Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge
Abstract
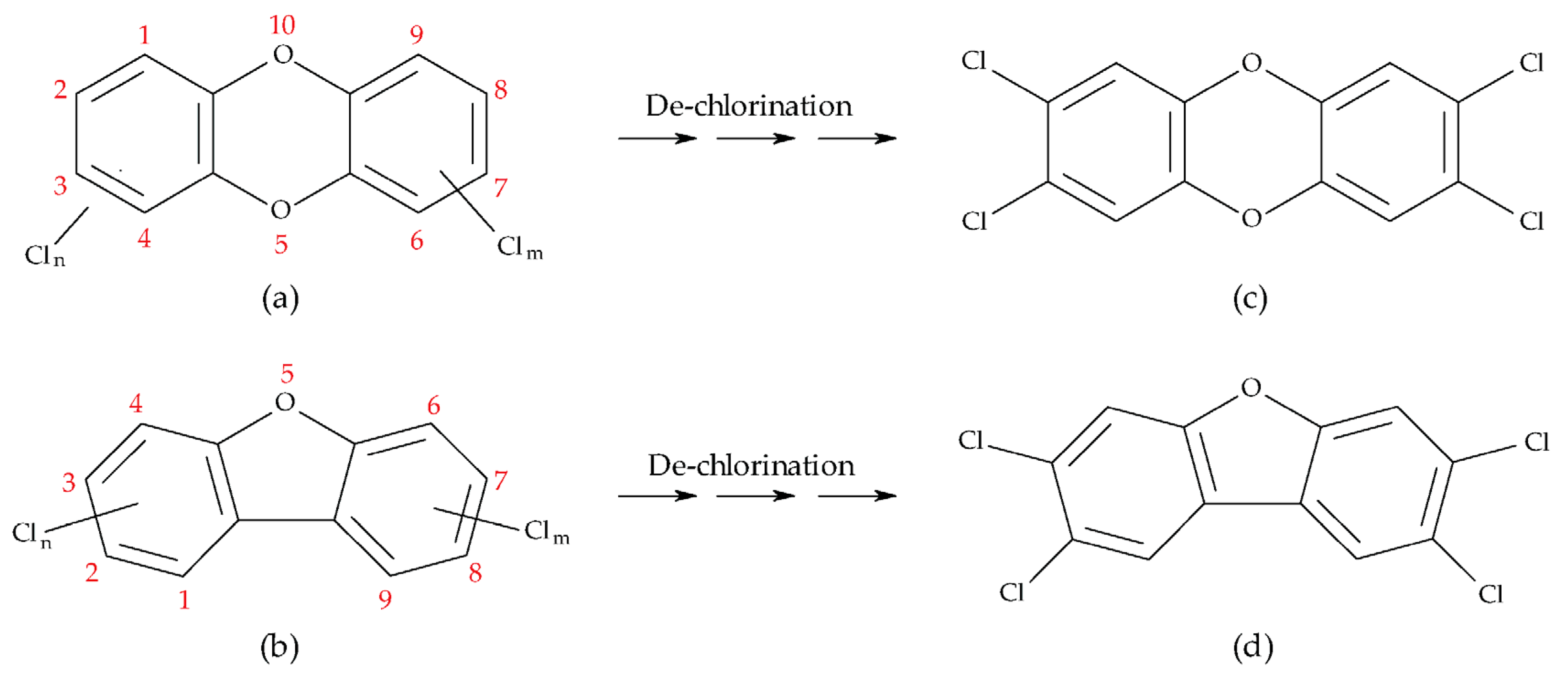
1. Introduction
2. Results
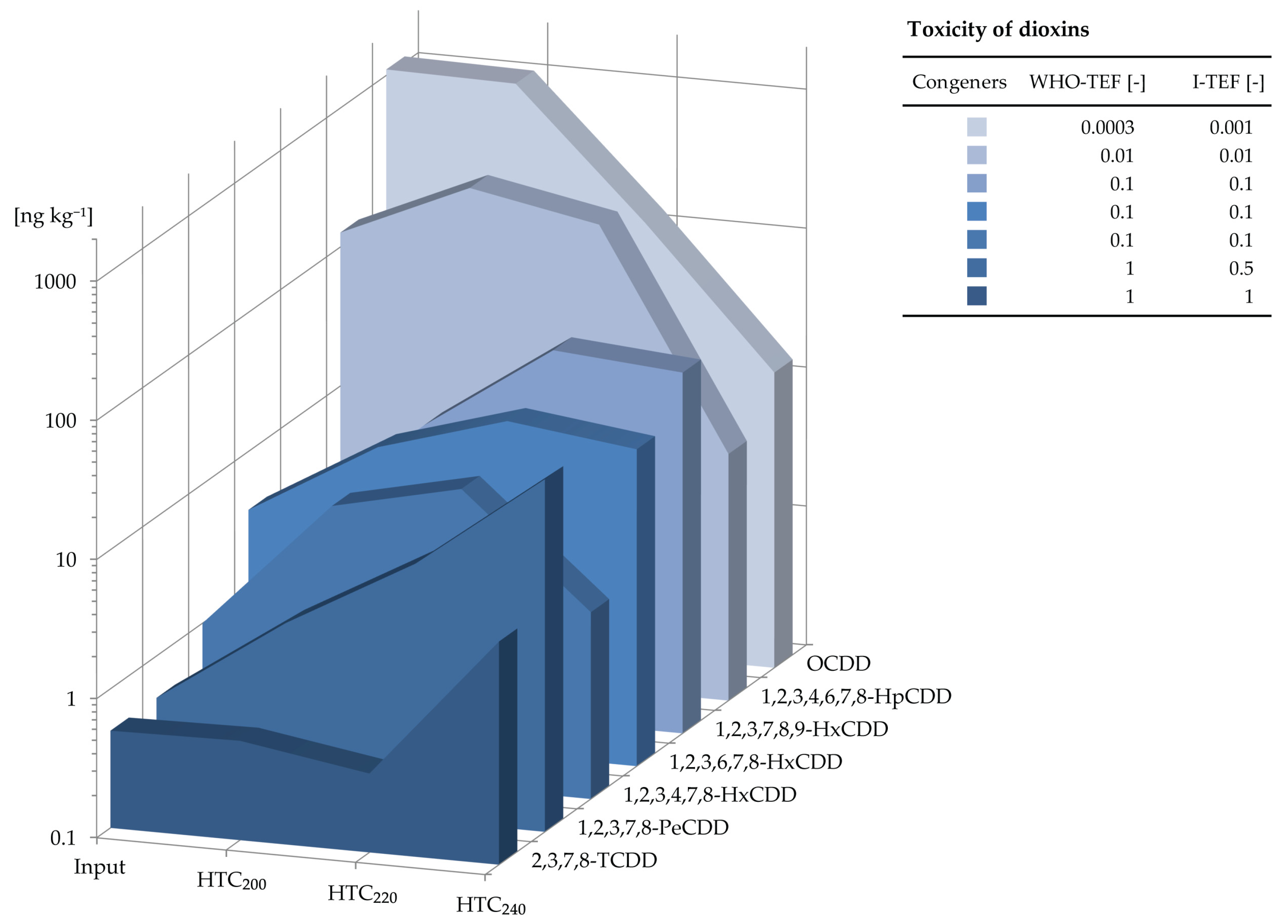
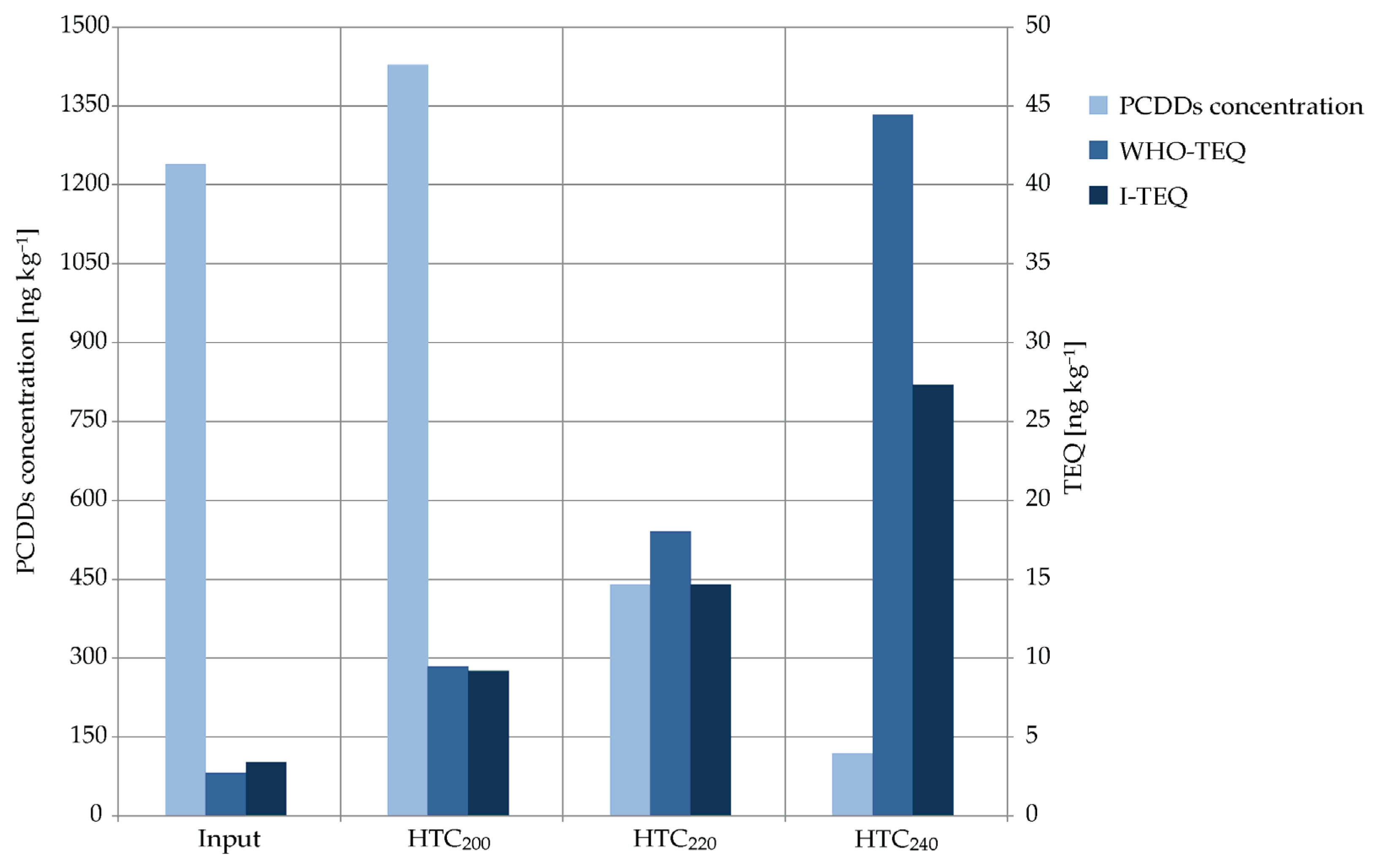
2.1. PCDD-Concentrations and Their Toxicity
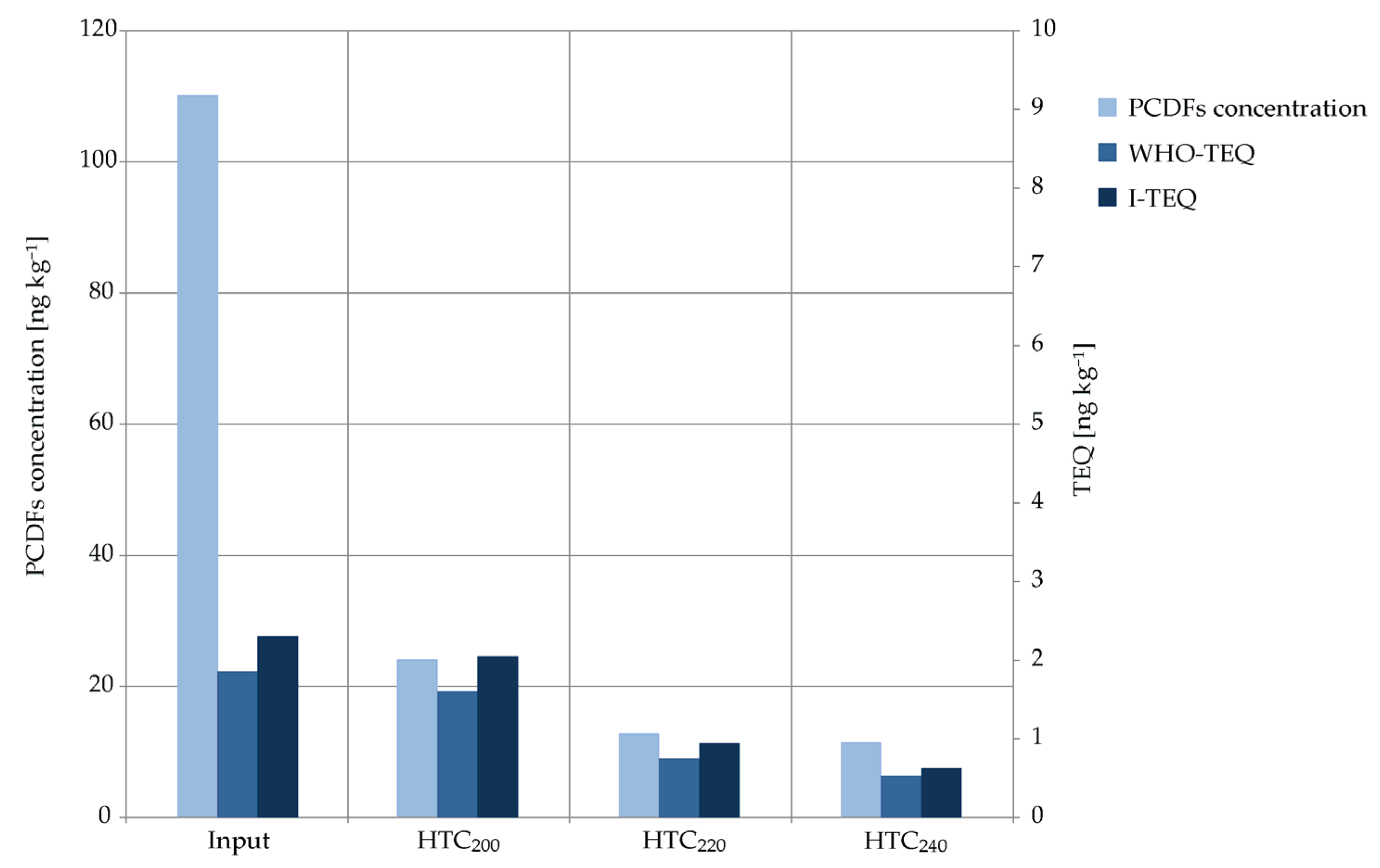
2.2. PCDF-Concentrations and Their Toxicity
2.3. PCB-Concentrations and Their Toxicity
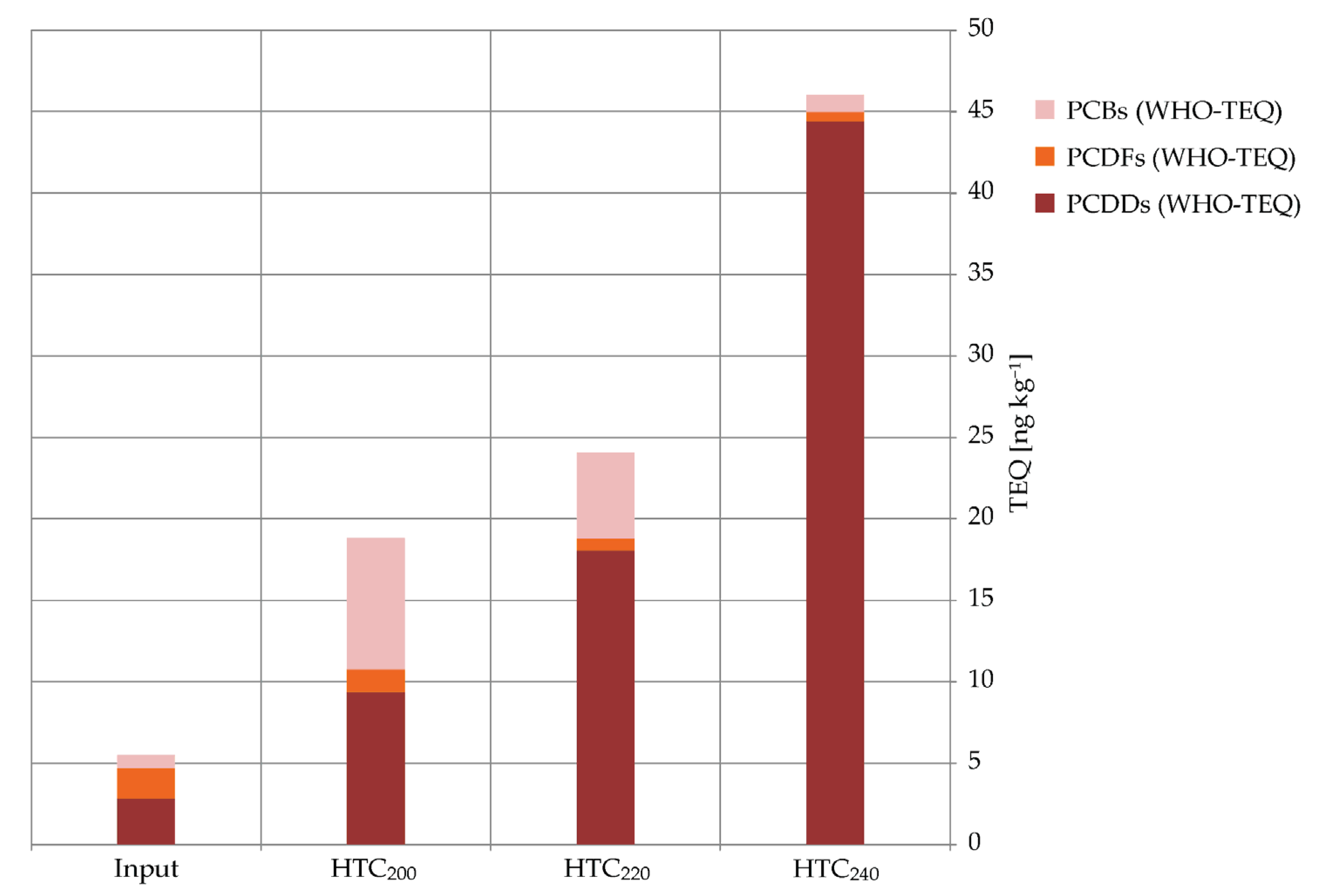
2.4. Total TEQ of the PCBs, PCDFs, and PCDD
3. Discussion
4. Materials and Methods
4.1. HTC-Autoclaves
4.2. Sewage Sludge
4.3. Analysis of PCDD/Fs and Coplanar PCBs
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Congeners | WHO-TEF [-] | I-TEF [-] | Congeners | WHO-TEF [-] | I-TEF [-] | |
|---|---|---|---|---|---|---|
| OCDD | 0.0003 | 0.001 | 2,3,3′,4,4′,5,5′-HpCB (189) | 0.00003 | - | |
| 1,2,3,4,6,7,8-HpCDD | 0.01 | 0.01 | 3,3′,4,4′,5,5′-HxCB (169) | 0.03 | - | |
| 1,2,3,7,8,9-HxCDD | 0.1 | 0.1 | 2,3′,4,4′,5,5′-HxCB (167) | 0.00003 | - | |
| 1,2,3,6,7,8-HxCDD | 0.1 | 0.1 | 2,3,3′,4,4′,5′-HxCB (157) | 0.00003 | - | |
| 1,2,3,4,7,8-HxCDD | 0.1 | 0.1 | 2,3,3′,4,4′,5-HxCB (156) | 0.00003 | - | |
| 1,2,3,7,8-PeCDD | 1 | 0.5 | 3,3′,4,4′,5-PeCB (126) | 0.1 | - | |
| 2,3,7,8-TCDD | 1 | 1 | 2′,3,4,4′,5-PeCB (123) | 0.00003 | - | |
| OCDF | 0.0003 | 0.001 | 2,3′,4,4′,5-PeCB (118) | 0.00003 | - | |
| 1,2,3,4,7,8,9-HpCDF | 0.01 | 0.01 | 2,3,4,4′,5-PeCB (114) | 0.00003 | - | |
| 1,2,3,4,6,7,8-HpCDF | 0.01 | 0.01 | 2,3,3′,4,4′-PeCB (105) | 0.00003 | - | |
| 2,3,4,6,7,8-HxCDF | 0.1 | 0.1 | 3,4,4′,5-TCB (81) | 0.00003 | - | |
| 1,2,3,7,8,9-HxCDF | 0.1 | 0.1 | 3,3′,4,4′-TCB (77) | 0.00001 | - | |
| 1,2,3,6,7,8-HxCDF | 0.1 | 0.1 | ||||
| 1,2,3,4,7,8-HxCDF | 0.1 | 0.1 | ||||
| 2,3,4,7,8-PeCDF | 0.05 | 0.05 | ||||
| 1,2,3,7,8-PeCDF | 0.5 | 0.5 | ||||
| 2,3,7,8-TCDF | 0.1 | 0.1 |
References
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Verordnung über das Inverkehrbringen von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln. (Düngemittelverordnung—DüMV); Bundesministerium der Justiz und für Verbraucherschutz: Berlin, Germany, 2017.
- Gemeinschaftsveröffentlichung der DWA (Deutsche Vereinigung für Wasserwirtschaft; Abwasser und Abfall e. V. und des Statistischen Bundesamtes). Abwasser und Klärschlamm in Deutschland-Statistische Betrachtungen: Teil 2: Klärschlamm, Klärgas, Rechen-und Sandfanggut; The Federal Statistical Office: Wiesbaden, Germany, 2015.
- Wiechmann, B.; Dienemann, C.; Kabbe, C.; Brandt, S.; Vogel, I.; Roskosch, A. Sewage Sludge Management in Germany; German Environmental Agency: Dessau-Roßlau, Germany, 2015.
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787–789. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Bioref. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Dahmen, N. Hydrothermal biomass conversion: Quo vadis? J. Supercrit. Fluids 2017. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lucian, M.; Fiori, L. Hydrothermal Carbonization of Waste Biomass: Process Design, Modeling, Energy Efficiency and Cost Analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Fiori, L.; Basso, A.; Castelloa, D.; Baratierib, M. Hydrothermal Carbonization of Biomass: Design of a Batch Reactor and Preliminary Experimental Results. Chem. Eng. Trans. 2014, 37. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef]
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.-D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Tirler, W.; Basso, A. Resembling a “natural formation pattern” of chlorinated dibenzo-p-dioxins by varying the experimental conditions of hydrothermal carbonization. Chemosphere 2013, 93, 1464–1470. [Google Scholar] [CrossRef] [PubMed]
- Conte, P.; Hanke, U.M.; Marsala, V.; Cimo, G.; Alonzo, G.; Glaser, B. Mechanisms of water interaction with pore systems of hydrochar and pyrochar from poplar forestry waste. J. Agric. Food Chem. 2014, 62, 4917–4923. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.; Schimmelpfennig, S.; Jansen-Willems, A.; Moser, G.; Kammann, C.; Müller, C. Microbial community shifts 2.6 years after top dressing of Miscanthus biochar, hydrochar and feedstock on a temperate grassland site. Plant Soil 2015, 397, 261–271. [Google Scholar] [CrossRef]
- Egle, L.; Rechberger, H.; Krampe, J.; Zessner, M. Phosphorus recovery from municipal wastewater: An integrated comparative technological, environmental and economic assessment of P recovery technologies. Sci. Total Environ. 2016, 571, 522–542. [Google Scholar] [CrossRef] [PubMed]
- Cordell, D.; Drangert, J.-O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus management in Europe in a changing world. Ambio 2015, 44 (Suppl. 2), 92. [Google Scholar] [CrossRef] [PubMed]
- Federal Law Gazette I Sewage Sludge Ordinance (Verordnung über Die Verwertung von Klärschlamm, Klärschlammgemisch und Klärschlammkompost. (Klärschlammverordnung—AbfKlärV)); Federal Law Gazette I: Berlin, Germany, 2017.
- Wiedner, K.; Naisse, C.; Rumpel, C.; Pozzi, A.; Wieczorek, P.; Glaser, B. Chemical modification of biomass residues during hydrothermal carbonization—What makes the difference, temperature or feedstock? Org. Geochem. 2013, 54, 91–100. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.-D. Hydrothermal carbonization of poly(vinyl chloride). Chemosphere 2015, 119, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, H. Existierende dioxininventare weltweit und neue methodik zur erstellung von vergleichbaren und vollständigen emissionsinventaren. UWSF Z Umweltchem Ökotox 2001, 13, 88–94. [Google Scholar] [CrossRef]
- Behnke, A.; Böhnhardt, A.; Bernd, M.; Bussian, K.B.; Dauert, U.; Fettig, I.; Gärtner, P.; Giese, E.; Juhrich, K.; Konietzka, R.; et al. Dioxine und Dioxinähnliche PCB in Umwelt und Nahrungsketten; Umweltbundesamt: Dessau, Germany, 2017. [Google Scholar]
- Rogers, H.R. Sources, behaviour and fate of organic contaminants during sewage treatment and in sewage sludges. Sci. Total Environ. 1996, 185, 3–26. [Google Scholar] [CrossRef]
- USEPA. Statistical Support Document for the Development of Round 2 Biosolids Use or Disposal Regulations; USEPA: Washington, DC, USA, 2002.
- Dai, J.; Xu, M.; Chen, J.; Yang, X.; Ke, Z. PCDD/F, PAH and heavy metals in the sewage sludge from six wastewater treatment plants in Beijing, China. Chemosphere 2007, 66, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; de Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L.; et al. The 2005 World Health Organization reevaluation of human and Mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Kutz, F.W.; Barnes, D.G.; Bottimore, D.P.; Greim, H.; Bretthauer, E.W. The international toxicity equivalency factor (I-TEF) method of risk assessment for complex mixtures of dioxins and related compounds. Chemosphere 1990, 20, 751–757. [Google Scholar] [CrossRef]
- Basso, D.; Ragazzi, M.; Rada, E.C.; Fiori, L. Release of PCDD/Fs during a hydrothermal carbonization process of organic waste residues. UPB Sci. Bull. Ser. D Mech. Eng. 2014, 76, 199–212. [Google Scholar]
- Janßen, E. Umweltanalytik; VDLUFA-Verl.: Darmstadt, Germany, 2011. [Google Scholar]

| Persistent Organic Pollutants | Input | Hydrochar 200 °C,5 h | Hydrochar 220 °C,5 h | Hydrochar 240 °C,5 h | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content | WHO-TEQ 2 | Content 1 | Δ Input | WHO-TEQ 2 | Content 1 | Δ Input | WHO-TEQ 2 | Content 1 | Δ Input | WHO-TEQ 2 | ||||||
| Congeners | [ng/kg] | [ng/kg] | [ng/kg] | [%] | [ng/kg] | [ng/kg] | [%] | [ng/kg] | [ng/kg] | [%] | [ng/kg] | |||||
| PCDDs | 2,3,7,8-TCDD | <0.5 | 0.25 | 0.5 | 104 | 0.52 | 0.369 | 74 | 0.37 | 4.02 | 804 | 4.02 | ||||
| 1,2,3,7,8-PeCDD | <0.5 | 0.25 | 2.1 | 423 | 2.12 | 6.92 | 1383 | 6.92 | 34.3 | 6861 | 34.3 | |||||
| 1,2,3,4,7,8-HxCDD | <1 | 0.05 | 8.5 | 854 | 0.85 | 13.8 | 1385 | 1.38 | 2.2 | 220 | 0.22 | |||||
| 1,2,3,6,7,8-HxCDD | 3.8 | 0.38 | 13.1 | 346 | 1.31 | 24.8 | 652 | 2.48 | 19 | 501 | 1.90 | |||||
| 1,2,3,7,8,9-HxCDD | 1.9 | 0.19 | 11 | 576 | 1.1 | 46.6 | 2451 | 4.66 | 39.4 | 2073 | 3.94 | |||||
| 1,2,3,4,6,7,8-HpCDD | 127 | 1.27 | 326 | 256 | 3.26 | 217 | 171 | 2.17 | 5.95 | 5 | 0.059 | |||||
| OCDD | 1104 | 0.331 | 1067 | 97 | 0.32 | 131 | 12 | 0.039 | 13.3 | 1 | 0.004 | |||||
| Σ PCDDs | 1238 | 2.72 | 1428 | 9.47 | 440 | 18 | 118 | 44.5 | ||||||||
| PCDFs | 2 ,3,7,8-TCDF | 1.7 | 0.17 | 2.18 | 128 | 0.218 | 2.28 | 134 | 0.228 | 2.03 | 119 | 0.203 | ||||
| 1,2,3,7,8-PeCDF | 1.1 | 0.033 | 1.16 | 106 | 0.035 | 0.575 | 52 | 0.017 | <0.79 | 72 | 0.012 | |||||
| 2,3,4,7,8-PeCDF | 1.9 | 0.57 | 2.07 | 109 | 0.622 | 0.909 | 48 | 0.273 | 0.436 | 23 | 0.131 | |||||
| 1,2,3,4,7,8-HxCDF | 2.5 | 0.25 | 1.83 | 73 | 0.183 | <1.1 | 44 | 0.055 | <0.5 | 20 | 0.025 | |||||
| 1,2,3,6,7,8-HxCDF | 2.1 | 0.21 | 1.69 | 80 | 0.169 | <0.5 | 24 | 0.025 | <0.5 | 24 | 0.025 | |||||
| 1,2,3,7,8,9-HxCDF | 2.3 | 0.23 | <0.68 | 30 | 0.034 | <0.5 | 22 | 0.025 | <1.26 | 55 | 0.063 | |||||
| 2,3,4,6,7,8-HxCDF | <1 | 0.05 | 2.72 | 272 | 0.272 | 1.02 | 102 | 0.102 | <1.02 | 102 | 0.051 | |||||
| 1,2,3,4,6,7,8-HpCDF | 30.4 | 0.304 | 6.17 | 20 | 0.062 | <2 | 7 | 0.01 | <2 | 7 | 0.01 | |||||
| 1,2,3,4,7,8,9-HpCDF | 2 | 0.02 | 2 | 100 | 0.01 | <2 | 100 | 0.01 | <2 | 100 | 0.01 | |||||
| OCDF | 65.6 | 0.02 | 10 | 15 | 0.0015 | <10 | 15 | 0.0015 | <10 | 15 | 0.0015 | |||||
| Σ PCDFs | 110 | 1.86 | 24.2 | 1.61 | 12.8 | 0.75 | 11.5 | 0.53 | ||||||||
| PCBs | PCB 77 | 154 | 0.015 | 226 | 146 | 0.023 | 335 | 218 | 0.034 | 861 | 559 | 0.086 | ||||
| PCB 81 | <20 | 0.003 | <20 | 100 | 0.003 | <20 | 100 | 0.003 | <20 | 100 | 0.003 | |||||
| PCB 105 | 689 | 0.021 | 609 | 88 | 0.018 | 731 | 106 | 0.022 | 679 | 99 | 0.020 | |||||
| PCB 114 | 53.8 | 0.002 | 56.7 | 105 | 0.002 | 49.3 | 92 | 0.001 | 20.3 | 38 | 0.001 | |||||
| PCB 118 | 2553 | 0.077 | 1909 | 75 | 0.057 | 2281 | 89 | 0.068 | 2168 | 85 | 0.065 | |||||
| PCB 123 | 49.1 | 0.001 | 19.5 | 40 | 0.001 | 60.6 | 123 | 0.002 | 126 | 257 | 0.004 | |||||
| PCB 126 | 5.81 | 0.581 | 77.4 | 1332 | 7.74 | 51.1 | 880 | 5.11 | <3 | 52 | 0.15 | |||||
| PCB 156 | 749 | 0.022 | 842 | 112 | 0.025 | 350 | 47 | 0.011 | 18.8 | 3 | 0.001 | |||||
| PCB 157 | 88.8 | 0.003 | 118 | 132 | 0.004 | 135 | 152 | 0.004 | 123 | 139 | 0.004 | |||||
| PCB 167 | 310 | 0.009 | 331 | 107 | 0.01 | 369 | 119 | 0.011 | 311 | 100 | 0.009 | |||||
| PCB 169 | <3 | 0.045 | 5.75 | 192 | 0.172 | <3 | 100 | 0.045 | 23.9 | 795 | 0.716 | |||||
| PCB 189 | 144 | 0.004 | 131 | 91 | 0.004 | 58.1 | 40 | 0.002 | <30 | 21 | 0.000 | |||||
| Σ PCBs | 4808 | 0.78 | 4334 | 8.06 | 4431 | 5.31 | 4357 | 1.06 | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brookman, H.; Gievers, F.; Zelinski, V.; Ohlert, J.; Loewen, A. Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge. Energies 2018, 11, 1582. https://doi.org/10.3390/en11061582
Brookman H, Gievers F, Zelinski V, Ohlert J, Loewen A. Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge. Energies. 2018; 11(6):1582. https://doi.org/10.3390/en11061582
Chicago/Turabian StyleBrookman, Heiner, Fabian Gievers, Volker Zelinski, Jan Ohlert, and Achim Loewen. 2018. "Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge" Energies 11, no. 6: 1582. https://doi.org/10.3390/en11061582
APA StyleBrookman, H., Gievers, F., Zelinski, V., Ohlert, J., & Loewen, A. (2018). Influence of Hydrothermal Carbonization on Composition, Formation and Elimination of Biphenyls, Dioxins and Furans in Sewage Sludge. Energies, 11(6), 1582. https://doi.org/10.3390/en11061582





