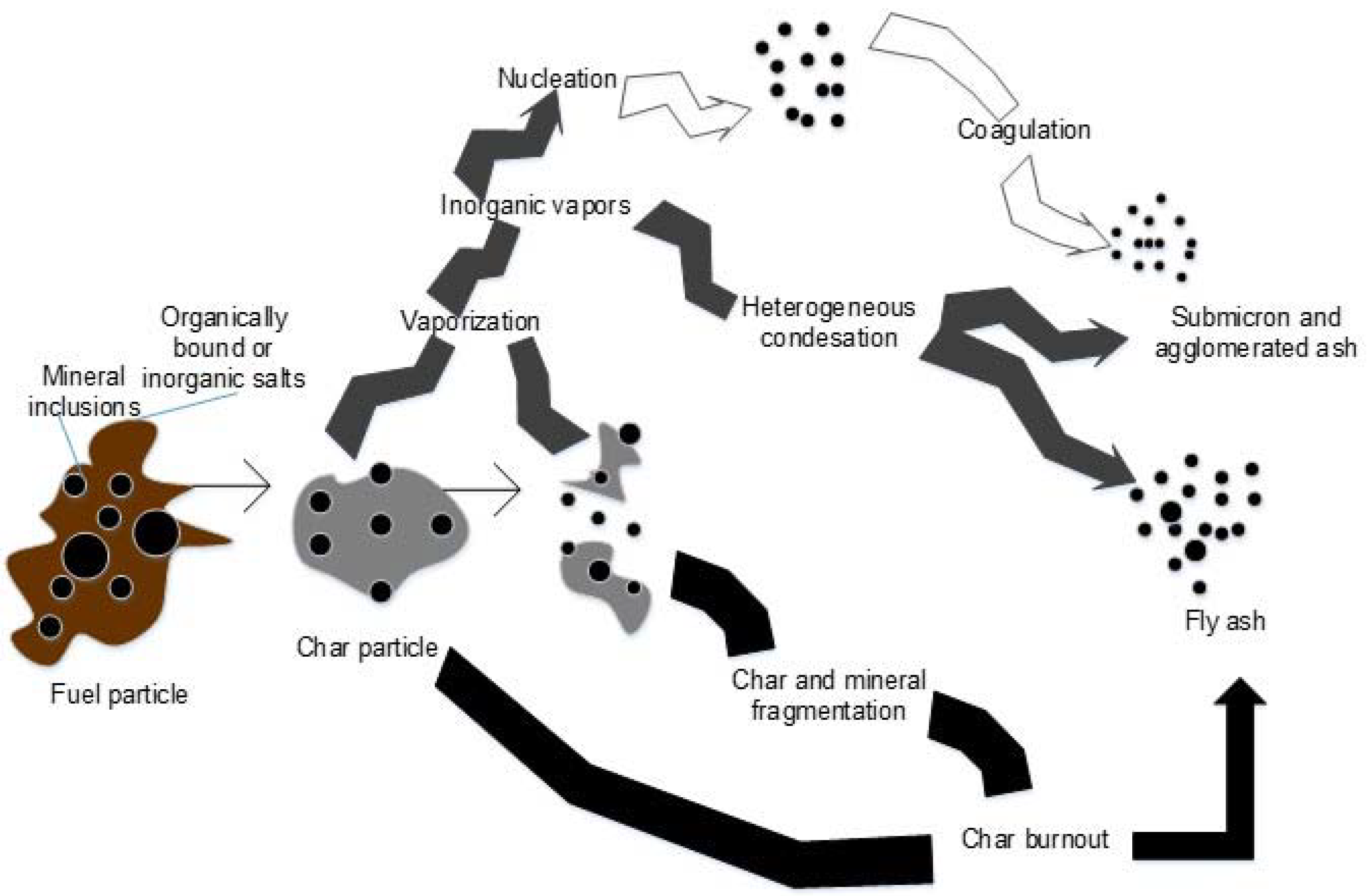
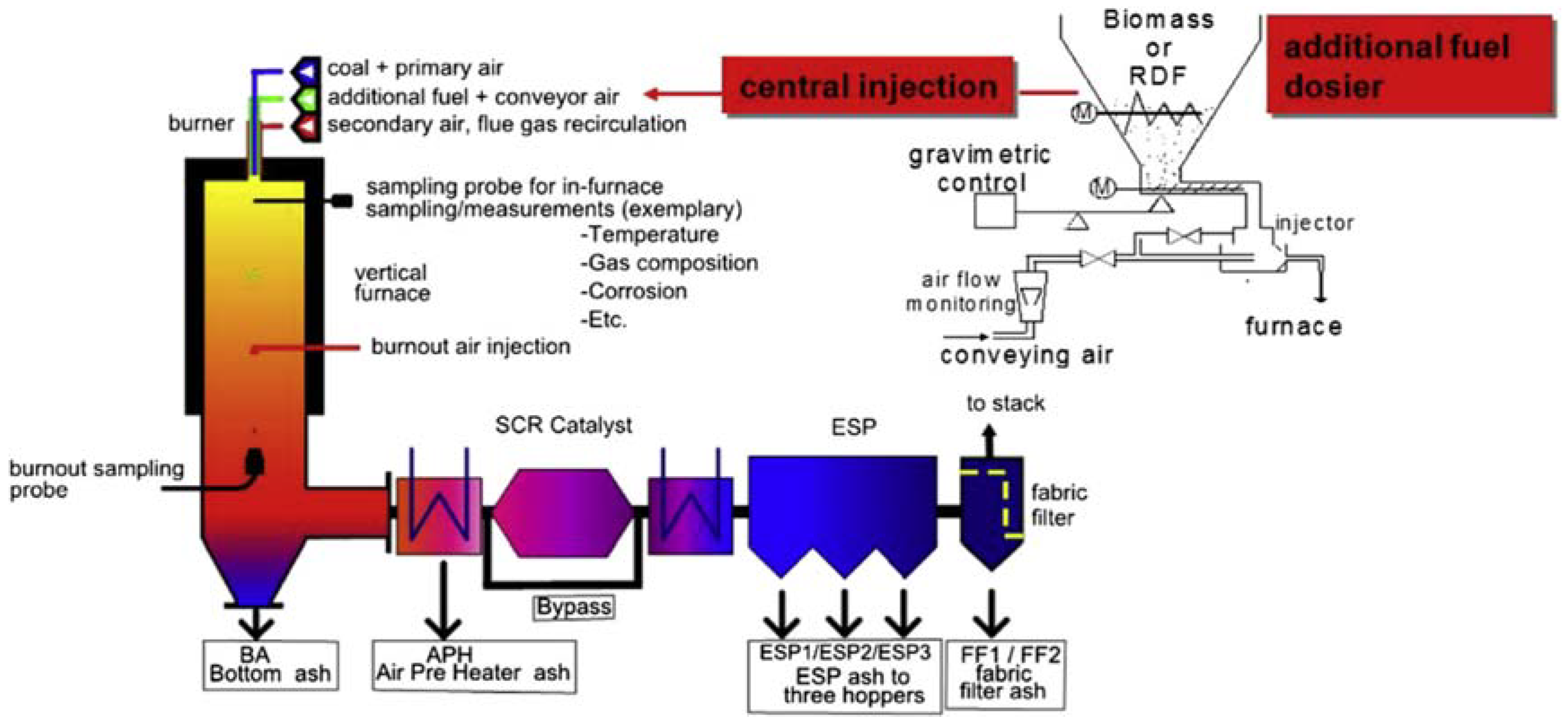
3.1. Chemical Characterization of Fuels
Table 2 shows the fuels proximate and ultimate analyses. As expected, there were noticeable differences in the moisture and ash contents of the lignite and the volatile content of the
Cynara.
The most noticeable difference of the fuels in
Table 2 is that the herbaceous biomass has a significantly lower water content and ash yield, and a correlating higher heating value. The
Cynara has a higher volatile content, about a 17% increase and a higher oxygen content. The
Cynara in the fuel blend indicates a much-improved regulation of combustion.
The ultimate analysis shows that the percentage of hydrogen and elemental oxygen are higher and the carbon lower in the
Cynara biomass when compared to the lignite. The lower value of nitrogen in the biomass shows that co-firing of
Cynara with the lignite will have the advantage of reducing the stack emissions of the conventional pollutant NOx that is regulated. Also, the lower nitrogen content of
Cynara means that co-firing may reduce the need for reagents to be injected into an selective non-catalytic reduction catalyst (SNCR) or high dust SCR equipment to reduce NOx emissions, if already occurring, which would decrease the propensity to absorb ammonia salt on fly ash (ammonia slip), as ammonia contamination of fly ash creates a host of potential odor issues when used in Portland cement-based projects [
29].
The calorific values illustrate the influence of the change in moisture content, and to a lesser extent, the volatile matter amount had on the heating value of the fuels. The net calorific value of the
Cynara is comparable to the values measured for other biomass types [
21]. Co-firing of the fuels would cause parameters to vary in proportion to their contribution to the fuel blend, e.g., energy and ash contents. Thus, the co-firing of
Cynara with the illustrated lignite quality may not only lead to improvements in the combustion performance, but also potentially in the ash quality.
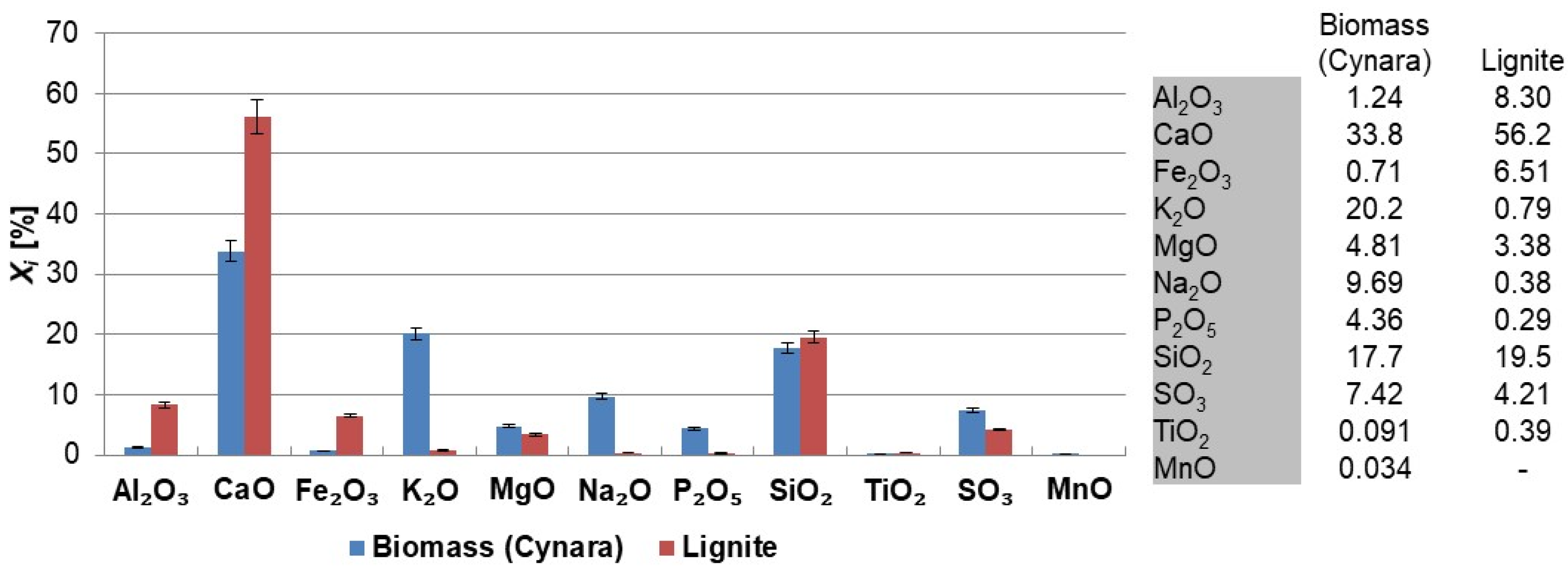
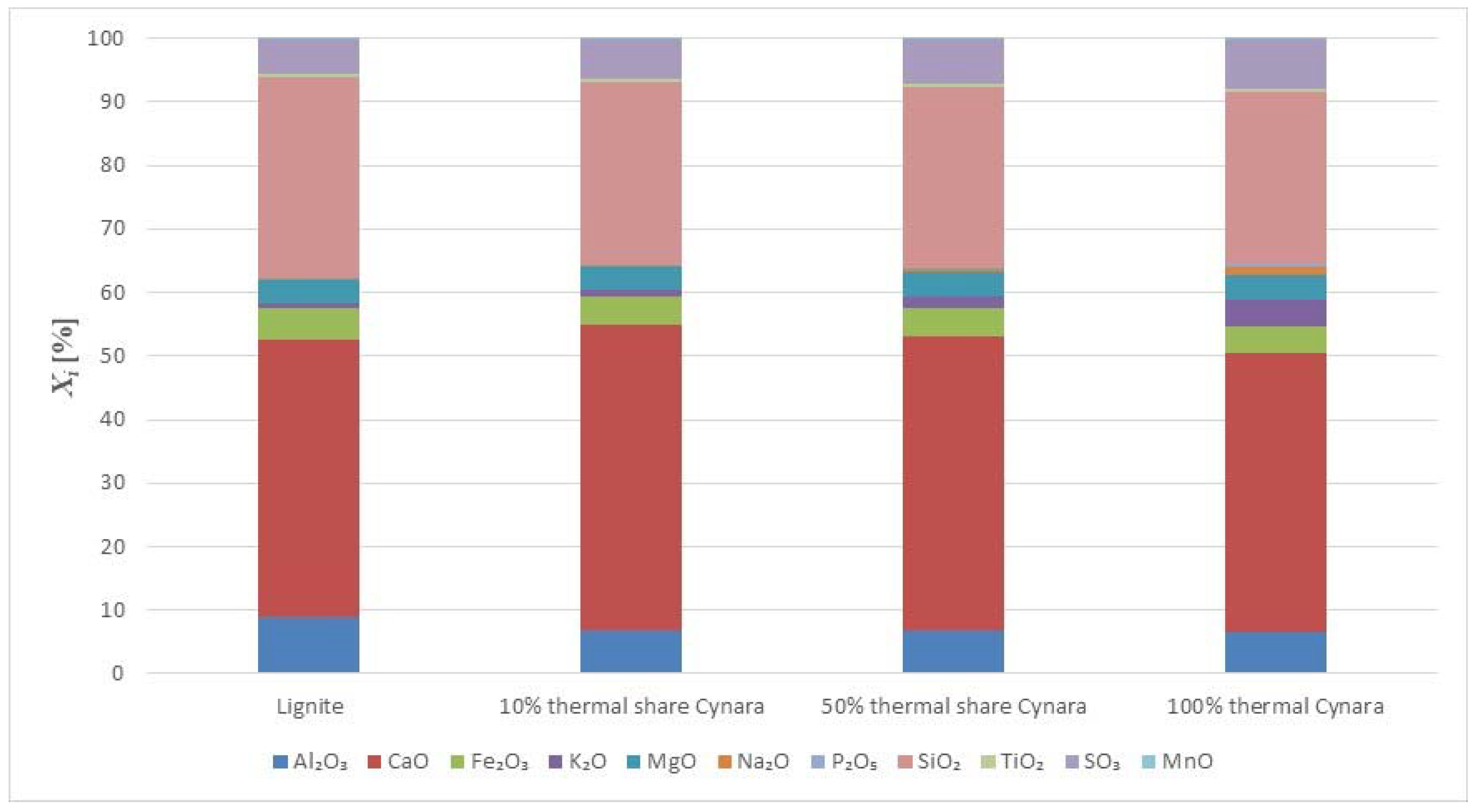
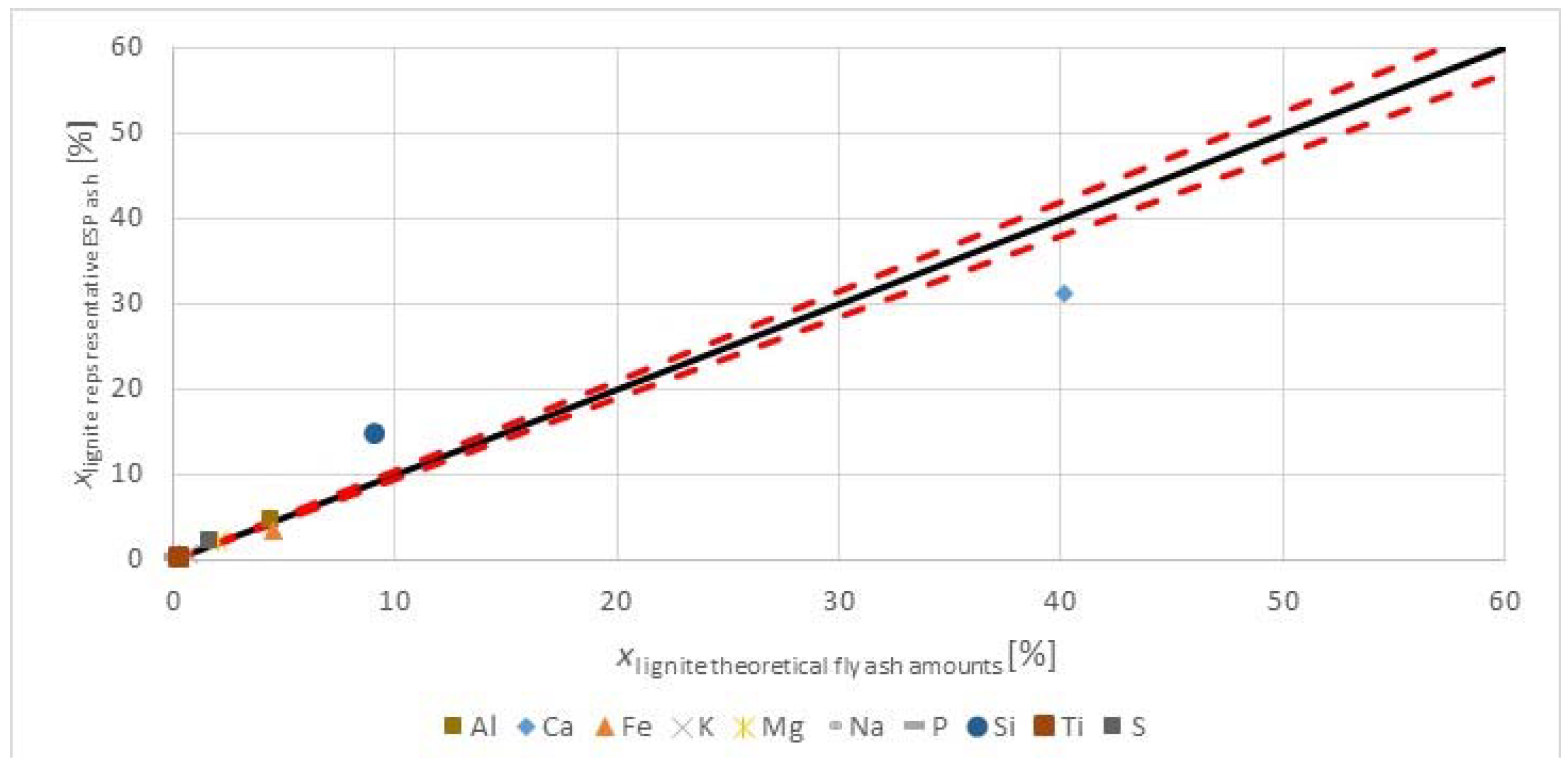
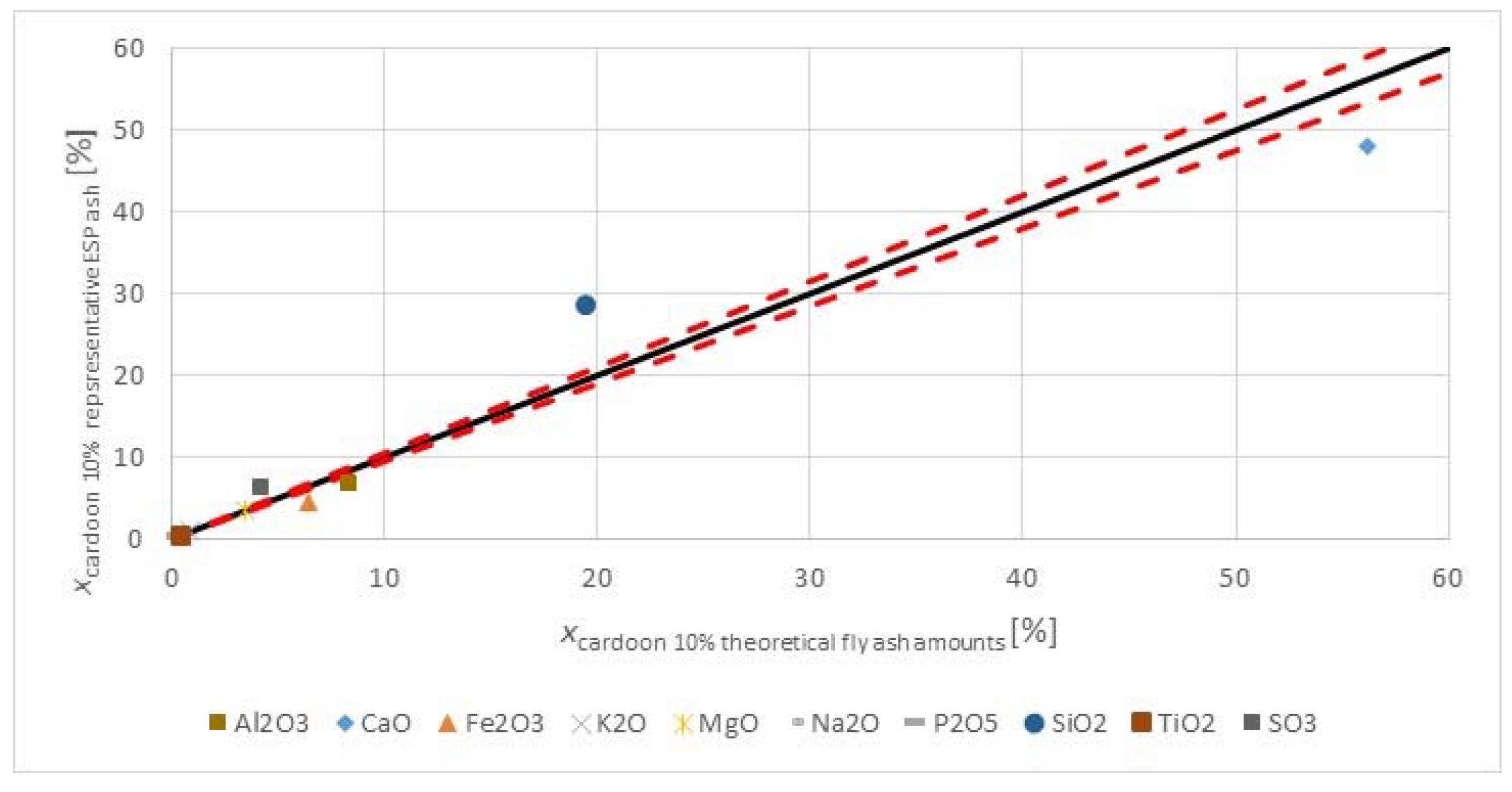
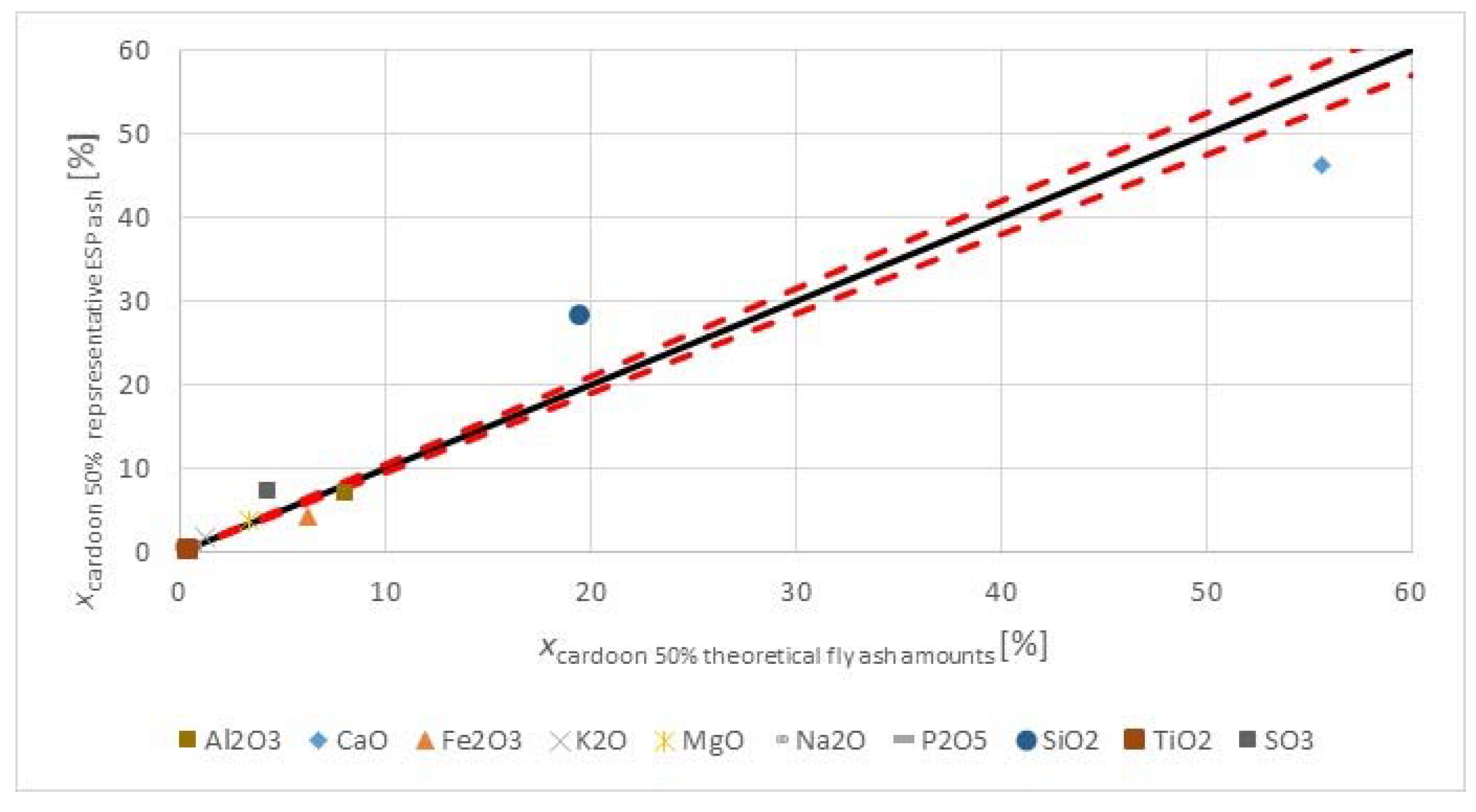
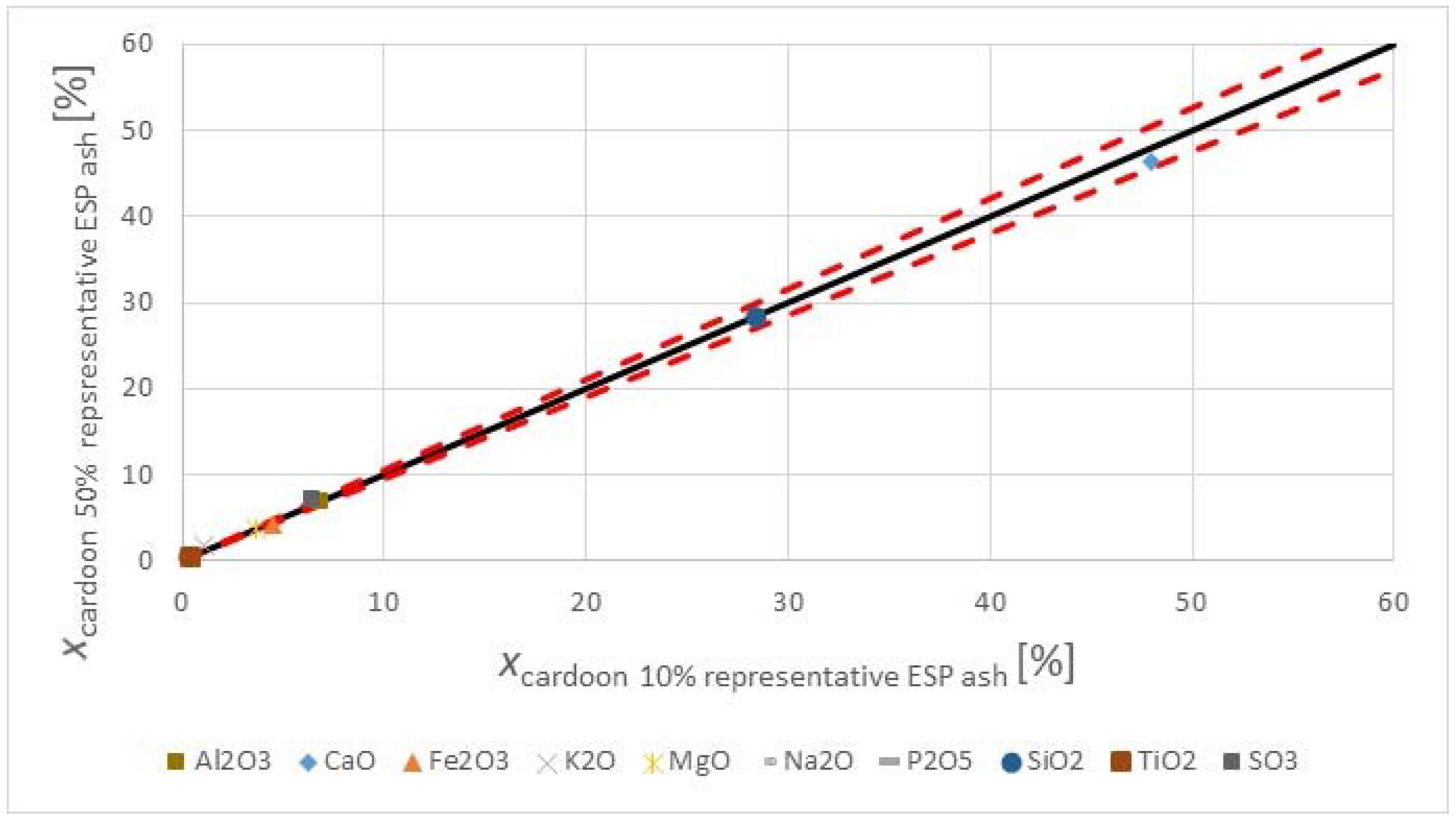
Figure 3 shows the major and minor oxides of the fuels on a carbon free basis.
The herbaceous biomass has less CaO than the lignite, but more K
2O, Na
2O, SO
3 and P
2O
5 contents. Generally, this trend is reported in literature for biomass fuels when compared to coal or lignite [
30], except for the SO
3 trend. The oxides of alkali, sulfur, and phosphorous are limited in concrete standards for applications in construction. However, when combusting the fuel with a lignite, having a significantly higher ash content, and at moderate thermal shares, the impact on the ash quality may be negligible too little. The SiO
2 contents in the fuels are comparable. The silica value for the
Cynara is aligned with values for herbaceous and agricultural biomass [
30]. The SiO
2 value in the lignite may reflect the quality from Greece, as the CaO oxide content is very high. Higher co-firing shares could change the ash quality for use in concrete, but masonry or mortar construction applications may be valuable options, as many of the concerns for concrete use with strict material provisions for conservative design are not warranted, and requirements are usually more performance based.
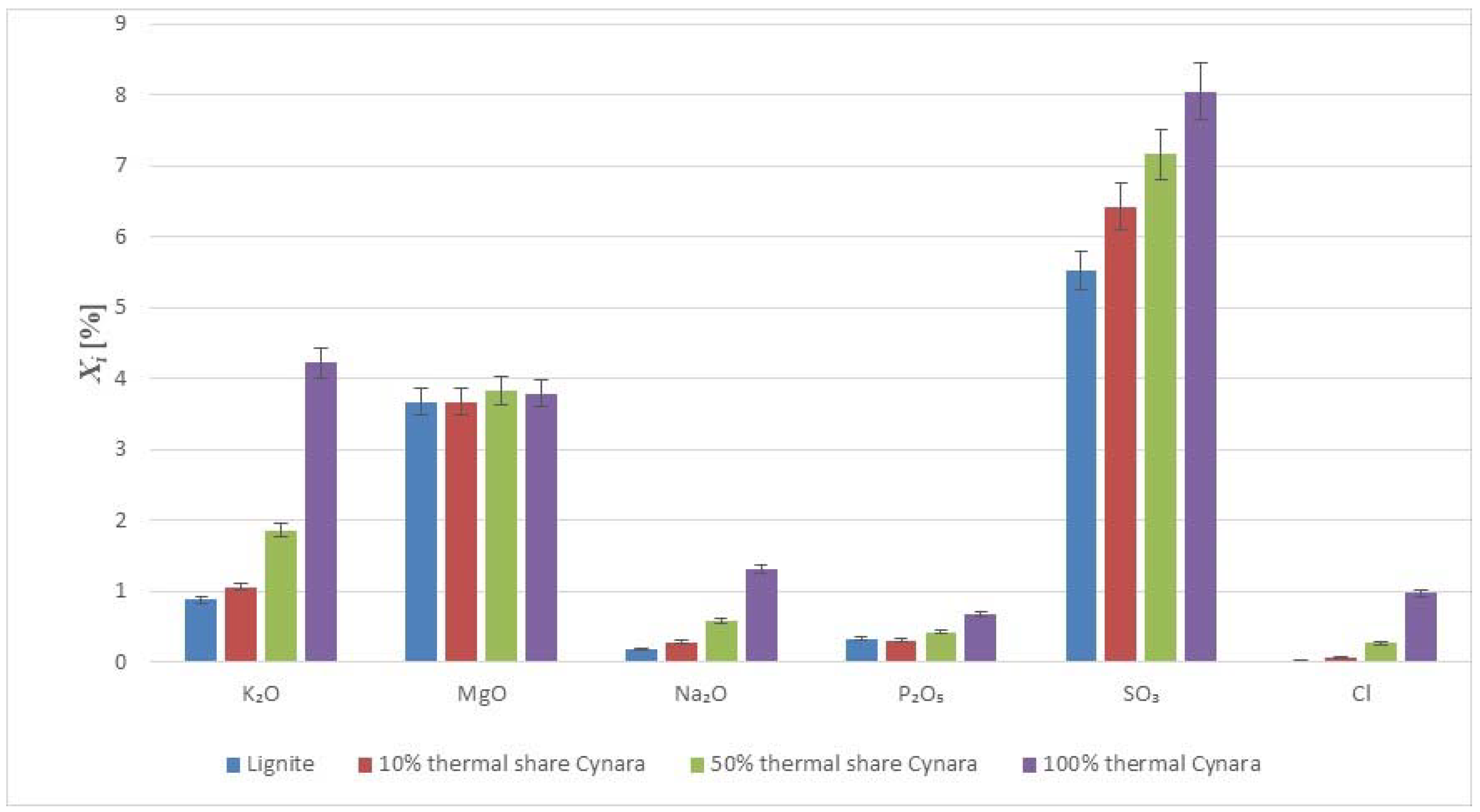
Table 3 shows values for the major and minor elements in the fuels.
Most of the ash-forming elements in annual plant biomass are noted as nutrients required for plant growth with inorganic impurities, occurring as a result of biomass being contaminated with soil during collection or handling [
31]. The diverse ash forming matter in biomass fuels depends on the biomass type, soil type, and harvesting conditions, with Ca, K, Na, Si and P being major ash forming inorganic chemical elements due to some of these needed as nutrients in biomass [
32]. Potassium and phosphorous are primary plant nutrients while sulfur, calcium, and magnesium are secondary plant nutrients [
33], so an increasing share of co-fired
Cynara would likely produce an ash quality very suitable for uses other than concrete, such as in low technical demand masonry applications, e.g., trafficked areas, parking lots, and in soil amelioration applications too.
3.3. Particle Size Distribution of Ashes
Fineness may be considered the most important property of fly ash in relation to its use in concrete, with the general rule the finer the better [
16]. The fineness of fly ash used as part of cement mixtures influences the rate of hydration since hydration occurs at the interface with water [
34,
35]. The fineness mainly impacts the short-term strength and only slightly affect the long-term strength [
36], which is due to the proposed occurrence of a more uniform microstructure expected for an increase in fineness [
35]. The pozzolanic quality of fly ash correlates to fineness and its glassy content (amorphous matter) [
37]. The strength increases as the granular distribution become narrow, which is predominantly an effect of a faster hydration under the conditions [
35].
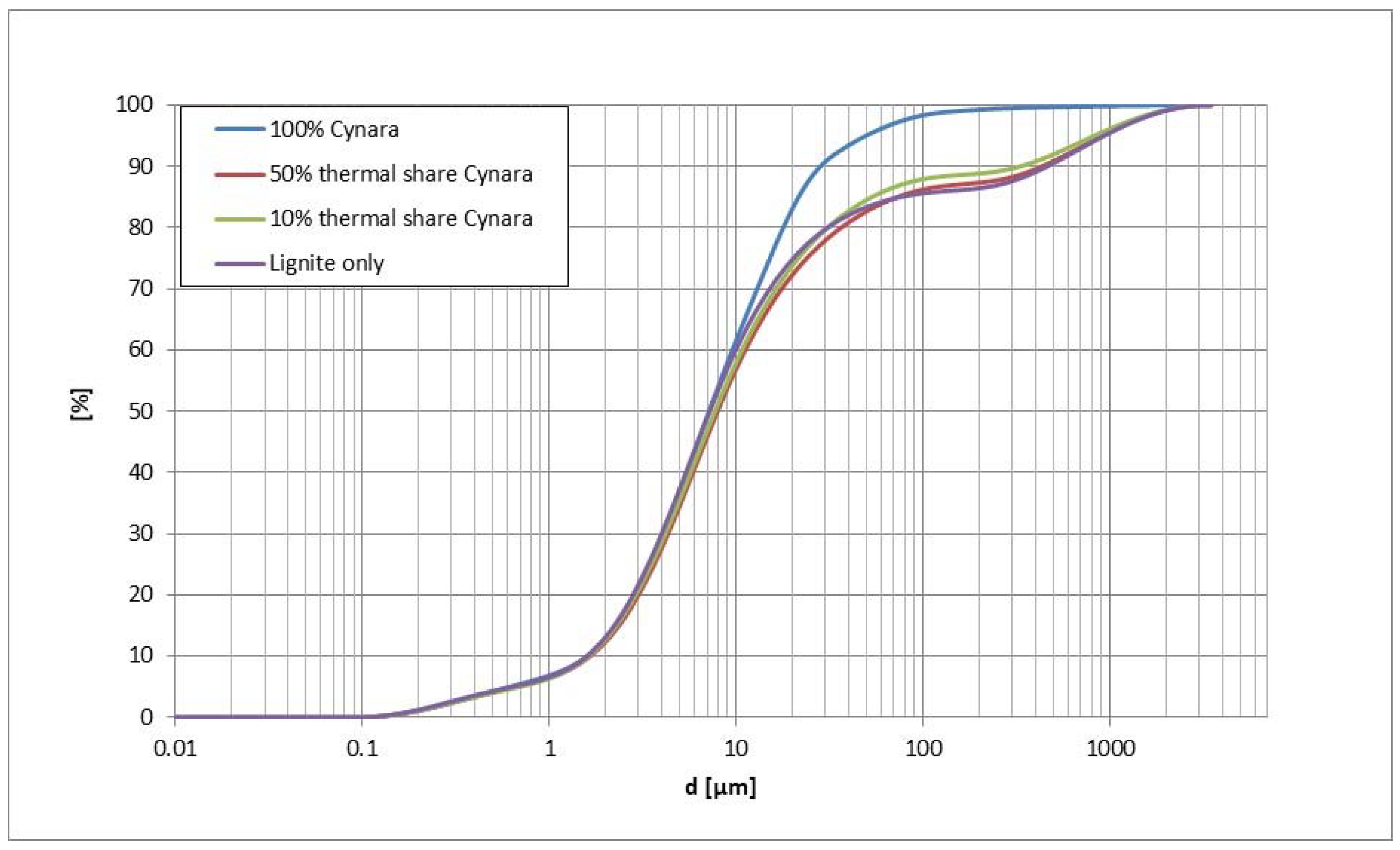
Compared in
Figure 5 is the particle size distribution (PSD) of the representative bulk fly ash samples from each
Cynara experimental trial. The pure
Cynara combustion fly ash is noticeably finer than the other ashes, while the other co-firing cases are similar to the lignite case.
The comparability of the co-fired fly ash particle sizes to the lignite case may be attributed to the percentage of
Cynara ash being much lower in the fuel mixture. In
Table 4 is shown the size distributions for
D10,
D50 and
D90 values.
The particle size distribution of the co-fired ashes are similar to the lignite case, and the
D90 value is improved. Thus, the ash reactivity is not negatively affected by the increased
Cynara thermal share in the fuel blend, regarding particle size distribution. The particle size is the most important physical characteristic determining the reactivity of fly ash [
3]. Fly ash particles in the range of 10 μm to 50 μm mainly are void-space fillers in concrete and particle sizes smaller than 10 μm are classified as pozzolanic reactive (hardening when reacted with Ca(OH)
2) [
3]. Thus, the larger biomass fuel particle size should not lead to reductions in the ash reactivity from the particle size distribution.
With the higher heating value of the
Cynara fuel, it is likely a rise in temperature may have contributed to improvements in char and mineral fragmentation and ultimately smaller particle sizes for the 100%
Cynara test scenario compared to the other cases. Furthermore, the alkali species are known to lower the eutectic temperature of compounds [
38] creating melts, which would be enhanced with the increase in the furnace temperature, and this likely creates an improved thermal contact due to melting of alkali compounds that enhances the heat transfer rate to other compounds [
23], e.g., via heterogeneous condensation. The
Cynara ash had much more K
2O content than the lignite. Thus, the herbaceous biomass fuel likely had a better particle fragmentation throughout the burning process. This would likely contribute to a finer particle size distribution of the fly ash produced from that test scenario. This needs further studying.
The very close gradation curves of the fly ash from the co-fired
Cynara tests compared to the lignite fly ash indicates no negative concerns for water requirement or cohesiveness of the fly ash from
Cynara in the fuel blend. The particle size distribution actually got somewhat finer with the 10% co-fired share of
Cynara. This slight change in fineness indicates a possible improvement in the workability when used in mixtures, as finer round shape particles can reduce the friction between ordinary Portland cement particles and among fly ash particles [
10].
It is believed that the
D90 value for the 10%
Cynara is caused by imperfect mixing of the ash before determination of the
D90. The ash formation for the co-firing of
Cynara is believed to be driven by the ash formation from the lignite, i.e., mineral fragmentation, coagulation, agglomeration, heterogeneous condensation, etc. Also, the burning of pure
Cynara, which has much lower ash and fixec-carbon contents along with a higher volatile matter amount, due to evaporation of alkali compounds and other organic matter the ash is finer. The PSD from the pilot-scale tests were compared to results from industrial experimental tests. This supports evaluations for the scale-up of pilot-scale test results. The industrial tests were conducted at the Kardia Power Plant located in Ptolemedia, Greece, and is described in the literature in [
39].
Table 5 shows those comparisons.
Table 5 shows a good correlation among the particle sizes from the pilot-scale fly ash and the industrial ground fly ashes. The pilot-scale results are very close to the ground industrial fly ashes, which is a practice in the industry. Thus, the pilot-scale observations can be considered to be transferable to industrial scale, regarding the PSD of the fly ashes, which has a very significant impact on the reactivity of ashes, and is one characteristic relied upon to assess fly ash pozzolanic behavior.
3.5. Scanning Electron Microscope (SEM) Analyses and EDX Results
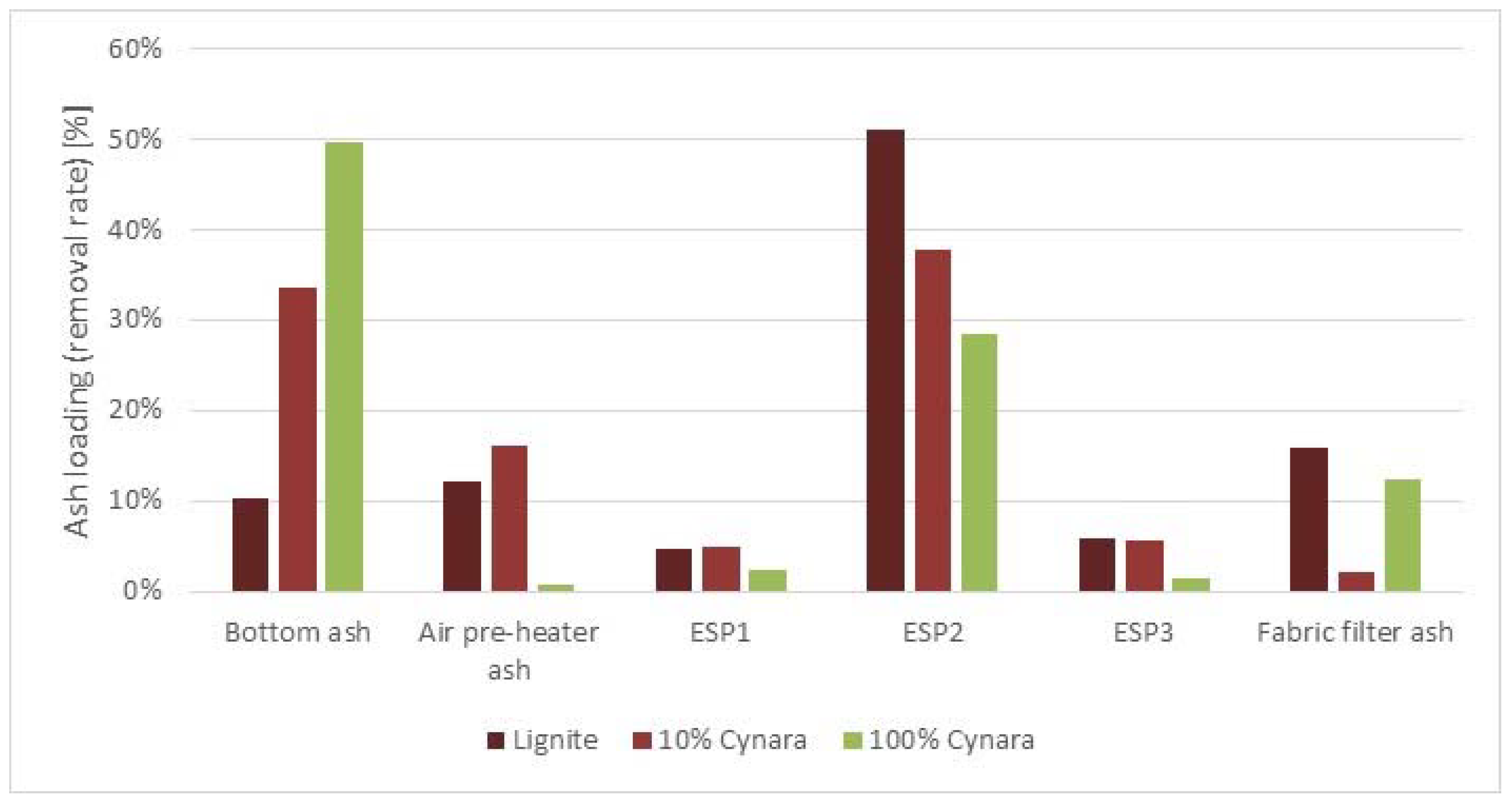
In the figures in this section, ESP hopper samples are numbered from 1 to 3, and a sample is identified as the bulk ash. The first three fly ash samples correspond to the three batches of ash precipitated at the three hoppers in the ESP (ranked from the coarser fraction, number 1, to fine the fraction, number 3). The bulk sample is a mixed representative fly ash of the ESP for each firing scenario.
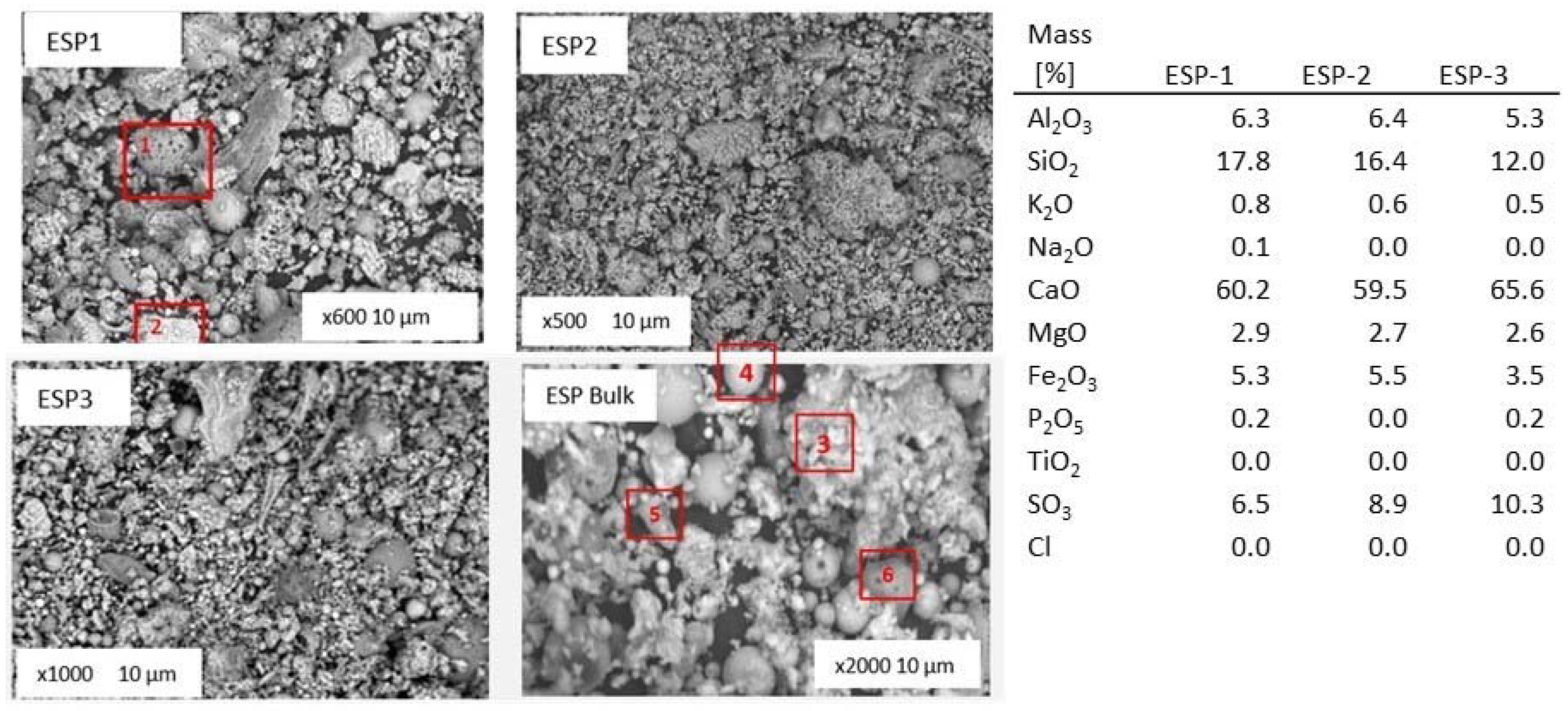
Figure 14 shows the general overview of collected fly ash samples during pure lignite combustion tests. The chemical composition of the fly ash from the three hoppers collected during pure lignite combustion from energy dispersive X-ray (EDX) analyses is given in the figure too. The EDX analyses are normalized on carbon and oxygen free basis, expressed in oxides.
An amorphous particle, number 1, represents an alumina-silicate compound enriched in iron and some potassium along with smaller amounts of calcium and magnesium. Particle number 2 is an example of a particle when calcium was primarily associated with oxygen and some sulfur.
The median particle size varied between 6 and 80 μm, with the larger particle sizes generally corresponding to fused molten agglomerates, see particle numbered 3. There is no significant difference in morphology of the particles collected at various hoppers, apart from the particle size. Obviously ash particles in the sample number 3 (from the third hopper) are smaller (see magnification). From the bulk sample, both mineral (particle number 4) and amorphous components (particle number 5) can be identified. Spherically and colorless particles indicate glassy textures or predominance of crystallinity and spherical, rounded light colored particles indicate a glassy surface [
65]. Rounded particles indicate a glassy particle, irregular shapes indicate partly crystalline particles, and angular shapes indicate a crystalline nature [
65,
66]. As determined by EDX, the predominant elements in the fly ash samples were calcium, silicon, aluminum, iron, magnesium and sulfur in various compounds. Calcium was observed to primarily be associated with oxygen, sulfur, and silica, or possibly with carbon (calcite). Particle number 6 shows a particle with holes, which represent such particles in the fly ash. Volatiles trapped in a particle cause it to have swelled surfaces, varying the shape of the particles and bubbles, which alters its surface [
67]. The bubbles formed may be attributed to the release of volatile matter from the internal zones of the particle as the solid particle’s surface is softening and melting [
67]. The bubbles can generate large cavities under the surface, which are smooth zones and considered to be less reactive for the subsequent steps of oxidation [
67]. This indicates a continuing combustion, which may have undesirable effects on the fly ash quality due to the presence of such apparent uncomplete combusted particles. Such particles were qualitatively observed to be more prevalent in the fly ash from the lignite scenario. This suggests the lignite case had the least optimal combustion performance.
Figure 15 shows the general overview of collected fly ash samples during 10% thermal share co-firing of
Cynara. The chemical composition of the fly ash from the three hoppers collected during 10% thermal share co-firing of
Cynara from EDX analyses is given in the figure too. There is no significant difference in morphology of the particles collected at various hoppers.
The coarser fraction (20–90 μm), in ESP1, is mainly composed of alumina silicates particles with potassium, calcium rich crystalline phase, and iron and magnesium oxides The SEM-EDX data showed calcium is associated with sulfur or oxygen in distinct particles, (see
Figure 15, particle number 2). Sulfur captured by lime, which is originating from fuel, is widely known as a self-desulfurization process. This process leads to a spontaneous reduction of SO
2 emissions in industrial boilers. The observation support the assertion noted in
Section 3.4.3, regarding the enrichment of elements in the fly ashes.
Particle 1 is an example of an amorphous alumina-silicate sphere. The majority of the iron-rich spheres observed consisted of an iron oxide mixed with amorphous alumina-silicate. Compared to the previous fly ash sample (100% lignite combustion), the fly ash samples from 10% thermal share of
Cynara co-firing contain less calcium, but more aluminum, silica, iron, and sulfur. Char particles with holes were detected in this fly ash too, see particle number 3 and particle number 4. Those particles indicate a swelling phenomenon and structural changes after devolatilization that led to melting and fusion into a mass with smooth surfaces. A particle with irregular and indented surfaces expose more active sites for gaseous reactant than a smooth surface, which influences the reactivity of the char [
67]. Thus, those char particles were not as reactive, which apparently led to their presence in the fly ash.
Necked and interlocked particles appear to be more prevalent in the 10%
Cynara bulk ash compared to the lignite scenario, which generally increased in the bulk ash with the increase in the co-firing share of
Cynara. The agglomeration phenomena may be the result of low melting compounds, mainly, alkali in biomass ashes, than can stick contacting surfaces of separate particles, causing them to cluster [
67]. Moreover, for this fly ash, the increase in irregular, elongated, flat, etc. particles may be associated with more particles considered as fissures. Fissures, holes, and superficial porosity represent the way volatile gases escape and for gaseous reactants to reach the active sites inside the char particle for oxidation [
67]. Fissures, which are associated with a scabrous particle surface with a wide distribution, lead to a more reactive particle [
67]. Thus, the apparent qualitative increase of irregular particles in this ash compared to the lignite scenario may suggest a better burning behavior of the fuel blend.
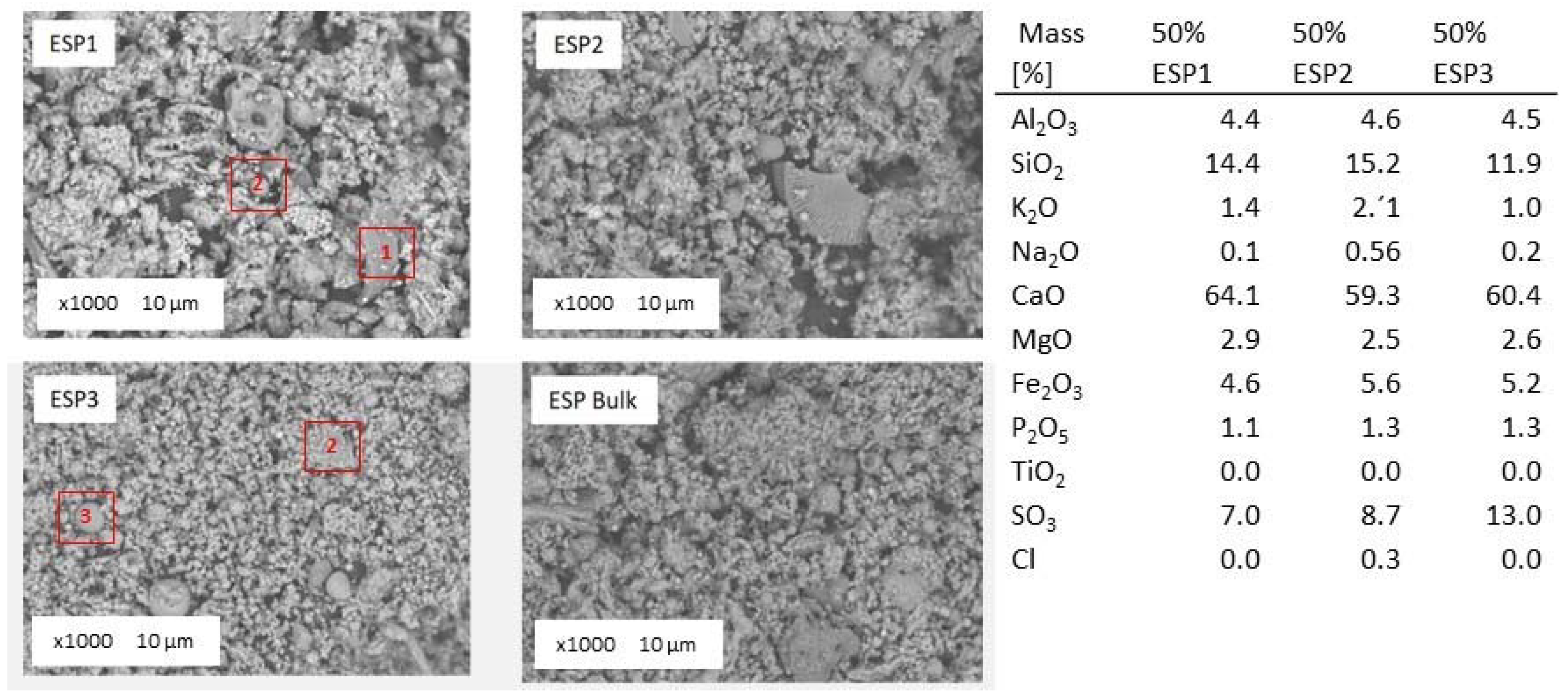
The general overview of collected fly ash samples during 50% thermal share co-firing of
Cynara is shown in
Figure 16. Mineral (particle numbers 1 and 2) and amorphous (particle numbers 3 and 4) components can be identified. The chemical composition of the fly ash from the three hoppers collected during 50% thermal share co-firing of
Cynara from EDX analyses is given in the figure too. There is no significant difference in the morphology or in the particle sizes between various hopper ashes.
The median particle size varied between 1 and 30 μm that means somewhat smaller sizes than in the lignite and 10% thermal share fly ashes, which is different than the particle size distribution of the bulk ash shown in
Section 3.3. The predominant elements in the fly ash samples were calcium, silicon, aluminum, iron, magnesium, sulfur and potassium in various compounds. Aluminum was observed with silicon and potassium. Apart from alumina-silicates, silica has been identified as a quartz too. Calcium was primarily associated with oxygen, sulfur, and phosphate. Again, this observation supports the assertion of the ash formation process in
Section 3.4.3 from the addition of natural gas.
For this fly ash quality, there was an apparent increase in irregular, fused, etc. particles. This may be attributed to more biomass particles in the fuel blend, giving rise to a higher temperature. At higher heating rates, from a higher temperature, particles can partially or completely melt, losing their distinction, fusing together, and forming completely or partially hollow particles and smoothed surface particles too [
68]. Also, a higher temperature implies not only a higher heating rate but also a rapid evolution of gases that build up pressure during volatile release, which may change the shape of some parts of the particle structure and cause some particle breaking (fragmentation) [
69]. The increase in pyrolysis pressure has the potential to lead to the formation of larger particles due to swelling as well as formation of particle clusters as a result of melting and subsequent fusion of particles [
68]. This is likely the mechanism of the clusters of particles formed with an increasing share of
Cynara in the fuel blend. This may support a better overall burning of the chars for co-firing cases, as the 50% thermal share of
Cynara had a lower LOI than the 10% thermal share of Cynara.
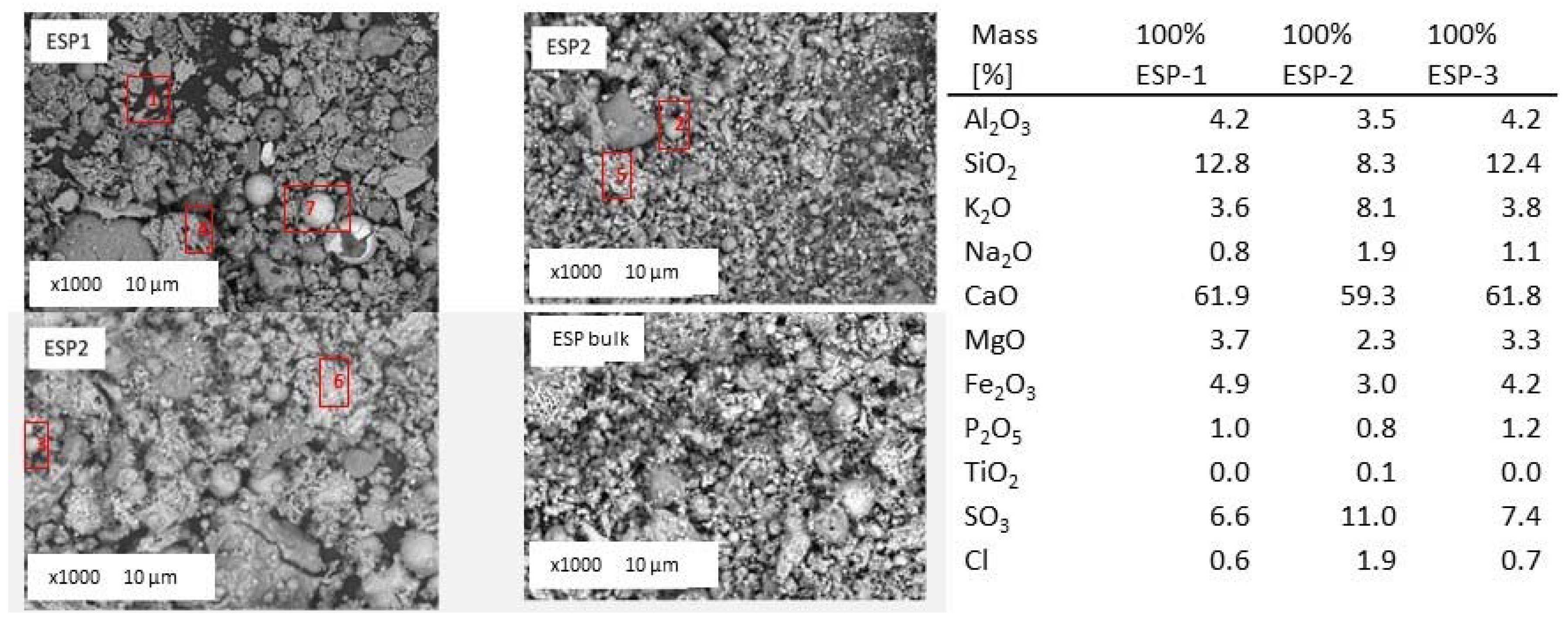
Figure 17 shows fly ash samples from the ESP hoppers and the mixed bulk fly ash collected during the 100% thermal share
Cynara tests. The chemical composition of the fly ash from the three hoppers collected during the 100% thermal share
Cynara tests is given in the Figure too from EDX analyses.
The fly ash contains amorphous (point numbers 1, 2 and 3) and crystalline ash particles of various shapes. The median particle size varied between 4 μm and 80 μm, with the larger particle sizes (particles numbers 4, 5 and 6,) generally corresponding to fused molten agglomerates. The sphere-shaped ash particles are composed mainly of aluminum and silicon, with potassium and calcium. The detection of alumina-silicate with potassium, as it was not detected in the other fly ash samples, could be attributed to the lower calcium content in the biomass fuel. Calcium is also the main ash forming element in the 100%
Cynara fly ash samples. Next to alumina-silicate cenospheres, similar particles, but then enriched in iron (ferrospheres) (particle number 7) were identified. Next to particle number 7, it can be seen a hollow ferrosphere as a result of the possible expansion of trapped volatile matter. Ferrospheres may be associated with heavy metals, such as Cu, Cr, Pb, and Zn due to inorganic, organic, and intermediate affinity of trace elements, which may make environmental pollution risks a paramount importance [
70]. The identification of ferrospheres give rise to a multi-utilization of the fly ash as they can be economically extracted by magnetic separation techniques that have industrial possibilities in metallurgy, ore and coal dressing processes, and dense concrete production [
70]. The iron oxide exhibits various textures even the dendritic pattern was found. The fine fraction of the ash deposit from the 100%
Cynara scenario is mainly associated with calcium, but also with potassium and sodium oxides, sulfates, phosphates and chlorides. Several crystalline particles containing alkali metals and sulfur with chlorine were identified. Since alkali chlorides crystal structure is octahedral as calcium oxide, it is difficult to clearly distinguish between various compounds. Nevertheless, the fly ash samples contain significantly higher amount of sulfur, potassium, and chloride compared to the other fly ashes. Compared to the 50%
Cynara co-firing scenario, there is more potassium, sodium, and chloride in the fly ash from 100%
Cynara combustion. The concentration of the sulfur remains comparable. Without the interaction of the lignite ash, i.e., more alumina-silicates and calcium available, the volatile species were more readily able to react with compounds of sulfur, silica, etc. that contributed to their capture in the fly ash. This is supported by the enrichment of elements for the 100%
Cynara combustion test scenario shown in
Section 3.4.3.
It is noted that for the influence of aggregates on concrete (an engineering construction material) shrinkage, flaky, elongated, angular, and rough aggregate particles have high voids, requiring more material to fill them, which increases water demand and shrinkage [
71]. Spherical and cubical aggregates with their less specific surface area than flat and elongated particles require less water for use, and they lead to lower shrinkage than flaky and elongated aggregates [
71]. The irregular particles can be overcome by applying a grinding fly ash post-treatment process to reduce the particles that are fused and create more spherical particles [
72]. Thus, the inclusion of
Cynara in the fuel blend may necessitate a small grinding post-treatment process to obtain an optimal fly ash for use in construction engineering materials, i.e., concrete and mortar for various masonry applications.
From the SEM analyses, it is seen that the fly ash samples consist of particles of various shapes and sizes. At temperatures in a pulverized fuel boiler, generally in excess of 1400 °C, the mineral matter within the fuel oxidizes, decompose, fuse, undergo fragmentation, agglomerate, etc. During the burning of coal, rapid cooling in the post-combustion zone results in the formation of spherical, amorphous (non-crystalline) particles, (≤90%), or glass and a small amount of crystalline material [
3]. Shown in
Section 3.4, the average chemical composition was relatively consistent for the pure lignite, 10%
Cynara thermal share, and the 50%
Cynara thermal share. The major element in all fly ash samples is calcium. The relatively bigger molten agglomerates are composed mainly of alumina silicates with large proportions of potassium. The coarse fly ash fraction may also contain significant amounts of calcium sulfates. Next to the coarse fraction, agglomerates of very fine particles (<<1 μm) clustered together were observed too. The fine fly ash fraction (ESP3) is formed from volatilized ash material that condenses in the cooling flue gas. The 100%
Cynara combustion test resulted in alkali salts formation in submicron particulates. Moreover, with the increasing share of
Cynara in the fuel blend, there was a reduction of amorphous particles (mainly associated with spherical particles) and an increase in irregular particles. Thus, the effects of irregular particles with more inner voids may need to be considered when deciding the utilization route of fly ash produced from
Cynara in the fuel blend, which will be dependent upon the application.
3.6. X-ray Powder Diffraction Analyses of the Ashes
Most elements in fly ash occur in both organic and inorganic matter, and each has a dominant association and affinity with some mineral phases [
73]. Elements in fly ash can occur in distinct phases that occur in different content, size, morphology, association, and generation in fly ashes, namely active, semi-active, pozzolanic, or inert mineral phases [
73]. Each crystal phase group has individual behaviors regarding hydration-dehydration and hydroxylation-dehydroxylation processes in fly ashes, which have a leading role in the production of construction materials that is missing information from the bulk chemical composition of fly ashes [
73]. Characterizing, identifying, and quantifying the phase mineral composition, along with the chemical content, of biomass ashes, are initial and necessary steps for assessing their utilization [
57]. Minerals are also essential for the building industry, where fly ash is likely to have a significant use, with lime and siliceous acid being among the most important for this industry [
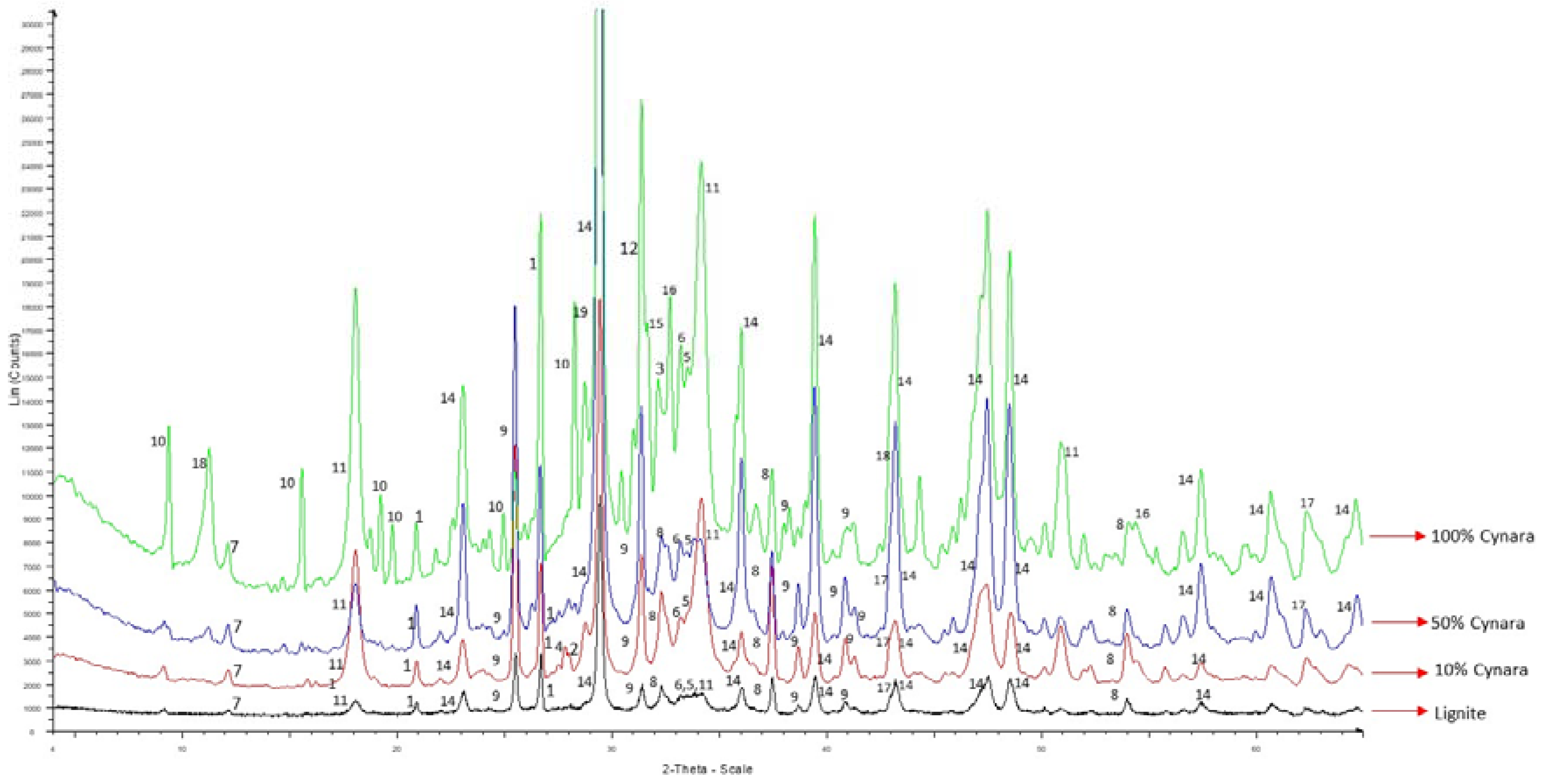
74]. The elements will form different minerals in co-fired biomass ash compared to coal ash or lignite ash. X-ray powder diffraction scans of the fly ashes from the different scenarios were compared among each other, as shown in
Figure 18.
Table 7 shows the minerals detected in the fly ashes for all scenarios.
Table 7 shows that fly ash qualities are dominated by calcium minerals, as expected. Dominated by calcium minerals are lignite fly ashes [
75]. The mineral phases in fly ashes are categorized as noted below, with calcium based minerals having active qualities and the most desirable in fly ashes for masonry uses. The following list highlight those categories [
57]:
Active (lime, periclase, anhydrite, bassanite, Ca and Ca-Mg silicates and alumina-silicates, ca-enriched glass, among others);
Semi-active (portlandite, brucite, gypsum, carbonates, clay and mica minerals, feldspars, Fe oxides, Ca-containing glass, among others),
Pozzolanic (glass); and
Inert or inactive (quartz, mullite, some char) behavior during hydration-dehydration and hydroxylation-dehydroxylation processes of biomass ashes.
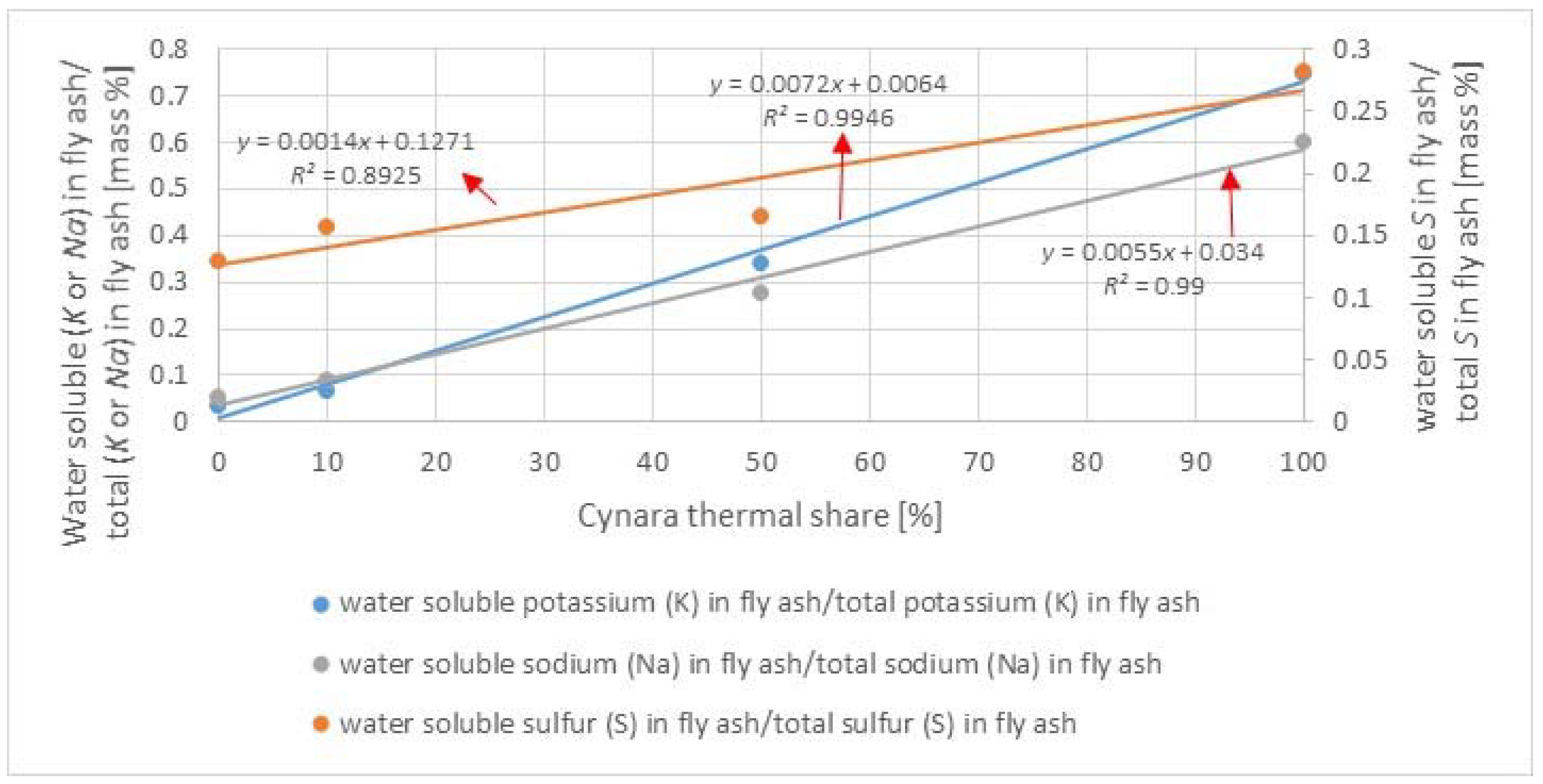
The XRD analyses showed that there were no alkali-sulfates detected in the fly ashes from the co-firing trials. The K in a sulfur phase detected in the 100%
Cynara combustion is in agreement with SEM-EDX observations. Thus, it is maintained that the inclusion of natural gas in the co-firing fuel blend led to more sulfur being transformed to the more reactive SO
3 species, as explained in
Section 3.4.3. The effect reduced the available sulfur to form alkali sulfates. This may be a major reason why the lignite ash and the co-fired ashes are more similar than anticipated. Therefore, an optimal co-firing of a low-quality lignite and an herbaceous biomass fuel may be with natural gas in order to mitigate ash related problems. More work is needed in this area.
It’s necessary to classify fly ashes according to their potential pozzolanic activity and cementitious properties, as well as others if needed, such as alkali-aggregate reactivity and sulfate resistance [
53]. Fly ash produced from combustion of low-rank lignites and sub-bituminous coals have both cementitious (self-hardening when reacted with water) and pozzolanic properties [
73]. The pozzolanic mineral phases undergo pozzolanic reactions, which are mainly diffusion controlled and begin at a much later period in the hardening system [
57]. Fly ashes with medium to little pozzolanic tendencies and generated from fuels with a variable rank, but mostly lignite, to a lesser extent bituminous coals and rarely anthracites, are active types [
73]. The primary key to the pozzolanic reaction is the structure of the silica, which must be in a glassy or amorphous phase with a disordered structure, being formed, like in the rapid cooling of a volcanic magma [
28]. If the silica is in a uniform crystalline structure, like found in silica sand, it is noted to not be chemically active [
28]. The fly ash qualities produced in this work are considered to have both cementitious and pozzolanic qualities as it is expected to be some amount of amorphous matter in fly ash produced in pulverized fuel boilers, as quenching retains high temperature phases and melts will appear as glass [
76], but below about 5%, it may not be detected by XRD analyses.
Primary minerals present do not undergo transformations during combustion, such as stable silicates [
73]. Quartz, which is a primary mineral and can be essentially non-reactive in combustion processes due to its high fusion temperature [
77]. Also, the quartz mineral, which may form by partial crystallization of the glassy phases in fly ash, decrease expansion from of alkali-aggregate (i.e., silica in the aggregates) reactions in concrete, and they are non-reactive [
49]. Quartz was found in all ash samples but had a qualitative decrease with increasing co-firing shares, which was an observance from peak intensities.
Anhydrite (CaSO
4), forming from the reaction of CaO, SO
2 and O
2 in the furnace or flue, plays a role in concrete’s hydration behavior [
75]. Shown below are the reactions of forming anhydrite (Equations (8) and (9)) [
78]:
Anhydrite may also be formed from the dehydration of gypsum [
1,
77], dehydration of bassanite (CaSO
4·1/2H
2O), or between a sulfuric acid (generated during sulfide oxidation) and calcium carbonates in the coal/lignite. Shown below is the reaction (Equation (10)) of gypsum decomposition [
1].
Anhydrite along with other soluble aluminates lead to the formation of ettringite (trisulfate of calcium aluminate) immediately upon addition of water to fly ash; the initial hydration reaction contributes much to the self-hardening characteristics of fly ash, which can participate and control the solubility of potentially hazardous trace elements [
75]. The decrease of quartz and increase of anhydrite, found in all samples, with the increasing co-firing share, implies an increased reactive fly ash quality. The lime decreased in fly ash with co-firing of
Cynara, which is a result of the lower level in the biomass. It is likely formed via the reaction of calcium in the fuel with oxygen, see below (Equation (11)) [
79]:
The lime may also be formed by the mechanism of the breakdown of calcium carbonate in the fuel. The strongly endothermic process leads to the breaking up of the chemical bond in the carbonate ion and the rearrangement of ions to form CaO crystals and the diffusion of CO
2 out of the cluster of lime crystals, as shown below (Equation (12)) [
25,
80]:
The lime may also be formed from calcium incorporated into the organic matter [
77]. The decreae in calcium oxide implies a decrease in any free lime (CaO) potential. Free CaO in excess amounts must be limited or unsoundness (undesirable volume change [
25]), i.e., expansion, could occur during hydration processes in concrete [
81]. Furthermore, free CaO in ash may react with water forming Ca(OH)
2, causing an increased volume and structural destruction of concrete [
82]. Probably formed via the reaction shown below (Equation (13)) was the portlandite mineral [
83]. The mineral’s presence in the fly ash increased with the 10%
Cynara co-firing, but decreased with the 50% share:
Formed via the reaction of CaO with CO
2 is calcite as shown below (Equation (14)) [
25,
84]. The reaction is widely known:
The periclase mineral may be formed via the general reaction shown below (Equation (15)) for the decomposition of carbonates in the fuel [
38]:
where
M equals Fe, Mg or Mn. The periclase may also be formed form the reaction indicated below (Equation (16)) [
85]:
Also, the periclase may be formed from incorporation into the organic matrix [
77]. The increasing co-firing shares led to a reduction of periclase, and it was not detected in fly ash from 100%
Cynara combustion. Deduced is the association of the mineral with the lignite fuel, and its absence in higher co-firing
Cynara shares suggests an improved fly ash quality. Free MgO present above a certain size [
34] slowly hydrates yielding brucite (Mg(OH)
2), causing vast and localized volume increases, leading to deleterious expansion [
34,
75].
The primary agents for hardened concrete’s expansion and cracking from sulfate attack are alumina-bearing hydrates (like calcium monosulfo-aluminate and calcium aluminate hydrate) [
32]. Those hydrates are attacked by the sulfate ion forming ettringite or calcium tri-sulfoaluminate [
32], a high sulfate form [
86], or by forming gypsum or wollastonite [
42]. The gypsum formed reacts with calcium aluminate hydrates (4CaO·Al
2O
3·19H
2O) and calcium alumino-sulfate hydrates (3CaO·CaSO
4·18H
2O), producing the insoluble ettringite that can lead to expansion (from reactions causing expandable pressure) causing deterioration of already hardened concrete due to high tensile stresses, according to the reactions that follow (Equations (17) and (18)) [
42]:
Thus, the co-firing of
Cynara with natural gas is believed to reduce the risks associated with calcium aluminates in the fly ash, as there were no detection of alkali sulfates, despite the extremely high amount of alkalis in the biomass fuels. Anhydrite also participates in the problematic reaction for concrete, but in masonry applications, anhydrite is an important mineral, e.g., for render, mortars, or binders on building sites [
74], as adhesion and binding properties have a precedence.
For the 50% thermal share of
Cynara co-firing, it was observed that mineral phases were similar to the 10% thermal share of fly ash. This is considered to be a significant finding due to the much higher content of problematic ash species in the biomass fuel. However, the merwinite and andradite phases had a relative decrease from peak intensities in the 50%
Cynara co-firing fly ash. Both of those phases are calcium bearing and would have contributions to the reactivity of the fly ash. A relative decrease with a relative increase in calcite may not change the fly ash reactive nature to any significant extent. Merwinite is formed by the reaction below, (Equation (19)) [
1]:
Lime had a somewhat lower peak intensity in the 50% Cynara co-firing case compared to the other scenarios. The observation may be due to interactions of the fuels leading to reduced reaction rates for this thermal share impacting conversion of species or attributed to the increase and formation of other minerals involving Ca and Si. Further work is needed to clarify the observation.
Dolomite and sylvite were detected in the pure biomass combustion fly ash. The presence of dolomite in the fly ash from the pure combustion of
Cynara is a testament to the fuel’s larger particle sizes that require a longer residence time to burnout than design of the boiler for coal particles. Dolomite is formed by the reaction below (Equation (20)) [
87]:
The presence of sylvite in the pure
Cynara combustion is due to the high amount of the species in the biomass and their subsequent reaction in the furnace and condensation in cooler regions. Also, the presence of KCl is attributed to the reduction of ash amount in the fuel blend containing silicates to form compounds with K. Sylvite is formed by the reaction sequences below (Equations (21) and (22)) [
21,
88]:
Ash composition changes during storage and under varying environmental conditions as carbon dioxide and moisture react with ash to form carbonates, bicarbonates, and hydroxides [
89]. Absorption of water on a molecular level is noted to be possibly due to the hydrophilic nature of ash and to small charge imbalances [
89]. Thus, the hydroxide mineral phases for the 100%
Cynara are attributed to the ash being hydrophilic.
The co-fired fly ash qualities generated may be useful were setting times are of concern, as the self-hardening properties can improve the setting time. Suitable applications may be pavements, parking lots, road embankments, and other trafficked areas due to self-hardening characteristics that improve the setting time [
54], bonding, and possibly adhesion qualities, characteristics associated with calcium minerals [
74]. The fly ashes appear to have a high value in masonry applications, such as making bricks, etc. Overall, co-firing a herbaceous biomass up to 50% thermal share with a low-quality lignite that has a high ash content and with natural gas in the fuel blend positively influence the fly ash quality.
3.7. Behavior Characteristics from Performance Test Applications of Some Fly Ashes
The technically quality of how a fly ash performs in a given application is generally good if the performance is equal to or better than the commercial available material for the same application [
16]. A key aspect in accepting the use of an industrial mineral like fly ash is its technical suitability and the ability of a power plant to supply a constant-quality product, which is guaranteed by specifications and certifications [
16]. Thus, the co-fired ash performance in certain applications was compared to the lignite ash.
Soundness testing gives an evaluation of possible impacts on durability in concrete from using the fly ash quality. This assessment of volume expansion (instability) is necessary when assessing long-term performance considerations of using the fly ash, for example, in concrete.
Table 8 shows results of soundness testing of ashes from co-firing 10% and 50% thermal shares of
Cynara compared to the lignite only case.
All fly ash samples tested met the limit for expansion. Shown is the addition of Cynara does not have a negative impact on the long-term stability characteristic compared to the lignite fly ash, and the 50% thermal share even improve the performance compared to the lignite. The apparent improvement shows that a 50% thermal share of co-firing Cynara with a low-quality lignite can improve the fly ash quality. Further long-term studies are needed.
Another technical parameter of importance is the initial setting time. The initial setting time is necessary for assessing when a concrete mixture can be expected to become rigid. The time of set of concrete mixes impacts a construction project schedule. Many factors influence the initial time of setting. Some factors are listed below [
49]:
The fly ash may contain high calcium amounts (self-hardening);
Fly ash may contain sulfates that react with cement;
The mixture may contain less water due to the presence of fly ash, and this will influence the process of hardening;
The fly ash may impede surface-active agents added to modify the rheology (water reducers) of concrete, and again this impacts the stiffness of mortar; and
Fly ash particles may act as nuclei for crystallization of cement hydration products.
In
Table 9 are shown initial setting times results of some tested fly ashes. A control sample is shown for enhancing comparisons. The impact of
Cynara in the fuel blend is apparent.
Table 9 shows that co-firing
Cynara improved the setting time performance of the fly ashes. The improvement is also expected with the 50% thermal share, although not tested due to the unavailable material. The improvement in setting time with
Cynara addition could be a result of reduced compounds in the fly ash that do not have good self-hardening properties an enhanced burning of the fuel blend with the biomass that contribute to an increase in mineral phases with hydraulic properties, i.e., anorthite (CaAl
2Si
2O
8) was shown in
Table 7, or an improved reactivity of the fly ash from a finer particle size distribution, as was shown in
Section 3.3.
Strength is the most important parameter used to characterize cement-based products; thus, the determination of compressive strength is the most important property of construction materials [
90], mainly concrete as a structural material [
27]. Compressive strength is widely used as an index of all other types of strength categories [
91]. The experience with using fly ash in structural concrete has seen about 30% substitution of the binder, where the possibility is shown for a similar or improved performance in comparison to OPC concrete of equivalent 28-day strength [
32]. The development of compressive strength on the fly ash generated from the lignite baseline case and the 10% thermal share of
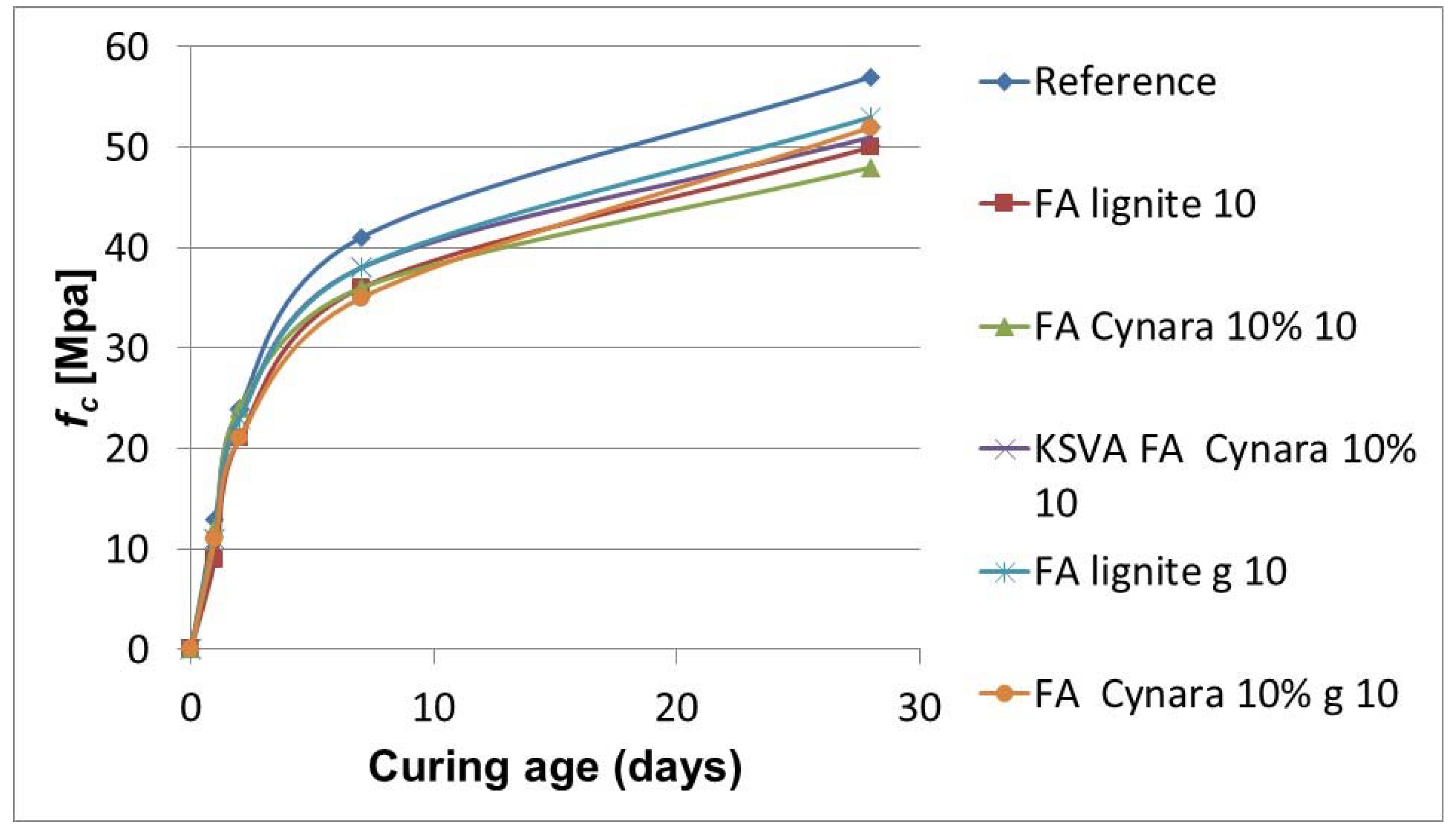
Cynara are evaluated in
Figure 19 for a 10% cement substitution. Also, a comparison of the results to the same type of tests for fly ash from the industrial Kardia Power Plant was performed. Characterization of strength development occurred for 1, 2, 7 and 28 days.
Figure 19 shows that the 10% cement substitution of
Cynara fly ash from a 10% thermal share produces a fly ash with nearly the same early strength development behavior and a similar 28-day strength development compared with the lignite fly ash. After grinding the fly ashes, the 28-day strength for the 10% thermal share of
Cynara fly ash at a 10% cement substitution has nearly the same strength value as the pure lignite combustion fly ash. Also, after grinding, the 28-day strength for the 10% thermal share of
Cynara at a 10% cement substitution has a 28-day strength development very close to the pilot-scale 10% thermal share
Cynara fly ash at a 10% cement substitution.
Grinding of fly ashes is a way to reveal their pozzolanic and hydraulic properties [
92]. Results indicate that a 10% thermal share of
Cynara in the fuel blend for a low-quality lignite would have the same behavior as a cement substitution where a lignite only fly ash applies. Also, from the results, ash obtained from the pilot-scale tests are considered to be representative of industrial fly ash behaviors.
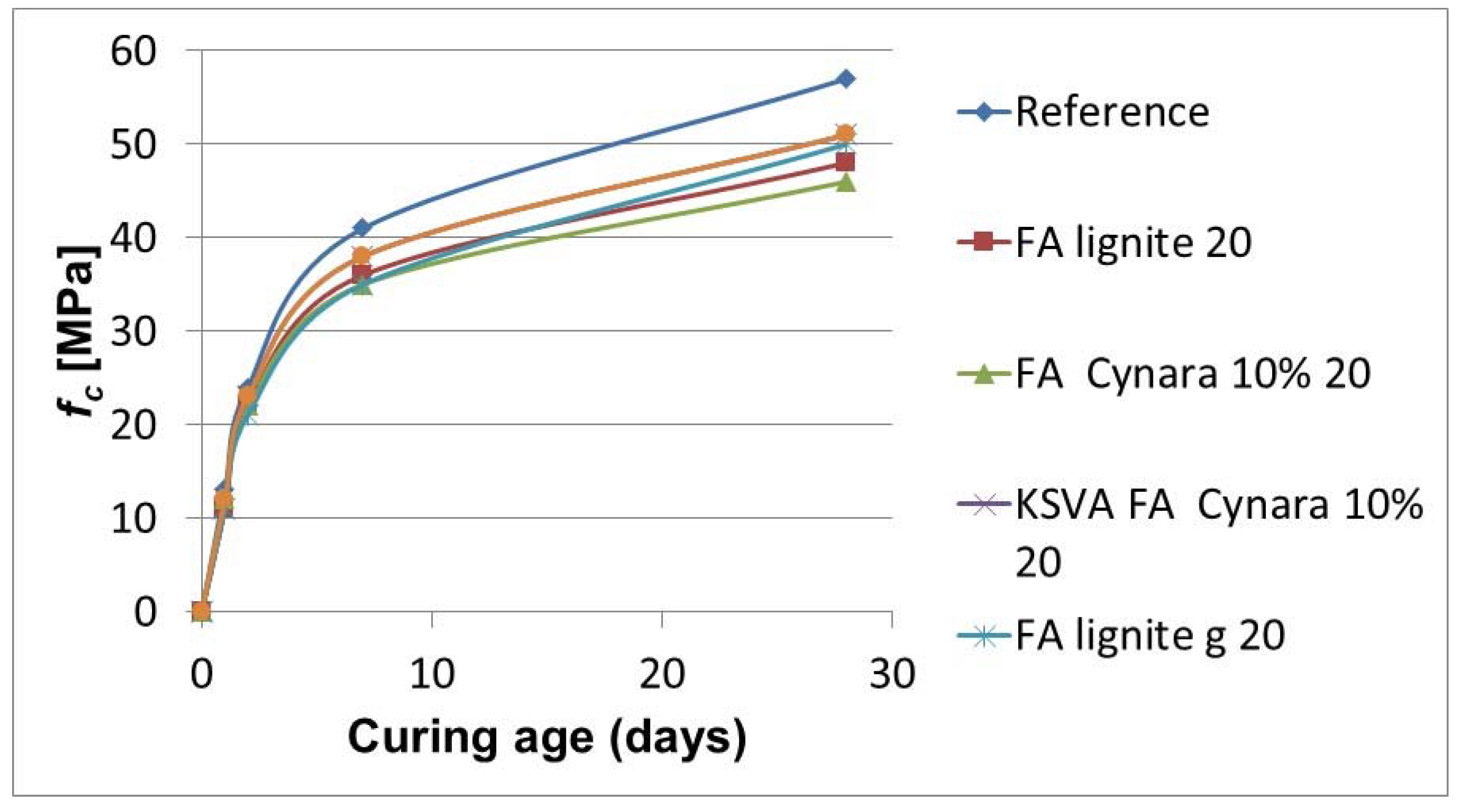
Figure 20 shows strength development with substituting 20% of the cement with fly ash.
The 20% cement substitution in
Figure 20 shows the same trend for strength development as the 10% cement replacement. Also, the values are very close, but with a noticeable slight increased variation. After grinding, the performance of the 10% thermal share fly ash at 20% cement substitution is the same as the lignite fly ash. Again, this shows the added value in a small co-firing proportion of a herbaceous biomass with a low-quality lignite on the fly ash quality. A higher cement substitution rate contributes more to mitigating CO
2 emissions from the manufacture of cement.
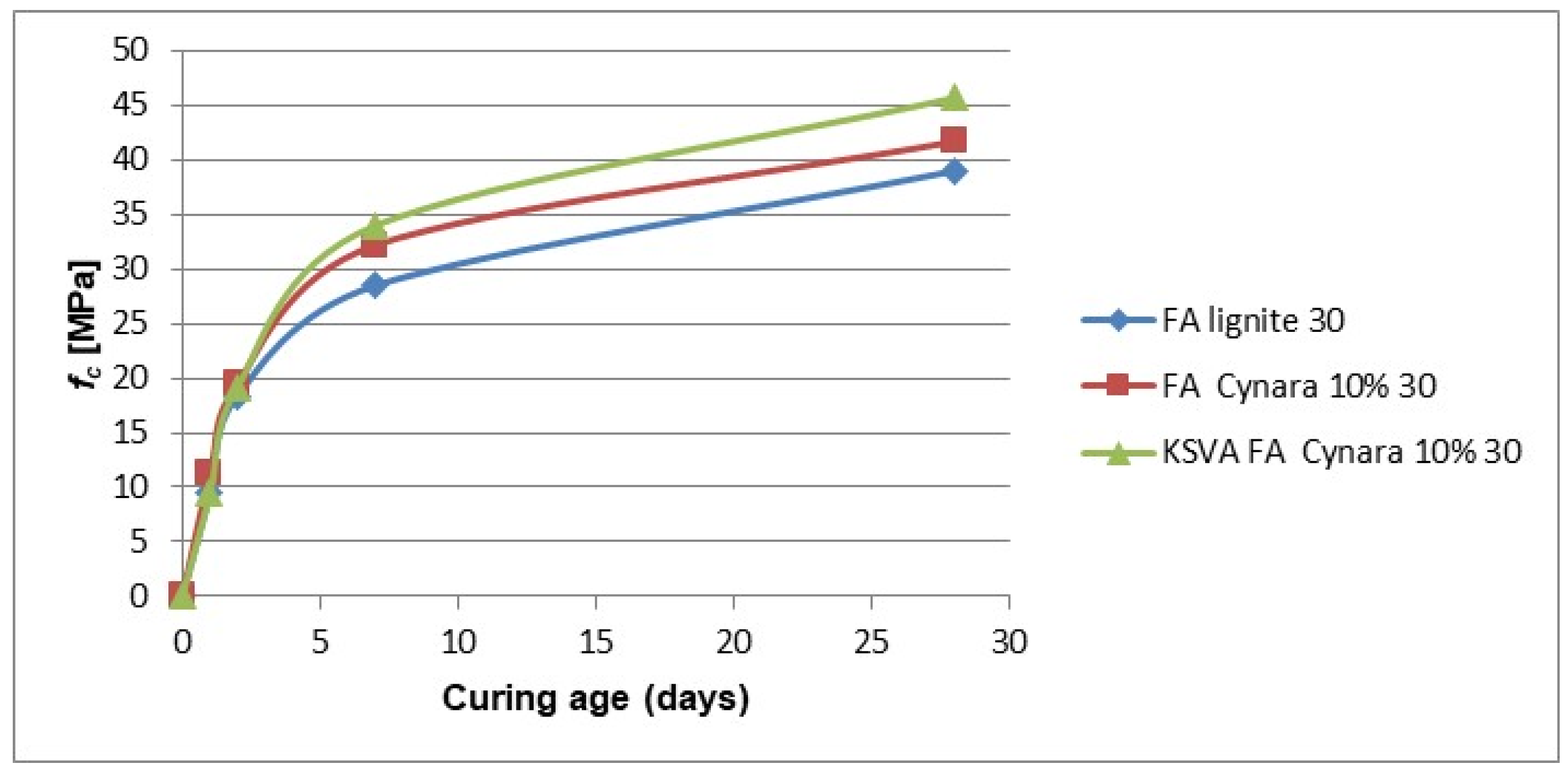
Figure 21 shows the comparison of strength development for 10% thermal share of
Cynara fly ash from KSVA with industrial fly ashes with a 30% cement substitution.
The values for the 30% cement substitution are lower than the other values, as expected due to the presence of less cement in the mix for hydration to supply Ca(OH)
2 needed to react with the pozzolanic fractions of the ash. So, it will take a longer time to start the reactions with fly ash, as the pH required is reached a later time compared to the other lower substitution rates. The industrial fly ash was not ground and compared, but the trend of exact values for the pilot-scale (KSVA) results should be the same, like the results for the other substitutions. An optimal replacement amount for utilization in concrete applications (with a technical approval) is likely at about 25% cement replacement, which is the practice according to EN 450-1 for bituminous coal ashes or ashes from co-firing particular green biomass fuel with bituminous coals. On the other hand, many masonry applications do not have the strict material provisions and conservative design like EN 450-1. Many masonry applications allow one to choose the components of a binder to meet specific performance requirements, e.g., EN 998-1, focusing on binding and adhesion properties. So, performance studies of the fly ash qualities in masonry applications could result in a significant higher amount of cement being replaced by the fly ash qualities. That would be a huge benefit as the total energy required based on kJ/kg of material is 372 and 0 for cement and fly ash, respectively [
75].
From the results, a high thermal share of Cynara does not appear to change the fly ash quality for economic utilization applications, despite the significantly higher contents of problematic ash components. At 50% thermal share of Cynara, the amount is likely able to meet a good fuel sustainable supply, easy handling and storage capabilities, efficient milling and feeding capabilities, and a normal boiler operation and performance. Thus, it is believed that a co-firing 50% thermal share of Cynara can be employed based upon the overall presented results without having negative impacts on the fly ash quality. This would also stimulate local and regional economies and may be a catalyst to promote trade between countries for such a biomass type to co-fire with lignite fuels. Quality fly ash standards requiring a minimum of co-firing of an herbaceous biomass with a low-quality lignite fuel on a thermal share basis would have far reaching positive benefits across environmental sustainability practices in lignite fuel combustion and biomass ash management. Such legislation can be employed regionally, nationally, EU-wide, or even internationally through European and ISO standards. However, such a practice needs assessment on a case basis for herbaceous biomass fuel types, since logistics and fuel supply would take priority, along with boiler performance concerns.
The largest use of ashes in Greece is in the cement industry to replace cement clinker and production of special cements, which include successful tests in road construction, mortars, waste treatment, embankments, and cement grouting [
92]. From results, it is believed a co-firing share of up to 50% of
Cynara biomass does not change those utilization options when potentially substituted up to 30% by mass of the cement content. Further long-term studies are needed.
From the technical performance test results, it is believed that co-firing of the
Cynara biomass with a low-quality lignite would be added value to a lignite power plant not operating at boiler load swings, which would be enhanced at a boiler de-rate with the inclusion of natural gas into the co-firing fuel mix. However, at lower loads there may be an impact on fuel/gas mixing due to changes in velocity and momentum [
60,
93]. Changes in running conditions of boilers result in variations of fly ash quality, as adjustments to the loading of boilers regularly have to take place to meet the changing demand for electricity, and the coexistence of alternative electricity generating options give rise to the policy of rapidly changing between supply sources for an optimal economy [
16]. Co-firing of
Cynara with a low-quality lignite in a boiler subjected to fluctuating operational conditions can possibly lead to keeping the variation of inhomogeneous fly ash to an acceptable condition, as the co-fired fly ashes had an equally comparable or better technical performance than the compared commercial material or baseline fly ash quality. This may be enhanced with the addition of natural gas into the fuel blend due to higher temperatures reached and a potential reduction of LOI values in the fly ash.