2018, 226, 263–277. [Google Scholar] [CrossRef]
- Li, Z.; Qiu, L.; Cheng, X.; Li, Y.; Wu, H. The evolution of soot morphology and nanostructure in laminar diffusion flame of surrogate fuels for diesel. Fuel 2018, 211, 517–528. [Google Scholar] [CrossRef]
- Liu, D.; Togbé, C.; Tran, L.-S.; Felsmann, D.; Oßwald, P.; Nau, P.; Koppman, J.; Lackner, A.; Glaude, P.-A.; Sirjean, B.; et al. Combustion chemistry and flame structure of furan group biofuels using molecular-beam mass spectrometry and gas chromatography—Part I: Furan. Combust. Flame 2014, 161, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-S.; Togbé, C.; Liu, D.; Felsmann, D.; Oßwald, P.; Glaude, P.-A.; Fournet, R.; Sirjean, B.; Battin-Leclerc, F.; Kohse-Höinghaus, K. Combustion chemistry and flame structure of furan group biofuels using molecular-beam mass spectrometry and gas chromatography—Part II: 2-Methylfuran. Combust. Flame 2014, 161, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Togbé, C.; Tran, L.-S.; Liu, D.; Felsmann, D.; Oßwald, P.; Glaude, P.-A.; Sirjean, B.; Fournet, R.; Battin-Leclerc, F.; Kohse-Höinghaus, K. Combustion chemistry and flame structure of furan group biofuels using molecular-beam mass spectrometry and gas chromatography—Part III: 2,5-Dimethylfuran. Combust. Flame 2014, 161, 780–797. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-S.; Wang, Z.; Carstensen, H.-H. Comparative experimental and modeling study of the low-tomoderate-temperature oxidation chemistry of 2,5-dimethylfuran, 2-methylfuran, and furan. Combust. Flame 2017, 181, 251–269. [Google Scholar] [CrossRef]
- Alexandrino, K.; Millera, A.; Bilbao, R.; Alzueta, M.U. Novel aspects in the pyrolysis and oxidation of 2,5-dimethylfuran. Proc. Combust. Inst. 2015, 35, 1717–1725. [Google Scholar] [CrossRef]
- Sirignano, M.; Conturso, M.; D’Anna, A. Effect of furans on particle formation in diffusion flames: An experimental and modeling study. Proc. Combust. Inst. 2015, 35, 525–532. [Google Scholar] [CrossRef]
- Russoa, C.; D’Annab, A.; Ciajoloa, A.; Sirignano, M. Analysis of the chemical features of particles generated from ethylene and ethylene/2,5 dimethyl furan flames. Combust. Flame 2016, 167, 268–273. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Zheng, Z.; Liu, H.; Xu, J.; Yao, M. Combustion and emissions of 2,5-dimethylfuran addition on a diesel engine with low temperature combustion. Fuel 2013, 103, 730–735. [Google Scholar] [CrossRef]
- Chen, G.; Shen, G.; Zhang, Q.; Yao, M.; Zheng, Z.; Liu, H. Experimental study on combustion and emission characteristics of a diesel engine fueled with 2,5-dimethylfuran–diesel, n-butanol–diesel and gasoline–diesel blends. Energy 2013, 54, 333–342. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.; Wei, L.; Liu, J.; Reitz, R.D.; Yao, M. Development of a reduced toluene reference fuel(TRF)-2,5-dimethylfuran-polycyclic aromatic hydrocarbon (PAH) mechanism for engine applications. Combust. Flame 2016, 165, 453–465. [Google Scholar] [CrossRef]
- Ueno, S.; Harada, K. Effects of magnetic fields on flames and gas flow. IEEE Trans. Magn. 1987, 23, 2752–2754. [Google Scholar] [CrossRef]
- Wakayama, N.I. Effect of a gradient magnetic field on the combustion of methane in Air. Chem. Phys. Lett. 1992, 188, 279–281. [Google Scholar] [CrossRef]
- Wakayama, N.I. Magnetic promotion of combustion in diffusion flames. Combust. Flame 1993, 93, 207–214. [Google Scholar] [CrossRef]
- Wakayama, N.I. Magnetic support of combustion in diffusion flames under microgravity. Combust. Flame 1996, 107, 187–192. [Google Scholar] [CrossRef]
- Mizuatani, Y.; Fuchihata, M.; Ohkura, Y. Premixed laminar flames in a uniform magnetic field. Combust. Flame 2001, 125, 1071–1073. [Google Scholar] [CrossRef]
- Baker, J.; Calvert, M.E. A study of the characteristics of slotted laminar jet diffusion flames in the presence of non-uniform magnetic fields. Combust. Flame 2003, 133, 345–357. [Google Scholar] [CrossRef]
- Jocher, A.; Pitsch, H.; Gomez, T.; Legros, G. Modification of sooting tendency by magnetic effects. Proc. Combust. Inst. 2015, 35, 889–895. [Google Scholar] [CrossRef]
- Jocher, A.; Bonnety, J.; Pitsch, H.; Gomez, T.; Legros, G. Dual magnetic effects on soot production in partially premixed lames. Proc. Combust. Inst. 2017, 36, 1377–1385. [Google Scholar] [CrossRef]
- Seong, H.J.; Boehman, A.L. Studies of soot oxidative reactivity using a diffusion flame burner. Combust. Flame 2012, 159, 1864–1875. [Google Scholar] [CrossRef]
- Jaramillo, I.C.; Gaddam, C.K.; Vander Wal, R.L.; Huang, C.-H.; Levinthal, J.D.; Lighty, J.S. Soot oxidation kinetics under pressurized conditions. Combust. Flame 2014, 161, 2951–2965. [Google Scholar] [CrossRef]
- Zhang, Y.; Boehman, A.L. Oxidation behavior of soot generated from the combustion of methyl 2-butenoate in a co-flow diffusion flame. Combust. Flame 2013, 160, 112–119. [Google Scholar] [CrossRef]
- Wu, W.; Qu, J.; Zhang, K.; Chen, W.; Li, B. Experimental Studies of Magnetic Effect on Methane Laminar Combustion Characteristics. Combust. Sci. Technol. 2016, 188, 472–480. [Google Scholar] [CrossRef]
- Kumar, M.; Agarwal, s.; Kumar, V.; Khan, G.S.; Shakher, C. Experimental investigation on butane diffusion flames under the influence of magnetic field. Appl. Opt. 2015, 54, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Kumar, M.; Shakher, C. Experimental investigation of the effect of magnetic field on temperature and temperature profile of diffusion flame using circular grating Talbot interferometer. Opt. Lasers Eng. 2015, 68, 214–221. [Google Scholar] [CrossRef]
- Shinoda, M.; Yamada, E.; Kajimoto, T.; Yamashita, H.; Kitagawa, K. Mechanism of magnetic field effect on OH density distribution in a methane–air premixed jet flame. Proc. Combust. Inst. 2005, 30, 277–284. [Google Scholar] [CrossRef]
- Wang, W.; Liu, D.; Ying, Y.; Liu, G.; Wu, Y. On the response of nascent soot nanostructure and oxidative reactivity to photoflash exposure. Energies 2017, 10, 961. [Google Scholar] [CrossRef]
- Botero, M.L.; Chen, D.; Gonzalez-Calera, S.; Jefferson, D.; Kraft, M. HRTEM evaluation of soot particles produced by the non-premixed combustion of liquid fuels. Carbon 2016, 96, 459–473. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. Development of an HRTEM image analysis method to quantify carbon nanostructure. Combust. Flame 2011, 158, 1837–1851. [Google Scholar] [CrossRef]
- Yehliu, K.; Vander Wal, R.L.; Boehman, A.L. A comparison of soot nanostructure obtained using two high resolution transmission electron microscopy image analysis algorithms. Carbon 2011, 49, 4256–4268. [Google Scholar] [CrossRef]
- Alfè, M.; Apicella, B.; Barbella, R.; Rouzaud, J.-N.; Tregrossi, A.; Ciajolo, A. Structure–property relationship in nanostructures of young and mature soot in premixed flames. Proc. Combust. Inst. 2009, 32, 697–704. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Tomasek, A.J.; Pamphlet, M.I.; Taylor, C.D.; Thompson, W.K. Analysis of HRTEM images for carbon nanostructure quantification. J. Nanopart. Res. 2004, 6, 555–568. [Google Scholar] [CrossRef]
- Gaddam, C.K.; Huang, C.-H.; Vander Wal, R.L. Quantification of nano-scale carbon structure by HRTEM and lattice fringe analysis. Pattern Recognit. Lett. 2016, 76, 90–97. [Google Scholar] [CrossRef]
- Dobiasova, L.; Stary, V.; Glogar, P.; Valvoda, V. Analysis of carbon fibers and carbon composites by asymmetric X-ray diffraction technique. Carbon 1999, 37, 421–425. [Google Scholar] [CrossRef]
- Ai, Y.L.; Yang, Y.Q.; Wang, X.X. Measurement of graphitization degree of carbon-carbon composites by X-ray diffraction. Coal Convers. 2009, 32, 72–74. [Google Scholar]
- Kholghya, M.R.; Afarina, Y.; Sediako, A.D.; Barba, J.; Lapuerta, M.; Chu, C. Comparison of multiple diagnostic techniques to study soot formation and morphology in a diffusion flame. Combust. Flame 2017, 176, 567–583. [Google Scholar] [CrossRef]
- Mao, Q.; Duin, A.C.T.V.; Luo, K.H. Formation of incipient soot particles from polycyclic aromatic hydrocarbons: A ReaxFF molecular dynamics study. Carbon 2017, 121, 380–388. [Google Scholar] [CrossRef]
- Parent, P.; Laffon, C.; Marhaba, I.; Ferry, D.; Regier, T.Z.; Ortega, I.K. Nanoscale characterization of aircraft soot: A high-resolution transmission electron microscopy, Raman spectroscopy, X-ray photoelectron and near-edge X-ray absorption spectroscopy study. Carbon 2016, 101, 86–100. [Google Scholar] [CrossRef]
- Russo, C.; Apicella, B.; Lighty, J.S.; Ciajolo, A.; Tregrossi, A. Optical properties of organic carbon and soot produced in an inverse diffusion flame. Carbon 2017, 124, 372–379. [Google Scholar] [CrossRef]
- Tang, Q.; Cai, R.; You, X.; Jiang, J. Nascent soot particle size distributions down to 1 nm from a laminar premixed burner-stabilized stagnation ethylene flame. Proc. Combust. Inst. 2017, 36, 993–1000. [Google Scholar] [CrossRef]
- Kholghy, M.R.; Veshkini, A.; Thomson, M.J. The core-shell internal nanostructure of soot—A criterion to model soot maturity. Carbon 2016, 100, 508–536. [Google Scholar] [CrossRef]

| Cases | Magnetic Conditions | Components (mol%) | Flow Rates (L/min) | ||||
|---|---|---|---|---|---|---|---|
| n-Heptane | DMF | Fuel (n-Heptane + DMF)/1000 (Liquid) | Air (Gas) | N2 (Carrier, Gas) | N2 (Protector, Gas) | ||
| O1 | Without magnet | 100 | 0 | 0.9833 | 0.7 | 0.1 | 9 |
| O2 | 75 | 25 | 0.9401 | ||||
| O3 | 50 | 50 | 0.9005 | ||||
| N1 | N pole of magnet | 100 | 0 | 0.9833 | 0.7 | 0.1 | 9 |
| N2 | 75 | 25 | 0.9401 | ||||
| N3 | 50 | 50 | 0.9005 | ||||
| S1 | S pole of magnet | 100 | 0 | 0.9833 | 0.7 | 0.1 | 9 |
| S2 | 75 | 25 | 0.9401 | ||||
| S3 | 50 | 50 | 0.9005 | ||||
| Cases | Average Fringe Length (nm) | Average Fringe Tortuosity |
|---|---|---|
| O1 | 0.94 ± 0.01 | 1.30 ± 0.01 |
| O2 | 1.06 ± 0.01 | 1.19 ± 0.01 |
| O3 | 1.03 ± 0.01 | 1.21 ± 0.01 |
| N1 | 1.02 ± 0.01 | 1.24 ± 0.01 |
| N2 | 1.12 ± 0.01 | 1.15 ± 0.01 |
| N3 | 1.08 ± 0.01 | 1.18 ± 0.01 |
| S1 | 0.98 ± 0.01 | 1.27 ± 0.01 |
| S2 | 1.08 ± 0.01 | 1.17 ± 0.01 |
| S3 | 1.05 ± 0.01 | 1.20 ± 0.01 |
| Cases | 2θ002/° | d002/nm | g/% |
|---|---|---|---|
| O1 | 24.420 | 0.3642 | −235.14 |
| O2 | 24.722 | 0.3598 | −184.11 |
| O3 | 24.551 | 0.3623 | −212.85 |
| N1 | 24.520 | 0.3627 | −217.99 |
| N2 | 24.773 | 0.3591 | −175.73 |
| N3 | 24.672 | 0.3606 | −192.52 |
| S1 | 24.493 | 0.3632 | −222.72 |
| S2 | 24.734 | 0.3597 | −182.09 |
| S3 | 24.591 | 0.3617 | −206.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, B.; Wang, P.; Ying, Y.; Luo, M.; Liu, D. Nanoscale Characteristics and Reactivity of Nascent Soot from n-Heptane/2,5-Dimethylfuran Inverse Diffusion Flames with/without Magnetic Fields. Energies 2018, 11, 1698. https://doi.org/10.3390/en11071698
Jiang B, Wang P, Ying Y, Luo M, Liu D. Nanoscale Characteristics and Reactivity of Nascent Soot from n-Heptane/2,5-Dimethylfuran Inverse Diffusion Flames with/without Magnetic Fields. Energies. 2018; 11(7):1698. https://doi.org/10.3390/en11071698
Chicago/Turabian StyleJiang, Bo, Pengfei Wang, Yaoyao Ying, Minye Luo, and Dong Liu. 2018. "Nanoscale Characteristics and Reactivity of Nascent Soot from n-Heptane/2,5-Dimethylfuran Inverse Diffusion Flames with/without Magnetic Fields" Energies 11, no. 7: 1698. https://doi.org/10.3390/en11071698
APA StyleJiang, B., Wang, P., Ying, Y., Luo, M., & Liu, D. (2018). Nanoscale Characteristics and Reactivity of Nascent Soot from n-Heptane/2,5-Dimethylfuran Inverse Diffusion Flames with/without Magnetic Fields. Energies, 11(7), 1698. https://doi.org/10.3390/en11071698






