One-Pot Hydrothermal Synthesis of Novel Cu-MnS with PVP Cabbage-Like Nanostructures for High-Performance Supercapacitors
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
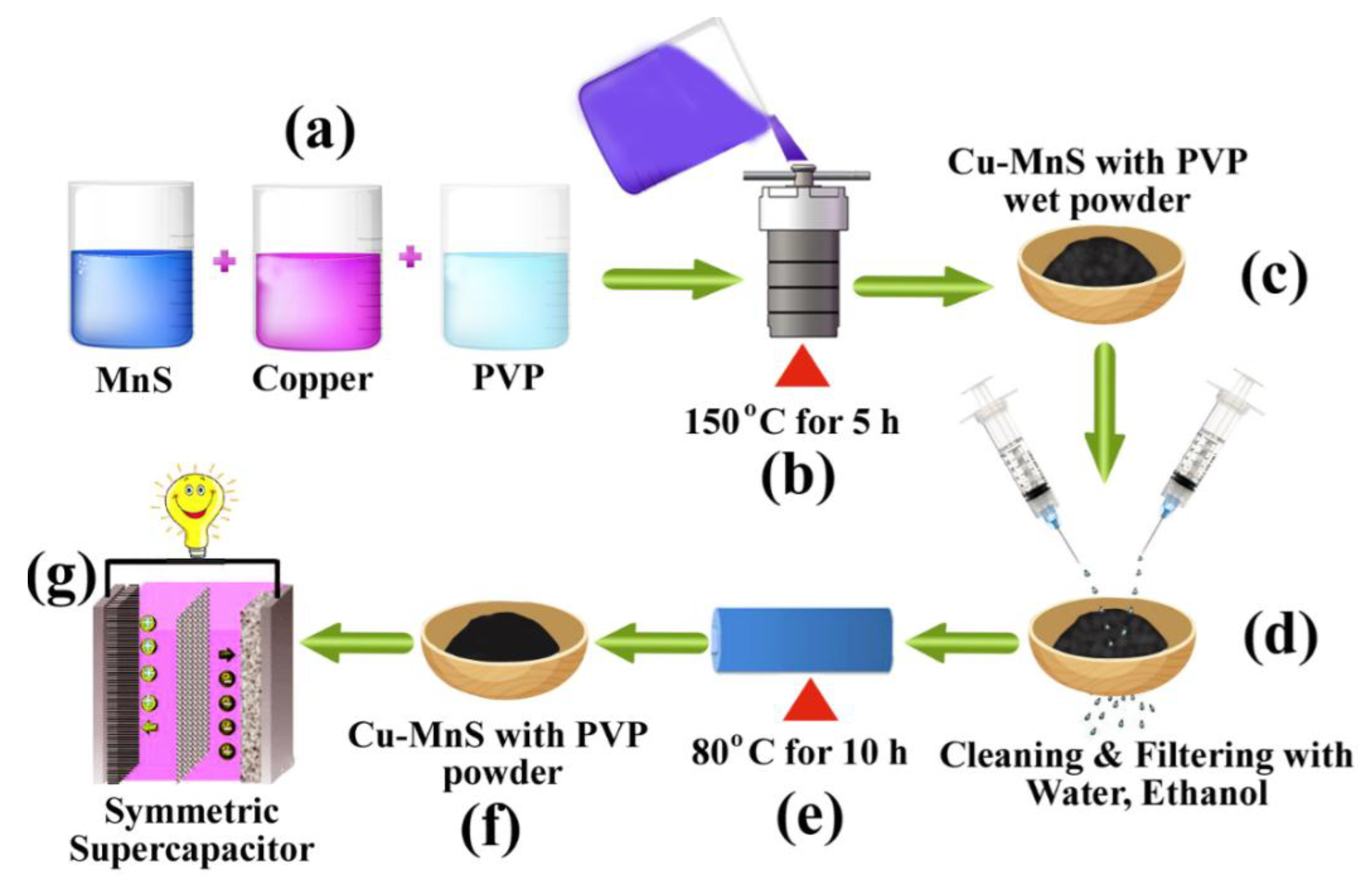
2.2. Synthesis of Cu-MnS with PVP Nanoparticles
2.3. Preparation of Working Electrode on Nickel Foam Substrate
2.4. Characterizations
2.5. Electrochemical Characterization
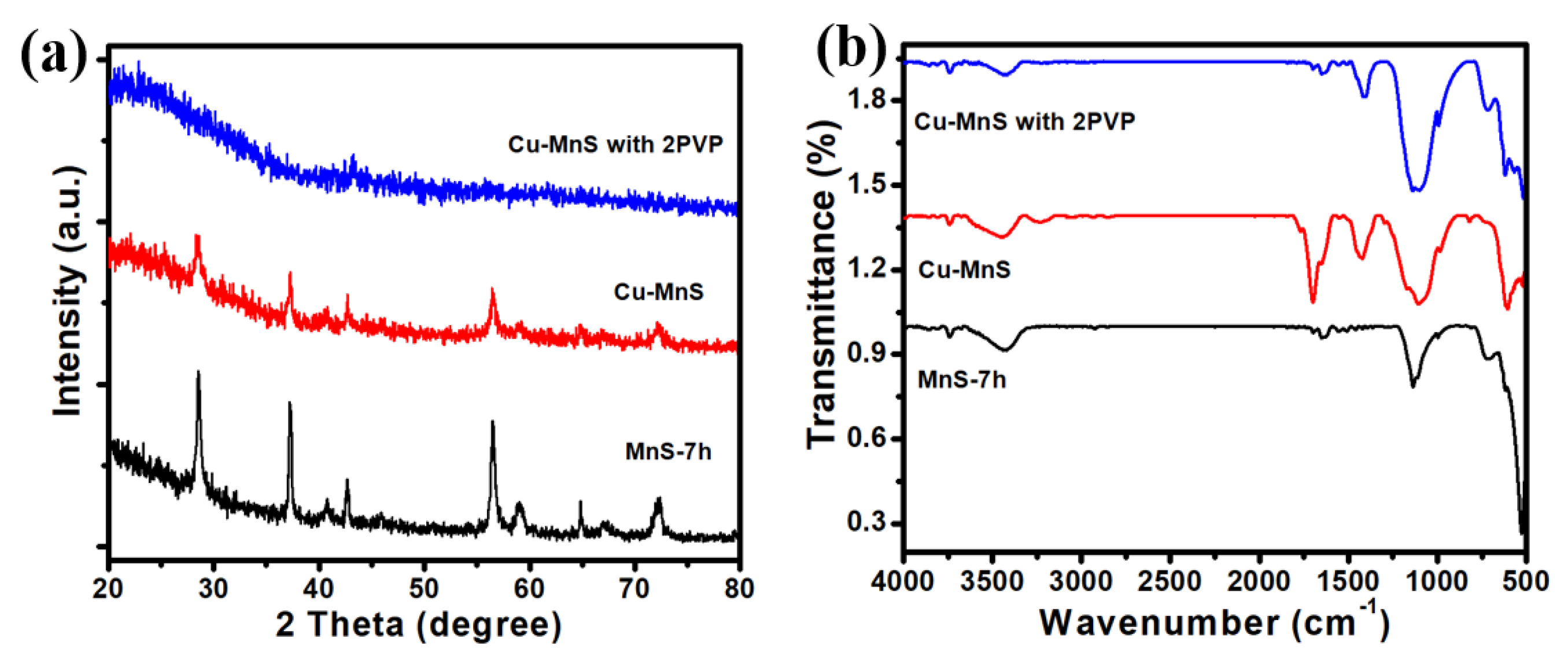
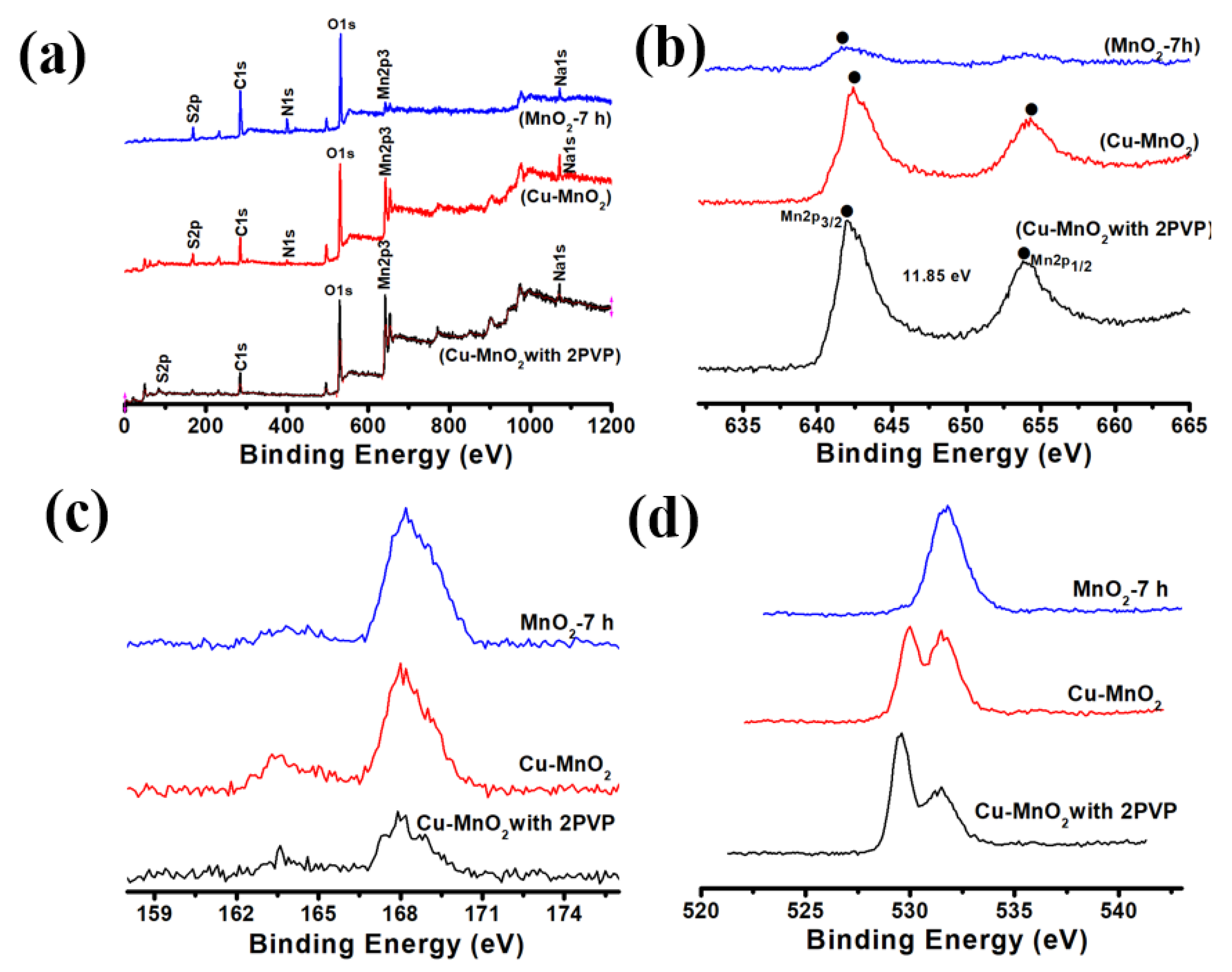
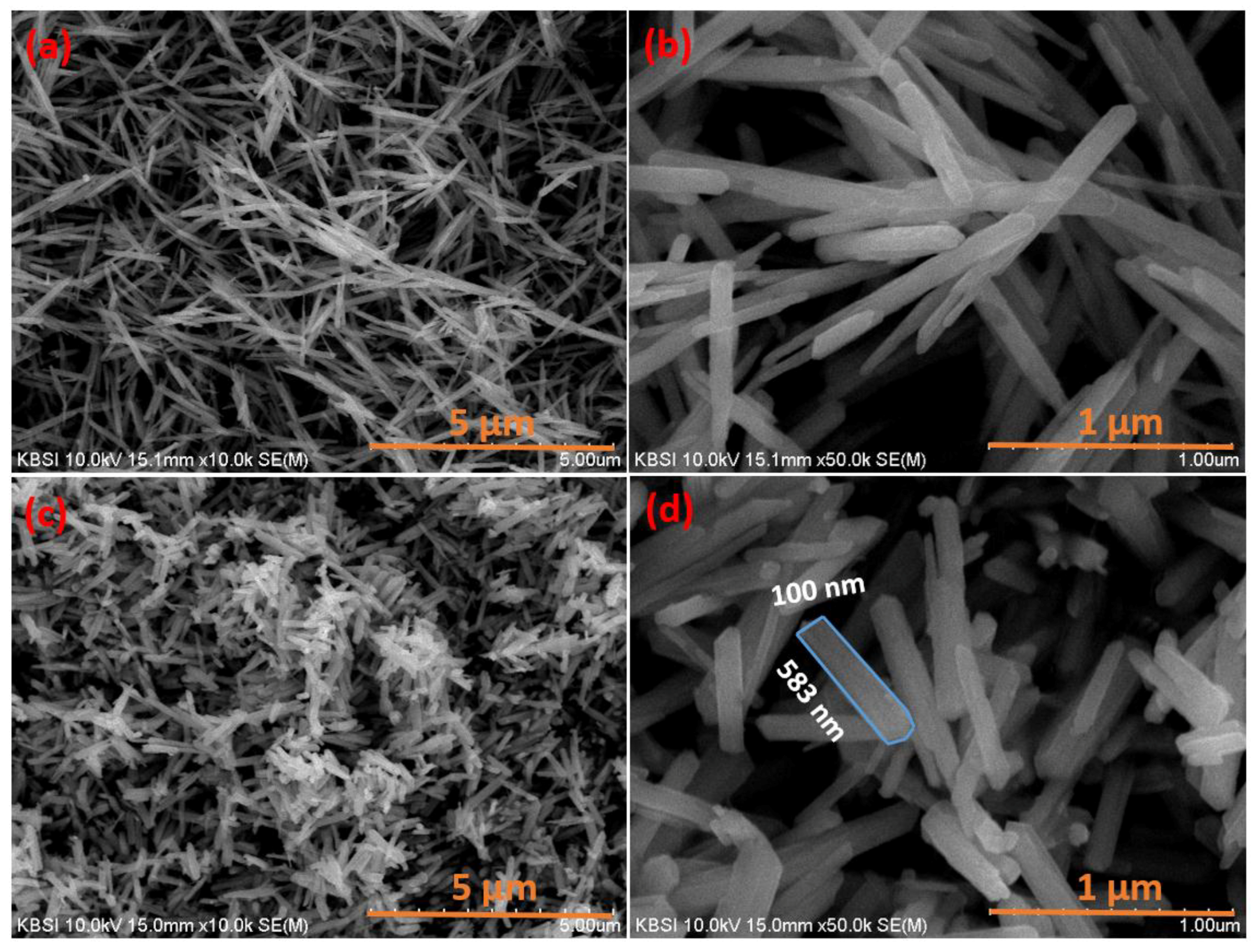
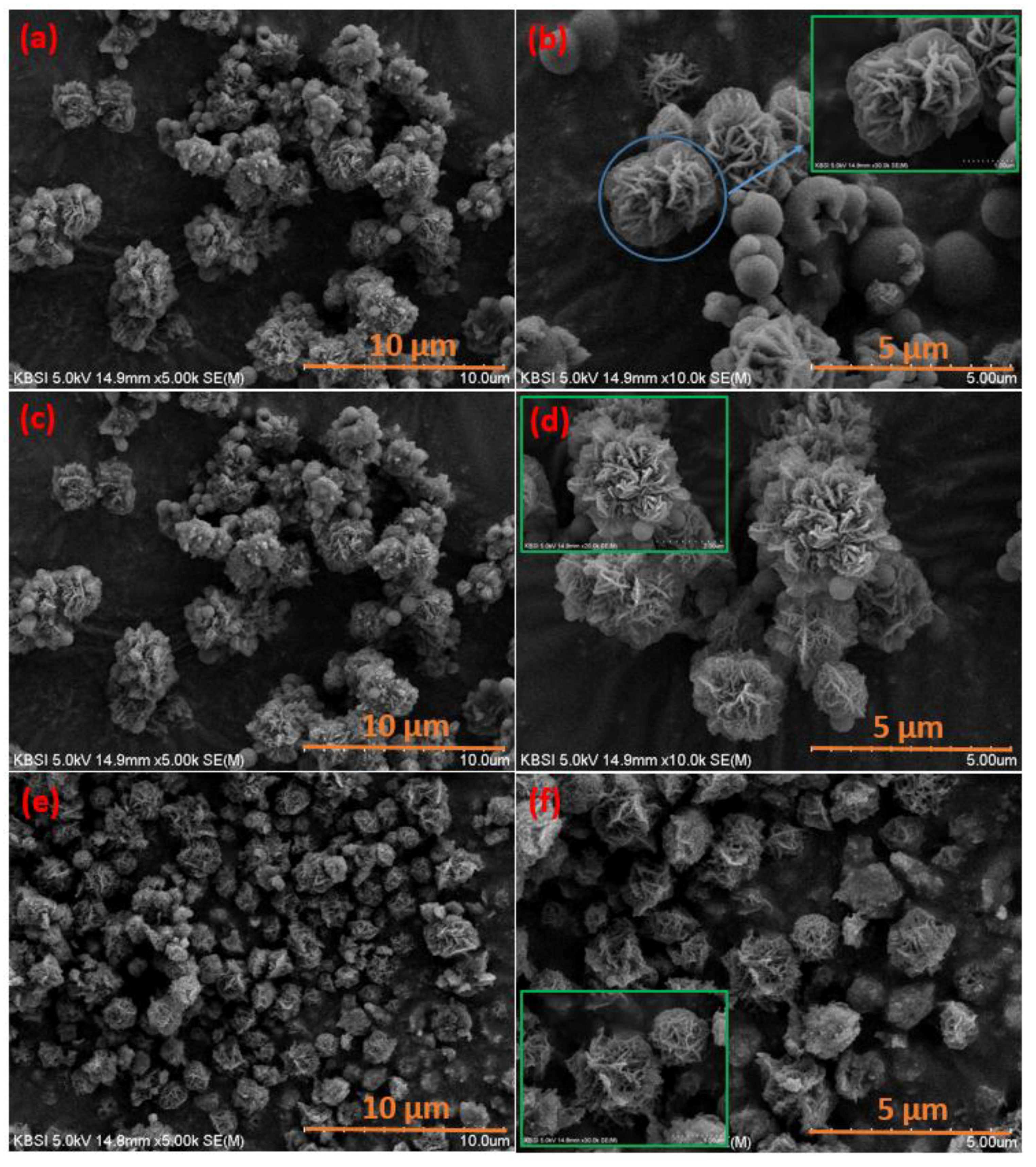
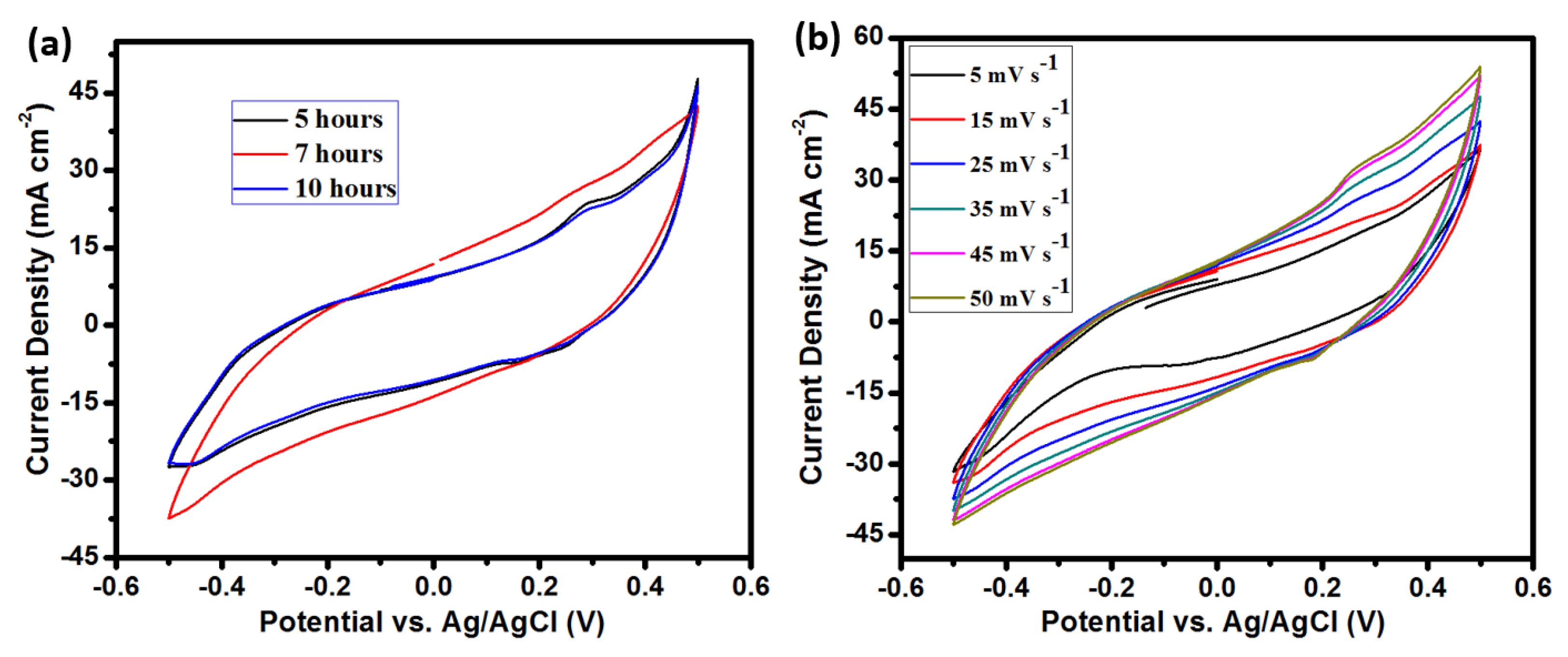
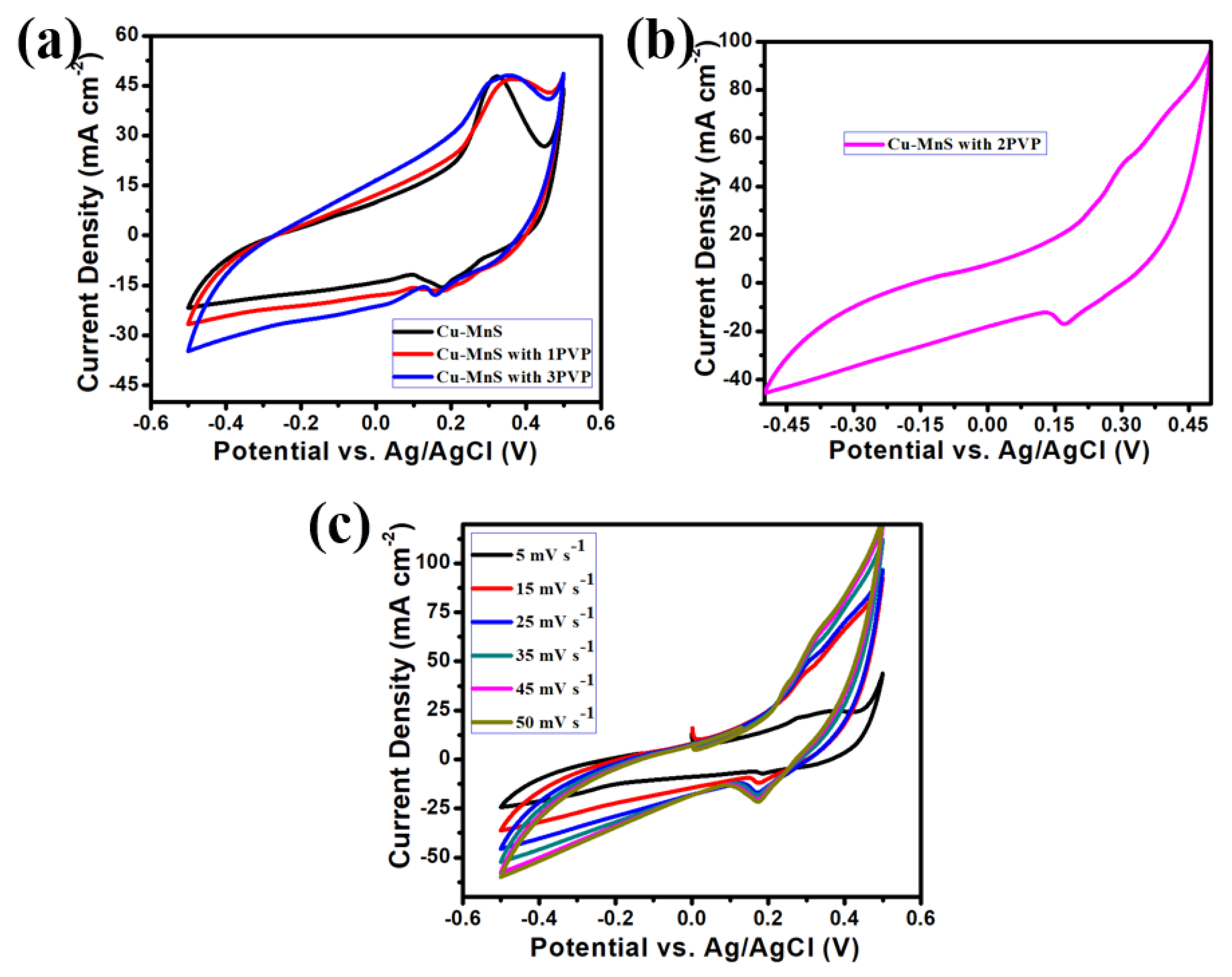
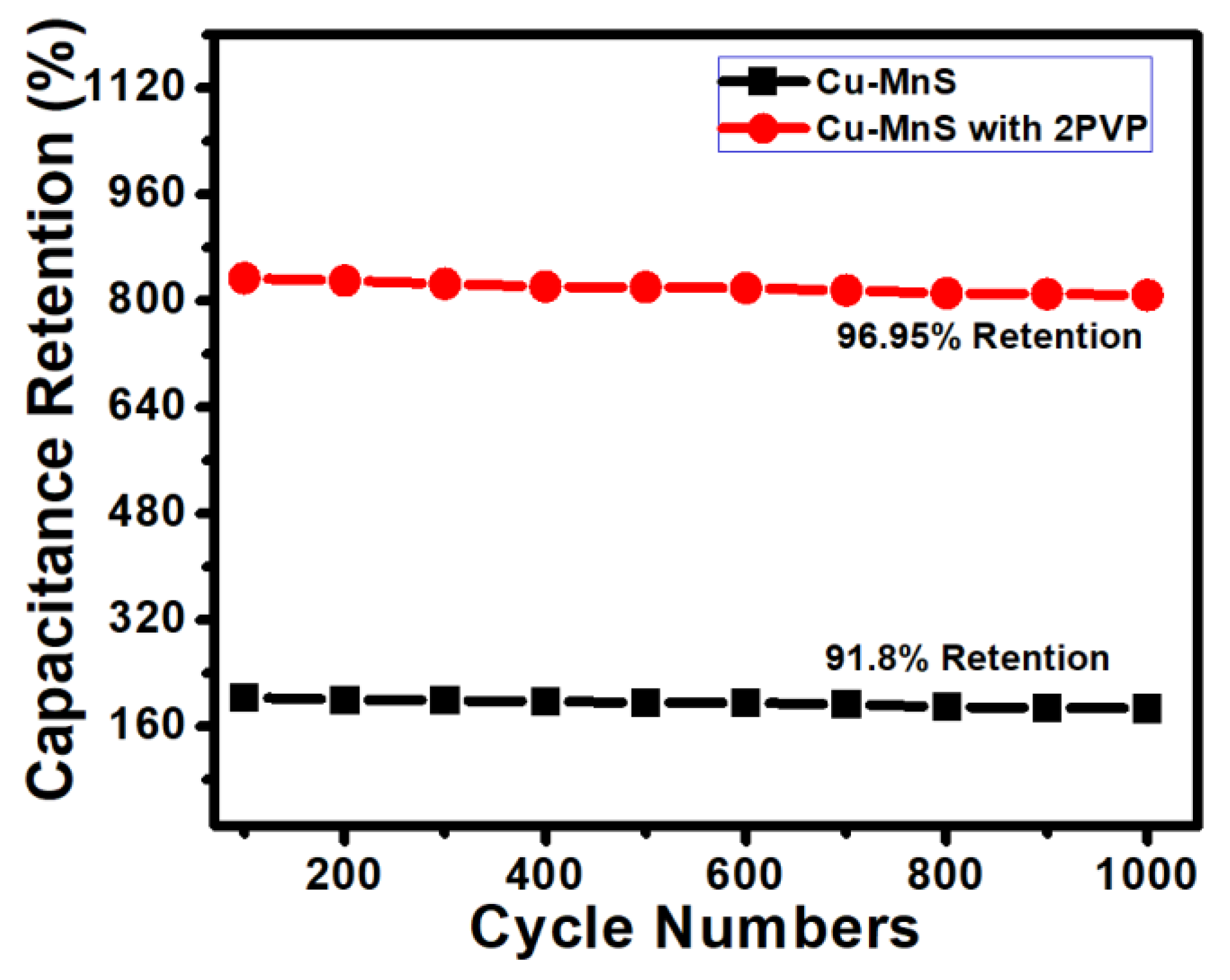
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, S.S.; Punnoose, D.; Bae, J.H.; Durga, I.K.; Varma, C.V.T.; Naresh, B.; Subramanian, A.; Raman, V.; Kim, H.J. Preparation and electrochemical performances of NiS with PEDOT:PSS chrysanthemum petal like nanostructure for high performance supercapacitors. Electrochim. Acta 2017, 254, 269–279. [Google Scholar]
- Rao, S.S.; Durga, I.K.; Kundakarla, N.; Punnoose, D.; Gopi, C.V.V.M.; Reddy, A.E.; Jagadeesh, M.; Kim, H.J. A hydrothermal reaction combined with a post anion-exchange reaction of hierarchically nanostructured NiCo2S4 for high-performance QDSSCs and supercapacitors. New J. Chem. 2017, 41, 10037–10047. [Google Scholar] [CrossRef]
- Xiang, D.; Bote, Z.; Liang, Z.; Zongping, S. Molten salt synthesis of nitrogen-doped carbon with hierarchical pore structures for use as high-performance electrodes in supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar]
- Bote, Z.; Lei, Z.; Qiaobao, Z.; Dongchang, C.; Yong, C.; Xiang, D.; Yu, C.; Ryan, M.; Xunhui, X.; Bo, S.; et al. Rational design of nickel hydroxide-based nanocrystals on graphene for ultrafast energy storage. Adv. Energy Mater. 2018, 8, 1702247. [Google Scholar]
- Shuge, D.; Bote, Z.; Chong, Q.; Dongchang, C.; Dai, D.; Bo, S.; Ben, M.D.; Jianwei, F.; Chenguo, H.; Ching-Ping, W.; et al. Controlled synthesis of three-phase NixSy/rGO nanoflake electrodes for hybrid supercapacitors with high energy and power density. Nano Energy 2017, 33, 522–531. [Google Scholar]
- Bote, Z.; Dongchang, C.; Xunhui, X.; Bo, S.; Renzong, H.; Qiaobao, Z.; Benjamin, H.R.; Gordon, H.W.; Dongxing, Z.; Yong, D.; et al. A high-energy, long cycle-life hybrid supercapacitor based on graphene composite electrodes. Energy Storage Mater. 2017, 7, 32–39. [Google Scholar]
- Gao, R.; Zhang, Q.; Soyekwo, F.; Lin, C.; Lv, R.; Qu, Y.; Chen, M.; Zhu, A.; Liu, Q. Novel amorphous nickel sulfide@CoS double-shelled polyhedral nanocages for supercapacitor electrode materials with superior electrochemical properties. Electrochim. Acta 2017, 237, 94–101. [Google Scholar] [CrossRef]
- Huang, L.; Hou, H.; Liu, B.; Zeinu, K.; Yuan, X.; Zhu, X.; He, X.; Wu, L.; Hu, J.; Yang, J. Phase-controlled solvothermal synthesis and morphology evolution of nickel sulfide and its pseudocapacitance performance. Ceram. Int. 2017, 43, 3080–3088. [Google Scholar] [CrossRef]
- Song, X.; Tan, L.; Wang, X.; Zhu, L.; Yi, X.; Dong, Q. Synthesis of CoS@rGO composites with excellent electrochemical performance for supercapacitors. J. Electroanal. Chem. 2017, 794, 132–138. [Google Scholar] [CrossRef]
- Subramani, K.; Sudhan, N.; Divya, R.; Sathish, M. All-solid-state asymmetric supercapacitors based on cobalt hexacyanoferrate-derived CoS and activated carbon. RSC Adv. 2017, 7, 6648–6659. [Google Scholar] [CrossRef]
- Adnan, Y.; Dewei, C.; Sean, L. Ethanol-directed morphological evolution of hierarchical CeOx architectures as advanced electrochemical capacitors. J. Mater. Chem. A 2015, 3, 13970–13977. [Google Scholar]
- Zhu, G.; He, Z.; Chen, J.; Zhao, J.; Feng, X.; Ma, Y.; Fan, Q.; Wang, L.; Huang, W. Highly conductive three-dimensional MnO2–carbon nanotube–graphene–Ni hybrid foam as a binder-free supercapacitor electrode. Nanoscale 2014, 6, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xu, S.; Zhang, C.; Zhu, Y.; Xiong, D.; Huang, R.; Qi, R.; Wang, L.; Chu, P.K. Three-dimensional homo-nanostructured MnO2/nanographene membranes on a macroporous electrically conductive network for high performance supercapacitors. J. Mater. Chem. A 2016, 4, 11317–11329. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Mondal, R.; Late, D.J.; Rout, C.S. Electrodeposited Nickel Cobalt Manganese based mixed sulfide nanosheets for high performance supercapacitor application. Microporous Mesoporous Mater. 2017, 244, 101–108. [Google Scholar] [CrossRef]
- Rao, S.S.; Durga, I.K.; Gopi, C.V.V.M.; Tulasivarma, C.V.; Kim, S.K.; Kim, H.J. The effect of TiO2 nanoflowers as a compact layer for CdS quantum-dot sensitized solar cells with improved performance. Dalton Trans. 2015, 44, 12852–12862. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Gopi, C.V.V.M.; Kim, S.K.; Son, M.K.; Jeong, M.S.; Savariraj, A.D.; Prabakar, K.; Kim, H.J. Cobalt sulfide thin film as an efficient counter electrode for dye-sensitized solar cells. Electrochim. Acta 2014, 133, 174–179. [Google Scholar]
- Cheng, J.; Yan, H.; Lu, Y.; Qiu, K.; Hou, X.; Xu, J.; Han, L.; Liu, X.; Kim, J.K.; Luo, Y. Mesoporous CuCo2O4 nanograsses as multi-functional electrodes for supercapacitors and electro-catalysts. J. Mater. Chem. A 2015, 3, 9769–9776. [Google Scholar] [CrossRef]
- Pang, H.; Wang, S.; Li, G.; Ma, Y.; Li, J.; Li, X.; Zhang, L.; Zhang, J.; Zheng, H. Cu superstructures fabricated using tree leaves and Cu–MnO2 superstructures for high performance supercapacitors. J. Mater. Chem. A 2013, 1, 5053–5060. [Google Scholar] [CrossRef]
- Cai, M.; Qian, H.; Wei, Z.; Chen, J.; Zheng, M.; Dong, Q. Polyvinyl pyrrolidone-assisted synthesis of a Fe3O4/graphene composite with excellent lithium storage properties. RSC Adv. 2014, 4, 6379–6382. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Jia, H.; Yin, B.; Ding, L.; Xu, Z.; Ji, Q. Polyvinyl pyrrolidone modified graphene oxide for improving the mechanical, thermal conductivity and solvent resistance properties of natural rubber. RSC Adv. 2016, 6, 54668–54678. [Google Scholar] [CrossRef]
- Joshi, K.V.; Joshi, B.K.; Harikrishna, U.; Patel, M.B.; Menon, S.K. Polyvinyl pyrrolidone modified ZnS nanoparticles as a highly selective and sensitive nanosensor for the iodide ion. Anal. Methods 2013, 5, 4973–4977. [Google Scholar] [CrossRef]
- Yang, G.W.; Xu, C.L.; Li, H.L. Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance. Chem. Commun. 2008, 48, 6537–6579. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Yuan, X.P.; Guan, X.H.; Wang, G.S. Synthesis of nickel chalcogenide hollow spheres using an l-cysteine-assisted hydrothermal process for efficient supercapacitor electrodes. J. Mater. Chem. A 2017, 5, 3621–3627. [Google Scholar] [CrossRef]
- Xia, Q.; Xu, M.; Xia, H.; Xie, J. Nanostructured Iron Oxide/Hydroxide-based electrode materials for supercapacitors. ChemNanoMat 2016, 2, 588–600. [Google Scholar] [CrossRef]
- Liu, R.M.; Jian, Z.X.; Liu, Q.; Zhu, X.D.; Liu, L.; Ni, L.; Shen, C.C. Novel red blood cell shaped α-Fe2O3 microstructures and FeO(OH) nanorods as high capacity supercapacitors. RSC Adv. 2015, 5, 91127–91133. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, C.W.; Punnoose, D.; Gopi, C.V.V.M.; Kim, S.K.; Prabakar, K.; Rao, S.S. Nickel doped cobalt sulfide as a high performance counter electrode for dye-sensitized solar cells. Appl. Surf. Sci. 2015, 328, 78–85. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Bunnak, N.; Juntaro, J.; Sain, M.; Manuspiya, H. Synthesis and luminescence properties of ZnS and metal (Mn, Cu)-doped-ZnS ceramic powder. Solid State Sci. 2012, 14, 299–304. [Google Scholar] [CrossRef]
- Ramachandran, R.; Saranya, M.; Grace, A.N.; Wang, F. MnS nanocomposites based on doped graphene: Simple synthesis by a wet chemical route and improved electrochemical properties as an electrode material for supercapacitors. RSC Adv. 2017, 7, 2249–2257. [Google Scholar] [CrossRef]
- Anothumakkool, B.; Torris, A.T.A.; Bhange, S.N.; Badiger, M.V.; Kurungot, S. Electrodeposited polyethylenedioxythiophene with infiltrated gel electrolyte interface: A close contest of an all-solid-state supercapacitor with its liquid-state counterpart. Nanoscale 2014, 6, 5944–5952. [Google Scholar] [CrossRef] [PubMed]
- Zhiguo, Z.; Xiao, H.; Huan, L.; Hongxia, W.; Yingyuan, Z.; Tingli, M. All-solid-state flexible asymmetric supercapacitors with high energy and power densities based on NiCo2S4@MnS and active carbon. J. Energy Chem. 2017, 26, 1260–1266. [Google Scholar]
- Moon, I.K.; Lee, J.; Ruoff, R.S.; Lee, H. Reduced graphene oxide by chemical graphitization. Nat. Commun. 2010, 1, 73. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.G.; Hong, J.; Hong, W.H.; Hammond, P.T.; Park, H. Facilitated ion transport in all-solid flexible supercapacitors. ACS Nano 2011, 5, 7205–7213. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Yao, Y.; Li, N.; Chen, T. Highly Stretchable supercapacitors based on aligned carbon nanotube/molybdenum disulfide composites. Angew. Chem. Int. Ed. 2016, 55, 9191–9195. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Lian, J. Arrays of hierarchical nickel sulfides/MoS2 nanosheets supported on carbon nanotubes backbone as advanced anode materials for asymmetric supercapacitor. J. Power Sources 2017, 343, 373–386. [Google Scholar] [CrossRef]
- Kumar, A.; Sager, A.; Kumar, A.; Mishra, Y.K.; Chandra, R. Performance of high energy density symmetric supercapacitor based on sputtered MnO2 nanorods. ChemistrySelect 2016, 1, 3885–3891. [Google Scholar] [CrossRef]
- Xia, H.; Zhu, D.; Luo, Z.; Yu, Y.; Shi, X.; Yuan, G.; Xie, J. Hierarchically structured Co3O4@Pt@MnO2 nanowire arrays for high-performance supercapacitors. Sci. Rep. 2013, 3, 2978. [Google Scholar] [CrossRef] [PubMed]
- Radhamani, A.V.; Shareef, K.M.; Rao, M.S.R. ZnO@MnO2 Core–Shell nanofiber cathodes for high performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30531–30542. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Zhang, Y.Z.; Yi, J.P.; Yang, L.; Zhang, J.D.; Lai, W.Y.; Huang, W. Inkjet-printed flexible, transparent and aesthetic energy storage devices based on PEDOT:PSS/Ag grid electrodes. J. Mater. Chem. A 2016, 4, 13754–13763. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Xin, L.; Wu, M.; Lou, X. Single-crystal β-NiS nanorod arrays with a hollowstructured Ni3S2 framework for supercapacitor applications. J. Mater. Chem. A 2016, 4, 7700–7709. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, Z.; Ding, S.; Chen, J.S.; Lou, X.W. Hierarchical nickel sulfide hollow spheres for high performance supercapacitors. RSC Adv. 2011, 1, 397–400. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.S.; Kanaka Durga, I.; Naresh, B.; Jin-Soo, B.; Krishna, T.N.V.; In-Ho, C.; Ahn, J.-W.; Kim, H.-J. One-Pot Hydrothermal Synthesis of Novel Cu-MnS with PVP Cabbage-Like Nanostructures for High-Performance Supercapacitors. Energies 2018, 11, 1590. https://doi.org/10.3390/en11061590
Rao SS, Kanaka Durga I, Naresh B, Jin-Soo B, Krishna TNV, In-Ho C, Ahn J-W, Kim H-J. One-Pot Hydrothermal Synthesis of Novel Cu-MnS with PVP Cabbage-Like Nanostructures for High-Performance Supercapacitors. Energies. 2018; 11(6):1590. https://doi.org/10.3390/en11061590
Chicago/Turabian StyleRao, S. Srinivasa, Ikkurthi Kanaka Durga, Bandari Naresh, Bak Jin-Soo, T.N.V. Krishna, Cho In-Ho, Jin-Woo Ahn, and Hee-Je Kim. 2018. "One-Pot Hydrothermal Synthesis of Novel Cu-MnS with PVP Cabbage-Like Nanostructures for High-Performance Supercapacitors" Energies 11, no. 6: 1590. https://doi.org/10.3390/en11061590
APA StyleRao, S. S., Kanaka Durga, I., Naresh, B., Jin-Soo, B., Krishna, T. N. V., In-Ho, C., Ahn, J.-W., & Kim, H.-J. (2018). One-Pot Hydrothermal Synthesis of Novel Cu-MnS with PVP Cabbage-Like Nanostructures for High-Performance Supercapacitors. Energies, 11(6), 1590. https://doi.org/10.3390/en11061590








