A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques
Abstract
1. Introduction
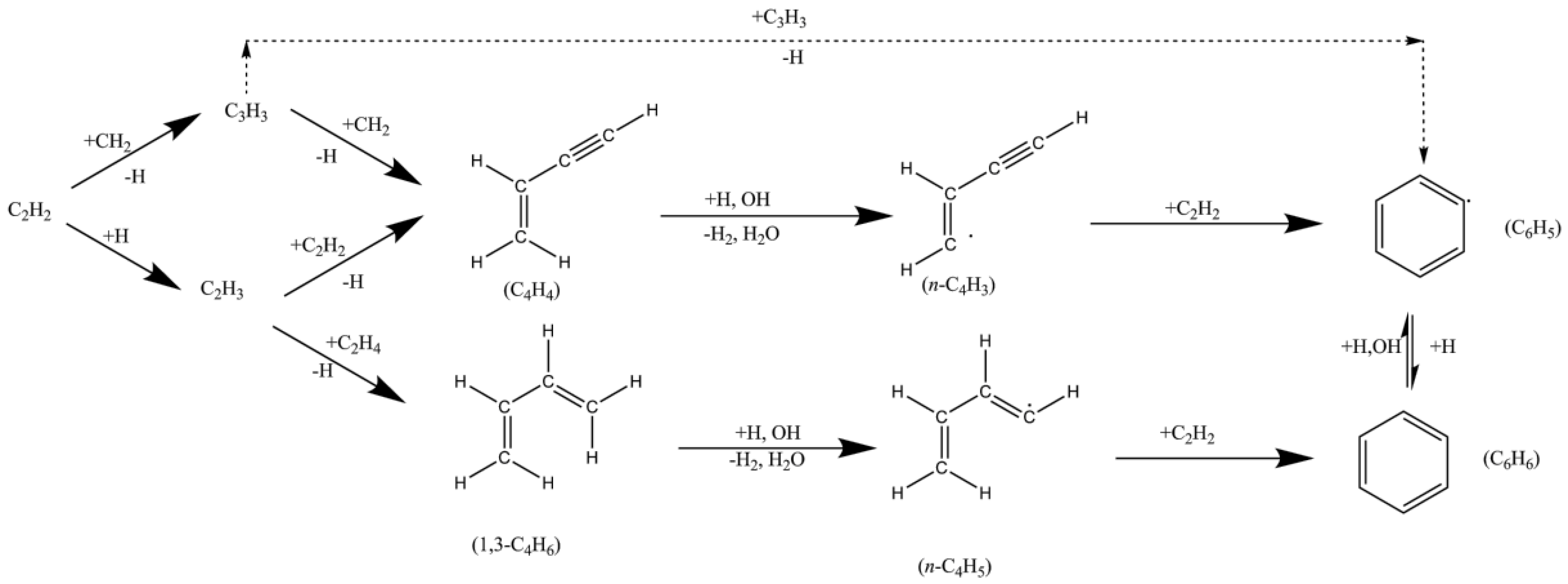
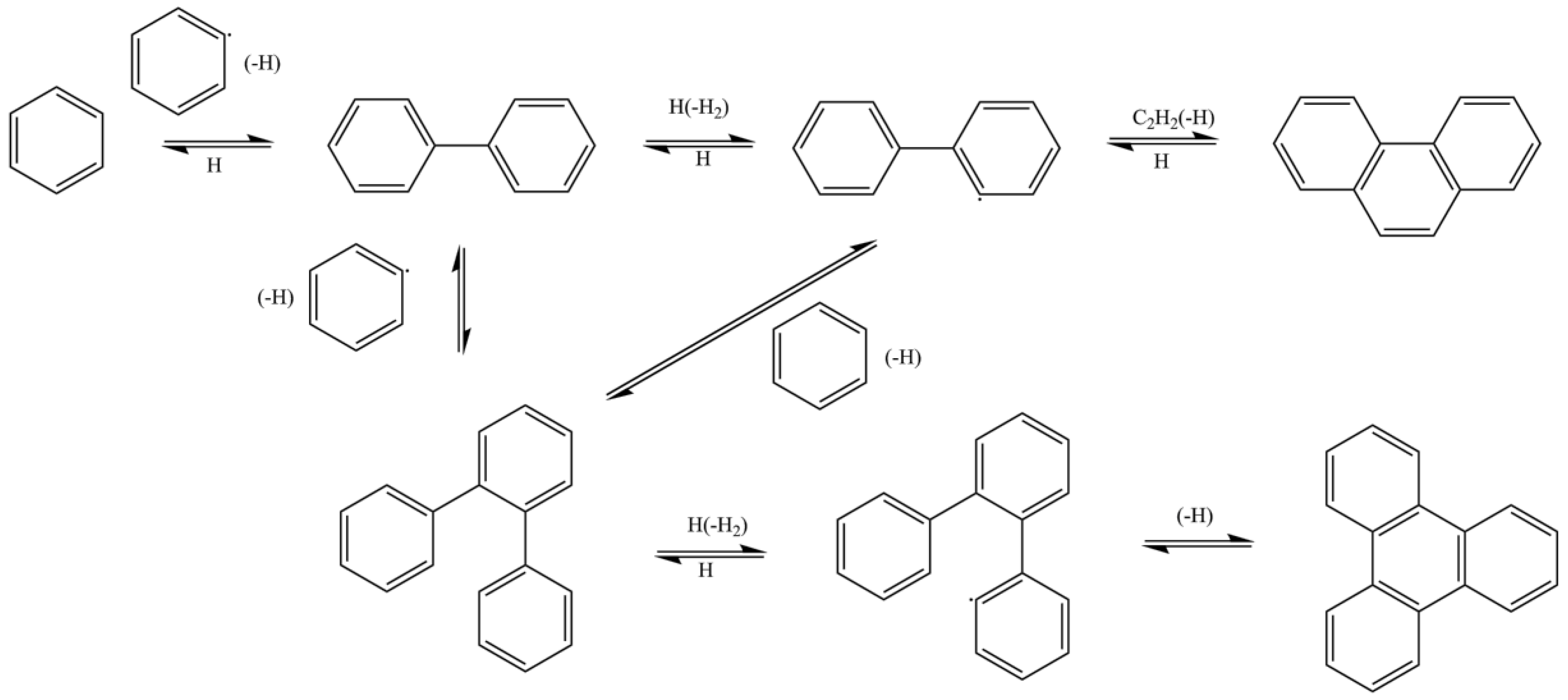
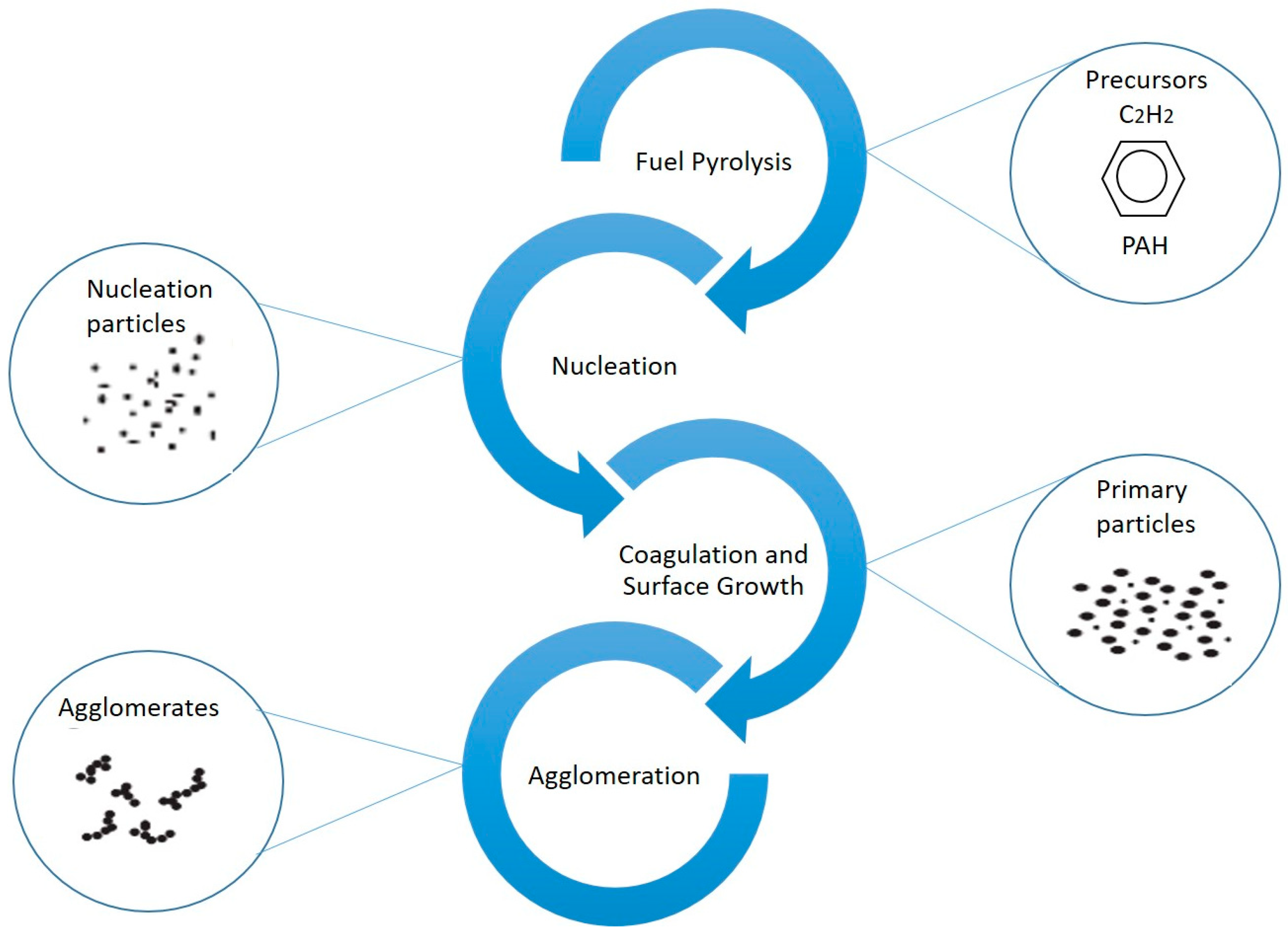
2. Particle Formation
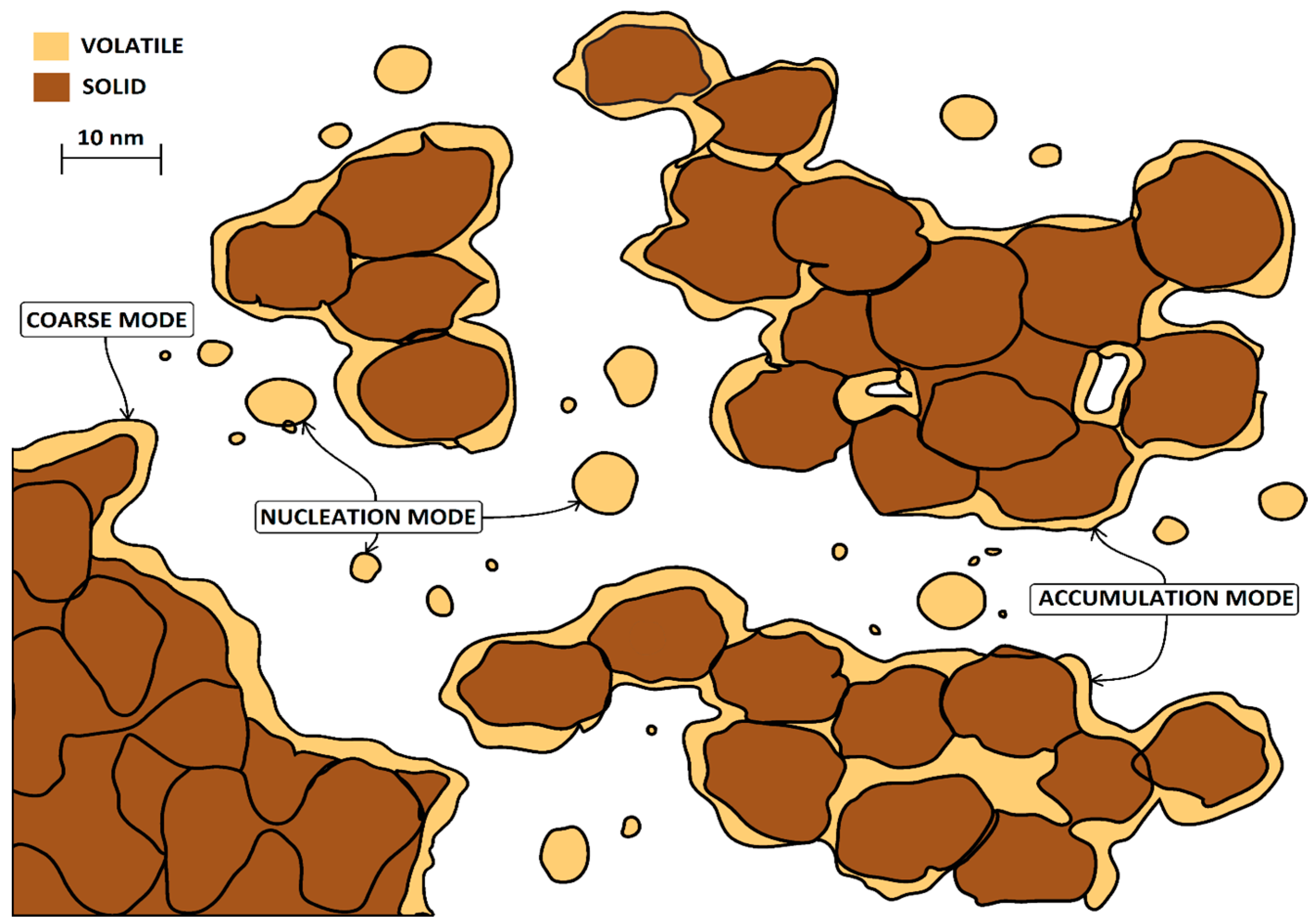
2.1. Formation Mechanisms
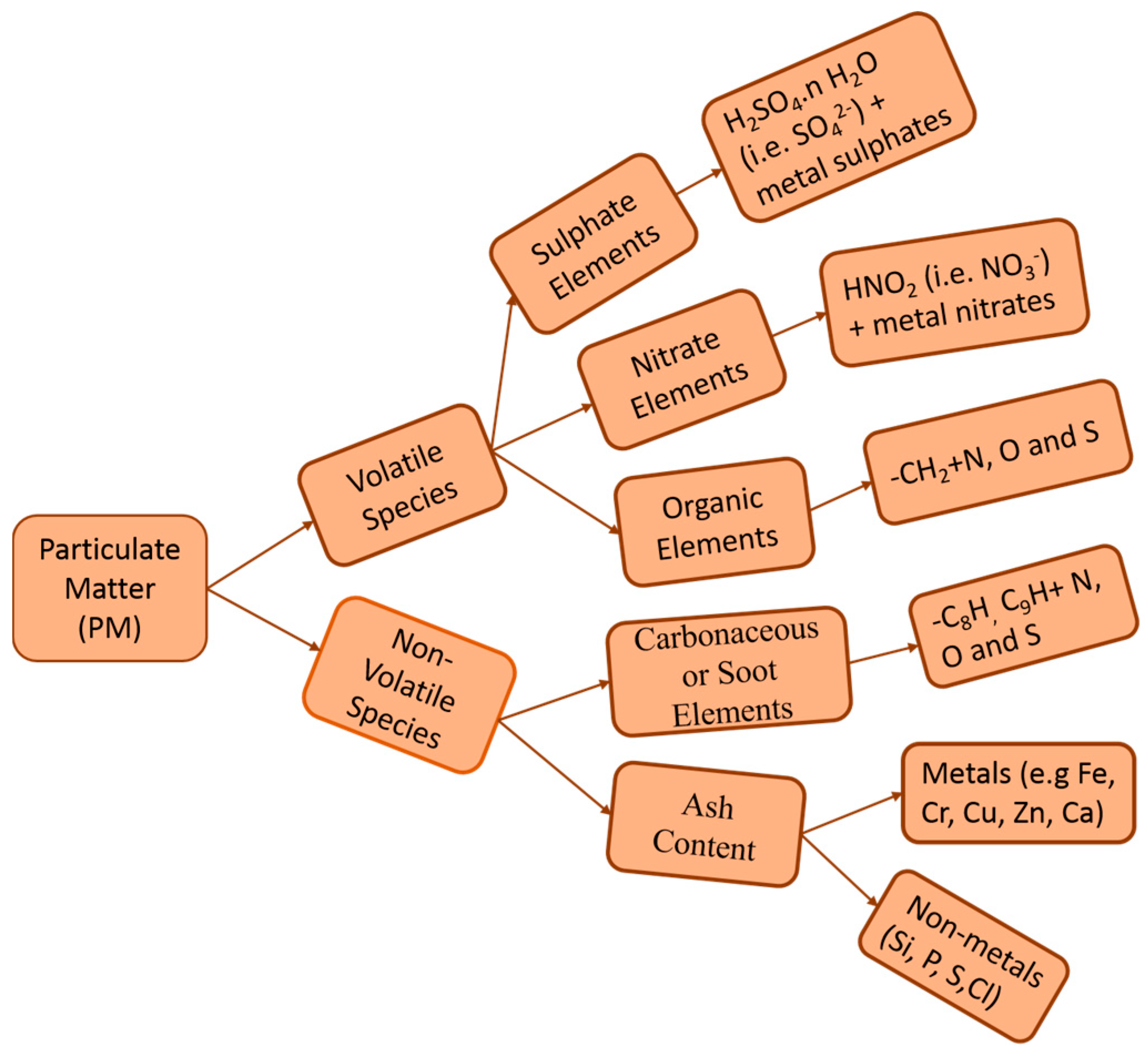
2.2. Non-Carbonaceous Particles
3. Gasoline Direct Injection (GDI) Engine PM Characteristics
3.1. Particle Composition
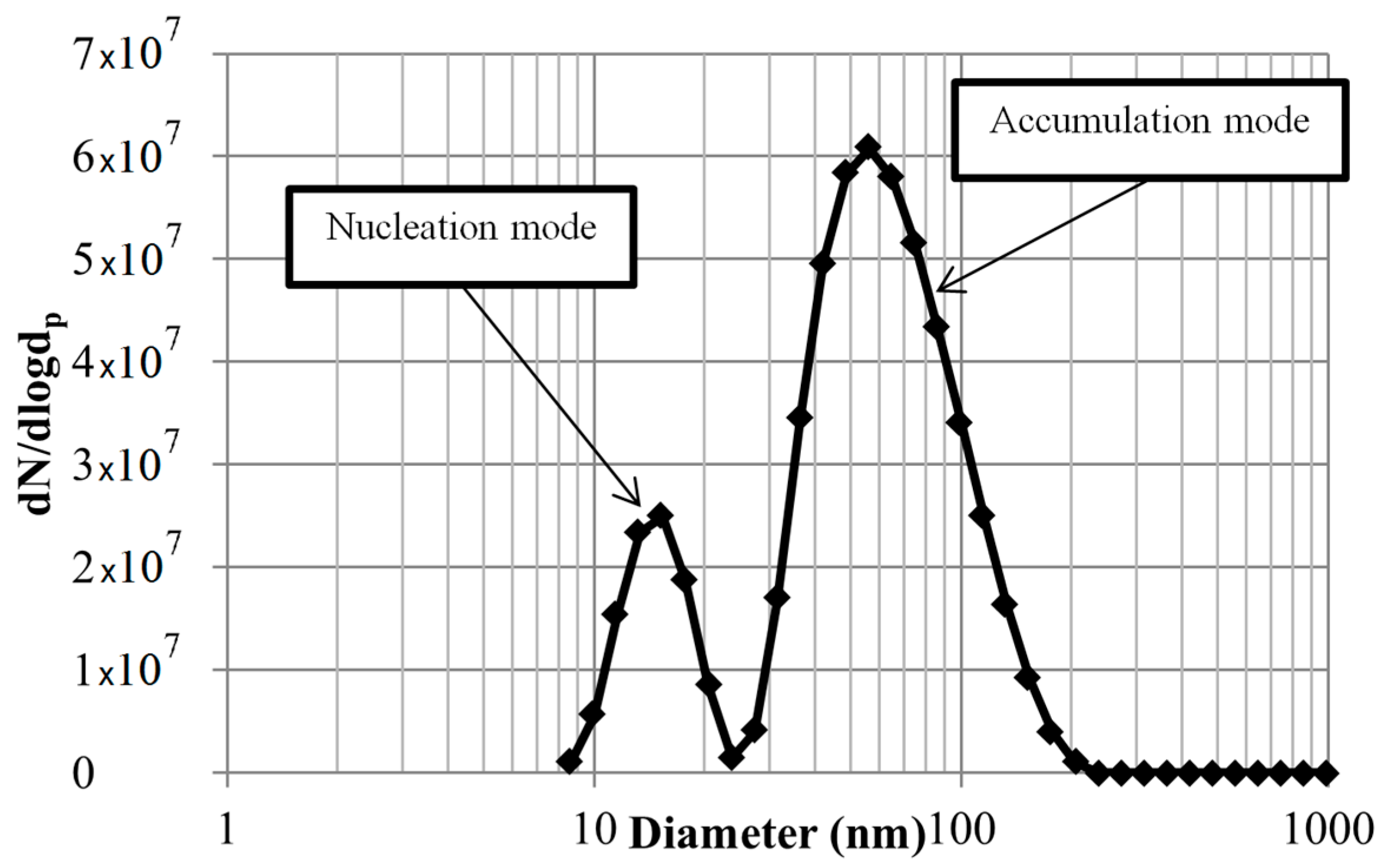
3.2. Particle Size Distribution
4. Effect of Engine Combustion Parameters on GDI PM Emissions
4.1. Pool Fires
4.2. Air-Fuel Ratio
4.3. Ignition Timing
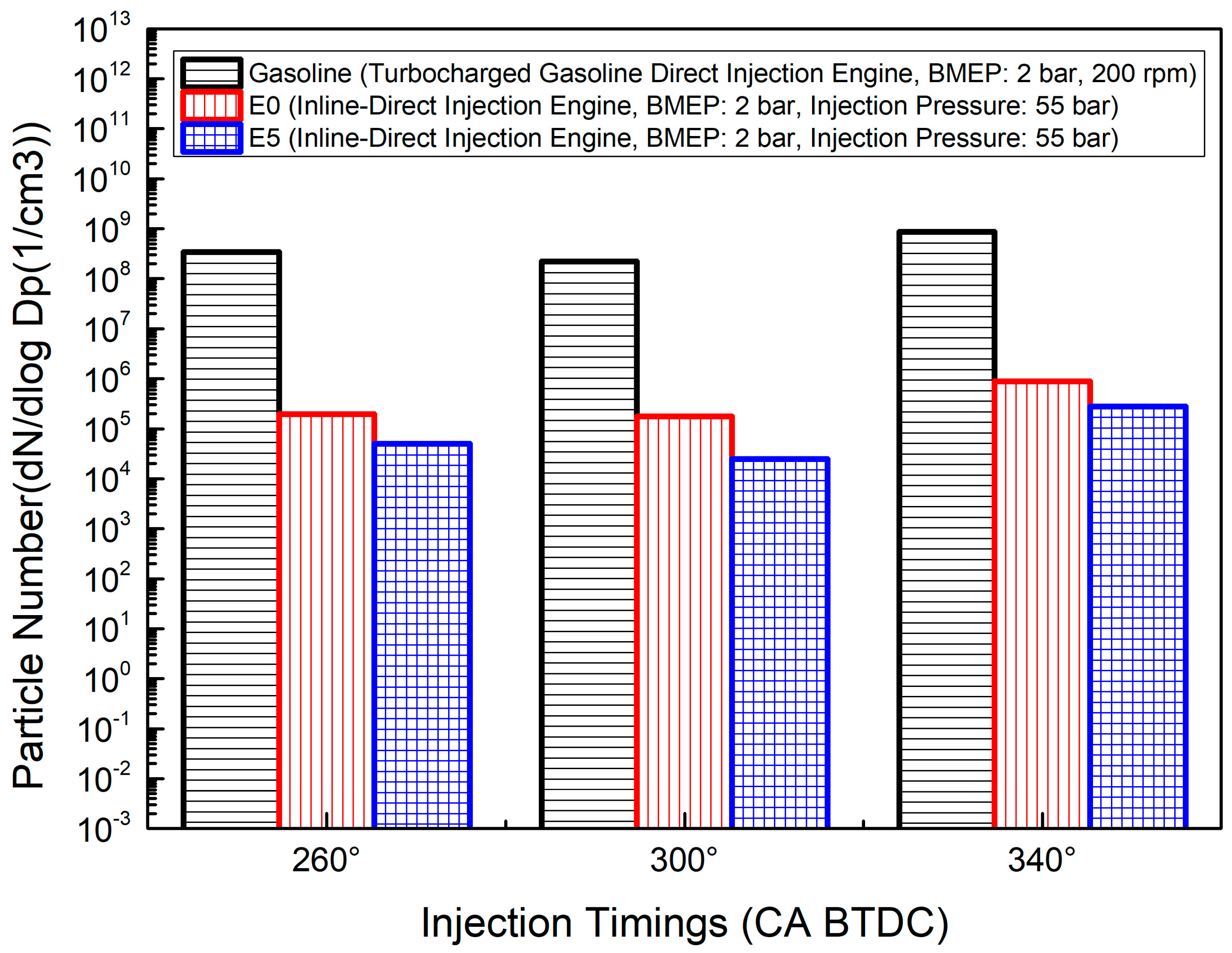
4.4. Fuel Injection Timing and Injection Strategies
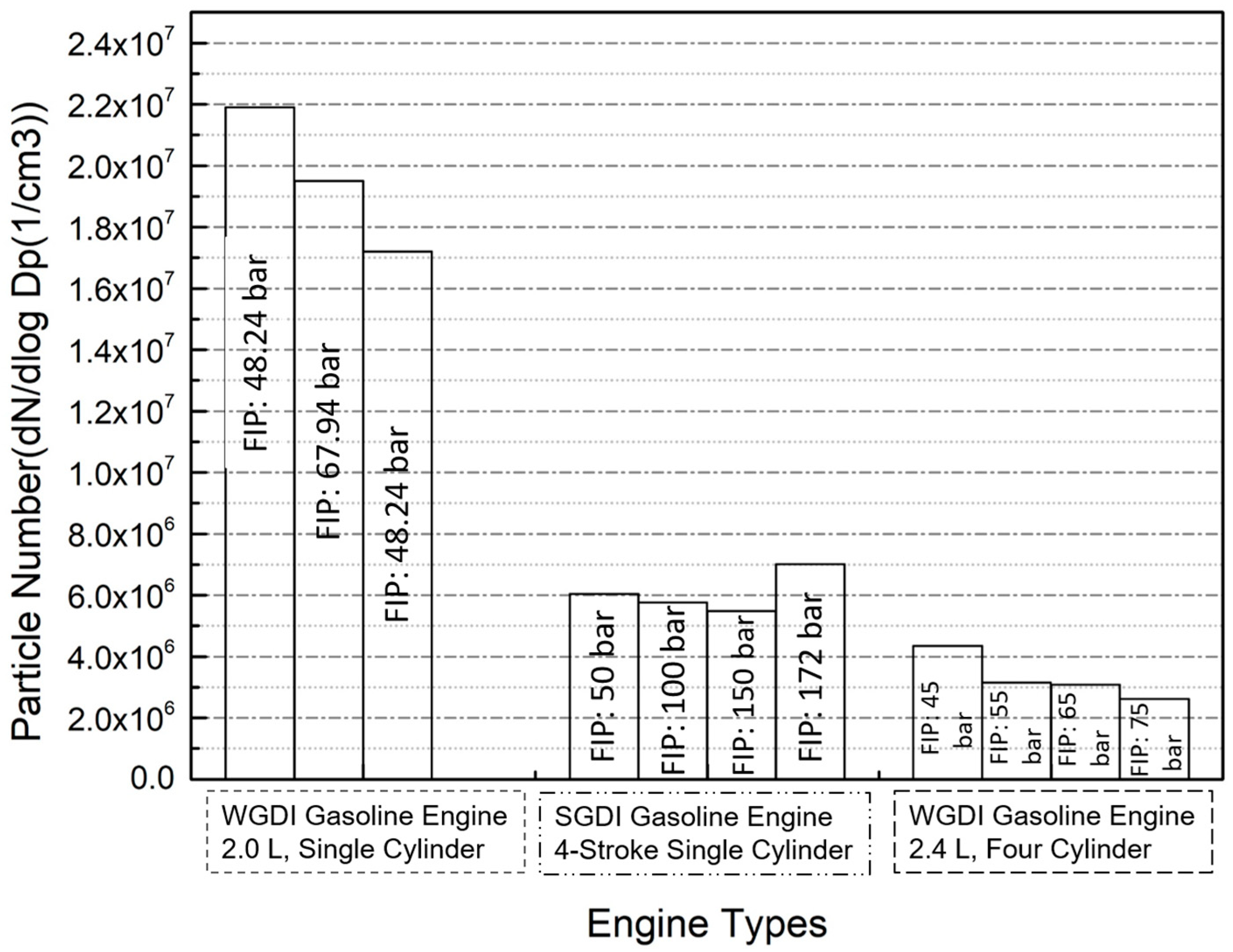
4.5. Fuel Injection Pressure
4.6. EGR and Inlet Air Conditions
4.7. Cold Start and Ambient Temperature Conditions
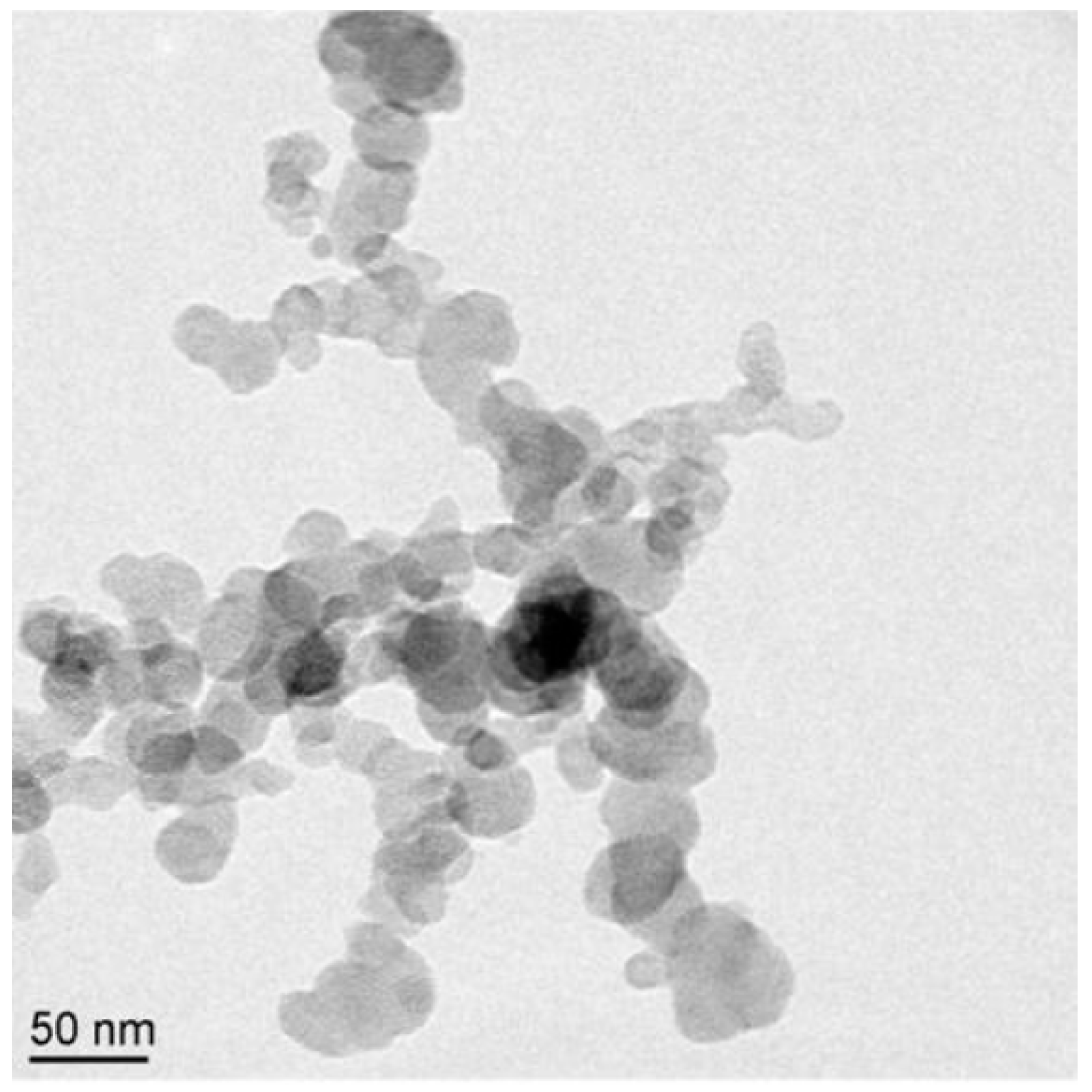
4.8. Factors Influencing the Particle Morphology, Nanostructure and Primary Particle Size
4.9. Boosted Engines
5. Fuel Effects on GDI PM Emissions
5.1. Effect of Gasoline Composition and Properties on GDI PM Emissions
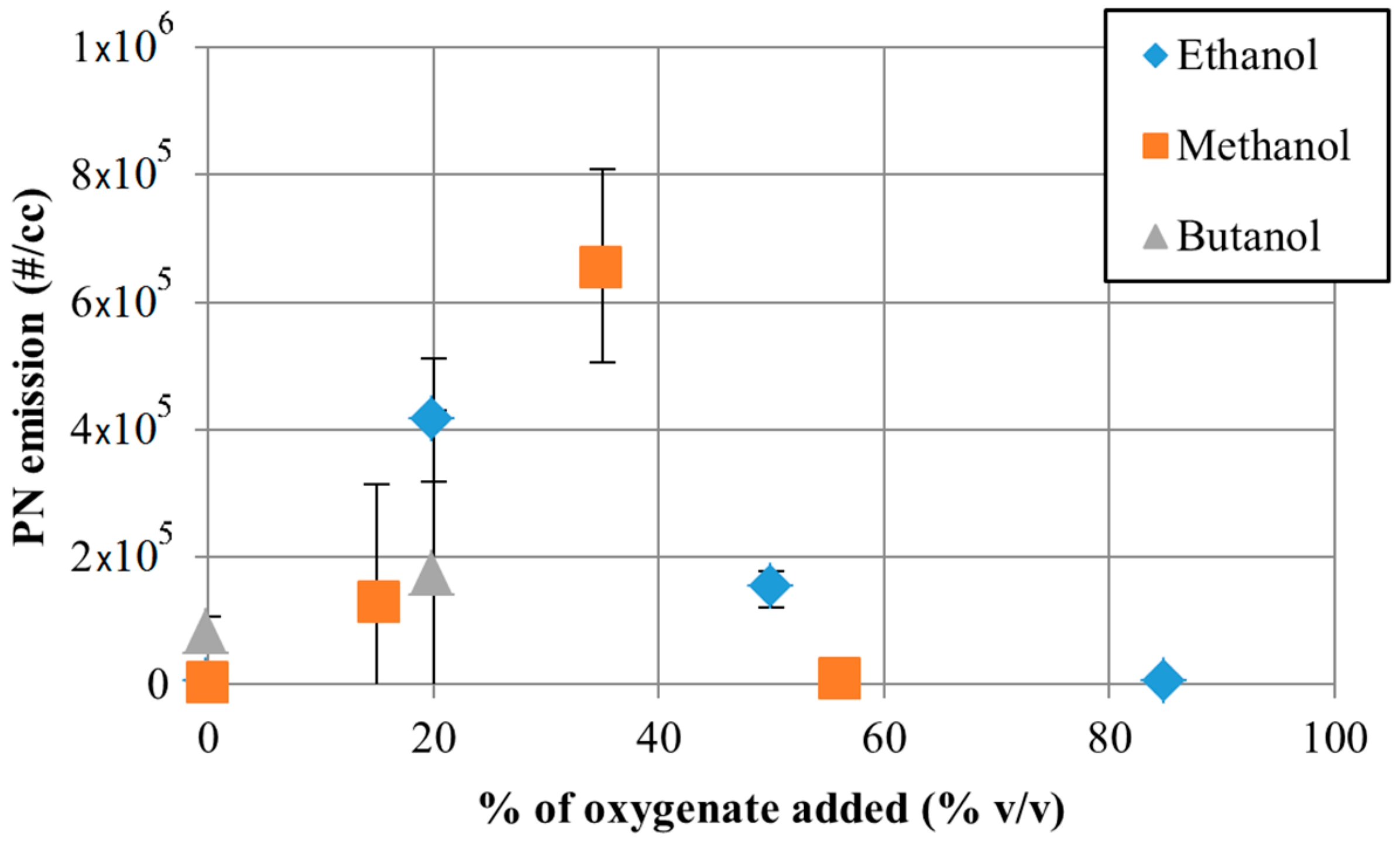
5.2. Impact of Oxygenated Fuels on GDI PM Emissions
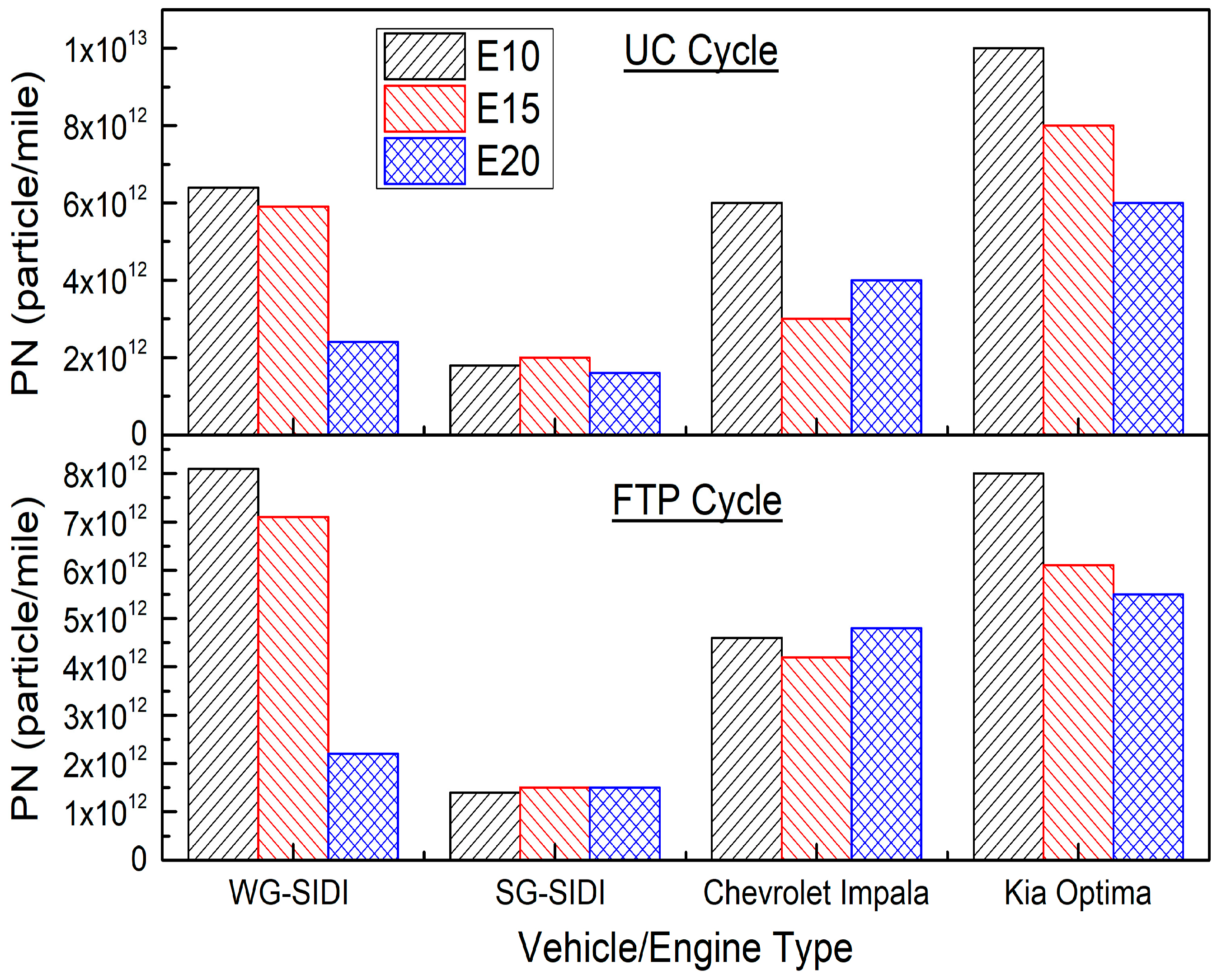
5.3. Impact of Ethanol Fuel Blends on GDI PM Emissions
5.4. Impact of Methanol Fuel Blends on GDI PM Emissions
5.5. Impact of Butanol Fuel Blends on GDI PM Emissions
6. Conclusions and Future Trends
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| AFR | air fuel ratio |
| bTDC | before top dead center |
| CARB | California air resources board |
| DPF | diesel particulate Filter |
| DISI | direct injection spark ignition |
| EC | elemental carbon |
| FTP | federal test procedure |
| GDI | gasoline direct injection |
| HAT | hydrogen atom transfer |
| MTBE | methyl tert-butyl ether |
| OC | organic carbon |
| PAHs | polycyclic aromatic hydrocarbons |
| PFI | port fuel injection |
| PM | particulate matter |
| PN | particle number |
| SGDI | spray guided direct injection |
| SIDI | spark ignition direct injection |
| T-GDI | turbo-charged gasoline direct injection |
| UC | unified cycle |
| UHCs | unburned hydrocarbons |
| VOCs | volatile organic carbons |
| WHO | world health organization |
| WGDI | wall guided direct injection |
| FIP | fuel injection pressure |
References
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.L.; Asa-Awuku, A.; Jung, H.; Johnson, K.C.; Durbin, T.D. The impact of ethanol and iso-butanol blends on gaseous and particulate emissions from two passenger cars equipped with spray-guided and wall-guided direct injection SI (spark ignition) engines. Energy 2015, 82, 168–179. [Google Scholar] [CrossRef]
- He, X.; Ratcliff, M.A.; Zigler, B.T. Effects of Gasoline Direct Injection Engine Operating Parameters on Particle Number Emissions. Energy Fuel 2012, 26, 2014–2027. [Google Scholar] [CrossRef]
- Chen, L.; Stone, R.; Richardson, D. A study of mixture preparation and PM emissions using a direct injection engine fuelled with stoichiometric gasoline/ethanol blends. Fuel 2012, 96, 120–130. [Google Scholar] [CrossRef]
- Chen, L.; Liang, Z.; Zhang, X.; Shuai, S. Characterizing particulate matter emissions from GDI and PFI vehicles under transient and cold start conditions. Fuel 2017, 189, 131–140. [Google Scholar] [CrossRef]
- Fatouraie, M.; Wooldridge, M.S.; Petersen, B.R.; Wooldridge, S.T. Effects of Ethanol on In-Cylinder and Exhaust Gas Particulate Emissions of a Gasoline Direct Injection Spark Ignition Engine. Energy Fuel 2015, 29, 3399–3412. [Google Scholar] [CrossRef]
- Bernstein, J.A.; Alexis, N.; Barnes, C.; Bernstein, I.L.; Bernstein, J.A.; Nel, A.; Peden, D.; Diaz-Sanchez, D.; Tarlo, S.M.; Williams, P.B. Health effects of air pollution. J. Allergy Clin. Immunol. 2004, 114, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Barone, T.L.; Storey, J.M.E.; Youngquist, A.D.; Szybist, J.P. An analysis of direct-injection spark-ignition (DISI) soot morphology. Atmos. Environ. 2012, 49, 268–274. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Z.; Lu, Y.; Zhang, C.; Zhang, X.; Zhang, C.; Roskilly, A.P. Experimental study of the gaseous and particulate matter emissions from a gas turbine combustor burning butyl butyrate and ethanol blends. Appl. Energy 2017, 195, 693–701. [Google Scholar] [CrossRef]
- Chen, L.; Liang, Z.; Liu, H.; Ding, S.; Li, Y. Sensitivity analysis of fuel types and operational parameters on the particulate matter emissions from an aviation piston engine burning heavy fuels. Fuel 2017, 202, 520–528. [Google Scholar] [CrossRef]
- Chen, L.; Raza, M.; Xiao, J. Combustion Analysis of an Aviation Compression Ignition Engine Burning Pentanol—Kerosene Blends under Different Injection Timings. Energy Fuels 2017, 31, 9429–9437. [Google Scholar] [CrossRef]
- Choi, S.; Myung, C.L.; Park, S. Review on characterization of nano-particle emissions and PM morphology from internal combustion engines: Part 2. Int. J. Automot. Technol. 2014, 15, 219–227. [Google Scholar] [CrossRef]
- Eastwood, P. Particulate Emissions from Vehicles; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008. [Google Scholar]
- State of California AIR RESOURCES BOARD. Available online: https://www.arb.ca.gov/msprog/levprog/leviii/meetings/051810/pm_disc_paper-v6.pdf (accessed on 25 April 2017).
- Überall, A.; Otte, R.; Eilts, P.; Krahl, J. A literature research about particle emissions from engines with direct gasoline injection and the potential to reduce these emissions. Fuel 2015, 147, 203–207. [Google Scholar] [CrossRef]
- Myung, C.L.; Park, S. Exhaust nanoparticle emissions from internal combustion engines: A review. Int. J. Automot. Technol. 2011, 13, 9. [Google Scholar] [CrossRef]
- Yao, Q.; Li, S.Q.; Xu, H.W.; Zhuo, J.K.; Song, Q. Reprint of: Studies on formation and control of combustion particulate matter in China: A review. Energy 2010, 35, 4480–4493. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, source and chemical composition as determinants of toxicity attributable to ambient particulate matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Sonntag, D.B.; Bailey, C.R.; Fulper, C.R.; Baldauf, R.W. Contribution of Lubricating Oil to Particulate Matter Emissions from Light-Duty Gasoline Vehicles in Kansas City. Environ. Sci. Technol. 2012, 46, 4191–4199. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kittelson, D.B.; Zachariah, M.R. The Influence of Engine Lubricating Oil on Diesel Nanoparticle Emissions and Kinetics of Oxidation; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Amirante, R.; Distaso, E.; Napolitano, M.; Tamburrano, P.; Iorio, S.D.; Sementa, P.; Vaglieco, B.M.; Reitz, R.D. Effects of lubricant oil on particulate emissions from port-fuel and direct-injection spark-ignition engines. Int. J. Engine Res. 2017, 18, 606–620. [Google Scholar] [CrossRef]
- Pirjola, L.; Karjalainen, P.; Heikkilä, J.; Saari, S.; Tzamkiozis, T.; Ntziachristos, L.; Kulmala, K.; Keskinen, J.; Rönkkö, T. Effects of Fresh Lubricant Oils on Particle Emissions Emitted by a Modern Gasoline Direct Injection Passenger Car. Environ. Sci. Technol. 2015, 49, 3644–3652. [Google Scholar] [CrossRef] [PubMed]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw Hill: New York, NY, USA, 1988. [Google Scholar]
- Köpple, F.; Jochmann, P.; Kufferath, A.; Bargende, M. Investigation of the Parameters Influencing the Spray-Wall Interaction in a GDI Engine—Prerequisite for the Prediction of Particulate Emissions by Numerical Simulation. SAE Int. J. Engines 2013, 6, 911–925. [Google Scholar] [CrossRef]
- Köpple, F.; Seboldt, D.; Jochmann, P.; Hettinger, A.; Kufferath, A.; Bargende, M. Experimental Investigation of Fuel Impingement and Spray-Cooling on the Piston of a GDI Engine via Instantaneous Surface Temperature Measurements. SAE Int. J. Engines 2014, 7, 1178–1194. [Google Scholar] [CrossRef]
- Velji, A.; Yeom, K.; Wagner, U.; Spicher, U.; Rossbach, M.; Suntz, R.; Bockhorn, H. Investigations of the Formation and Oxidation of Soot Inside a Direct Injection Spark Ignition Engine Using Advanced Laser-Techniques; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Smith, O.I. Fundamentals of soot formation in flames with application to diesel engine particulate emissions. Prog. Energy Combust. Sci. 1981, 7, 275–291. [Google Scholar] [CrossRef]
- Glassman, I. Soot formation in combustion processes. In Symposium (International) on Combustion; Elsevier: New York, NY, USA, 1989; Volume 22, pp. 295–311. [Google Scholar]
- Dobbins, R.A.; Fletcher, R.A.; Lu, W. Laser microprobe analysis of soot precursor particles and carbonaceous soot. Combust. Flame 1995, 100, 301–309. [Google Scholar] [CrossRef]
- An, Y.Z.; Teng, S.P.; Pei, Y.Q.; Qin, J.; Li, X.; Zhao, H. An experimental study of polycyclic aromatic hydrocarbons and soot emissions from a GDI engine fueled with commercial gasoline. Fuel 2016, 164, 160–171. [Google Scholar] [CrossRef]
- Law, C.K. Combustion Physics; Cambridge University Press: Cambridge, MA, USA, 2006. [Google Scholar]
- Oh, K.C.; Lee, C.B.; Lee, E.J. Characteristics of soot particles formed by diesel pyrolysis. J. Anal. Appl. Pyrolysis 2011, 92, 456–462. [Google Scholar] [CrossRef]
- Dastanpour, R.; Rogak, S.N. Observations of a Correlation between Primary Particle and Aggregate Size for Soot Particles. Aerosol. Sci. Technol. 2014, 48, 1043–1049. [Google Scholar] [CrossRef]
- Wen, J.Z. Chemical and Physical Aspects of Soot/Nano-Particle Formation in Combustion; University of Toronto: Toronto, ON, Canada, 2005. [Google Scholar]
- Kholghy, M.; Saffaripour, M.; Yip, C.; Thomson, M.J. The evolution of soot morphology in a laminar flow diffusion flame of a surrogate for Jet A-1. Combust. Flame 2013, 160, 2119–2130. [Google Scholar] [CrossRef]
- Park, S.H.; Rogak, S.N. A One-Dimensional Model for Coagulation, Sintering, and Surface Growth of Aerosol Agglomerates. Aerosol. Sci. Technol. 2003, 37, 947–960. [Google Scholar] [CrossRef]
- Park, S.H.; Rogak, S.N.; Bushe, W.K.; Wen, J.Z.; Thomson, M.J. An aerosol model to predict size and structure of soot particles. Combust. Theory Model. 2005, 9, 499–513. [Google Scholar] [CrossRef]
- Appel, J.; Bockhorn, H.; Frenklach, M. Kinetic modeling of soot formation with detailed chemistry and physics: Laminar premixed flames of C2hydrocarbons. Combust. Flame 2000, 121, 122–136. [Google Scholar] [CrossRef]
- Bisetti, F.; Blanquart, G.; Mueller, M.E.; Pitsch, H. On the formation and early evolution of soot in turbulent nonpremixed flames. Combust. Flame 2012, 159, 317–335. [Google Scholar] [CrossRef]
- Mayer, A.; Czerwinski, J.; Kasper, M.; Ulrich, A.; Mooney, J.J. Metal Oxide Particle Emissions from Diesel and Petrol Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2012. [Google Scholar]
- Thiruvengadam, A.; Besch, M.C.; Yoon, S.; Collins, J.; Kappanna, H.; Carder, D.K.; Ayala, A.; Herner, J.; Gautam, M. Characterization of Particulate Matter Emissions from a Current Technology Natural Gas Engine. Environ. Sci. Technol. 2014, 48, 8235–8242. [Google Scholar] [CrossRef] [PubMed]
- Andrews, G.E.; Abbass, M.K.; Williams, P.T.; Bartle, K.D. Factors influencing the composition of the organic fraction of diesel particulates. J. Aerosol. Sci. 1989, 20, 1373–1376. [Google Scholar] [CrossRef]
- Fushimi, A.; Kondo, Y.; Kobayashi, S.; Fujitani, Y.; Saitoh, K.; Takami, A.; Tanabe, K. Chemical composition and source of fine and nanoparticles from recent direct injection gasoline passenger cars: Effects of fuel and ambient temperature. Atmos. Environ. 2016, 124, 77–84. [Google Scholar] [CrossRef]
- Jimenez, J.L.; Canagaratna, M.R.; Donahue, N.M.; Prevot, A.S.; Zhang, Q.; Kroll, J.H.; DeCarlo, P.F.; Allan, J.D.; Coe, H.; Ng, N.L.; et al. Evolution of organic aerosols in the atmosphere. Science 2009, 326, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Fuzzi, S.; Andreae, M.O.; Huebert, B.J.; Kulmala, M.; Bond, T.C.; Boy, M.; Doherty, S.J.; Guenther, A.; Kanakidou, M.; Kawamura, K.; et al. Critical assessment of the current state of scientific knowledge, terminology, and research needs concerning the role of organic aerosols in the atmosphere, climate, and global change. Atmos. Chem. Phys. 2006, 6, 2017–2038. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Enviorn. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef]
- Hiura, T.S.; Kaszubowski, M.P.; Li, N.; Nel, A.E. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J. Immunol. 1999, 163, 5582–5591. [Google Scholar] [PubMed]
- Seagrave, J.; McDonald, J.D.; Gigliotti, A.P.; Nikula, K.J.; Seilkop, S.K.; Gurevich, M.; Mauderly, J.L. Mutagenicity and in Vivo Toxicity of Combined Particulate and Semivolatile Organic Fractions of Gasoline and Diesel Engine Emissions. Toxicol. Sci. 2002, 70, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.W.; Lee, W.W.; Li, C.H.; Lee, C.C.; Kang, J.J. Genotoxicity of motorcycle exhaust particles in vivo and in vitro. Toxicol. Sci. 2004, 81, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Castranova, V.; Ma, J.Y.; Yang, H.M.; Antonini, J.M.; Butterworth, L.; Barger, M.W.; Roberts, J.; Ma, J.K. Effect of exposure to diesel exhaust particles on the susceptibility of the lung to infection. Enviorn. Health Perspecct. 2001, 109, 609–612. [Google Scholar] [CrossRef]
- Leach, F. Particulate Emissions from Gasoline Direct Injection Engines. Ph.D. Thesis, Engineering Science, University of Oxford, Oxford, UK, 2014. [Google Scholar]
- Lee, D.; Miller, A.; Kittelson, D.; Zachariah, M.R. Characterization of metal-bearing diesel nanoparticles using single-particle mass spectrometry. J. Aerosol. Sci. 2006, 37, 88–110. [Google Scholar] [CrossRef]
- Kittelson, D.B.; Watts, W.F.; Johnson, J.P. On-road and laboratory evaluation of combustion aerosols—Part 1: Summary of diesel engine results. J. Aerosol. Sci. 2006, 37, 913–930. [Google Scholar] [CrossRef]
- Filippo, A.D.; Maricq, M.M. Diesel nucleation mode particles: Semivolatile or solid? Enviorn. Sci. Technol. 2008, 42, 7957–7962. [Google Scholar] [CrossRef]
- Rönkkö, T.; Virtanen, A.; Kannosto, J.; Keskinen, J.; Lappi, M.; Pirjola, L. Nucleation mode particles with a nonvolatile core in the exhaust of a heavy duty diesel vehicle. Enviorn. Sci. Technol. 2007, 41, 6384–6389. [Google Scholar] [CrossRef]
- Lähde, T.R.T.; Virtanen, A.; Solla, A.; Kytö, M.; Söderström, C.; Keskinen, J. Dependence between nonvolatile nucleation mode particle and soot number concentrations in an EGR equipped heavy-duty Diesel engine exhaust. Enviorn. Sci. Technol. 2010, 44, 3175–3180. [Google Scholar] [CrossRef] [PubMed]
- Sgro, L.A.; Sementa, P.; Vaglieco, B.M.; Rusciano, G.; D’Anna, A.; Minutolo, P. Investigating the origin of nuclei particles in GDI engine exhausts. Combust. Flame 2012, 159, 1687–1692. [Google Scholar] [CrossRef]
- Burtscher, H. Physical characterization of particulate emissions from diesel engines: A review. J. Aerosol. Sci. 2005, 36, 896–932. [Google Scholar] [CrossRef]
- Eggersdorfer, M.L.; Pratsinis, S.E. The Structure of Agglomerates Consisting of Polydisperse Particles. Aerosol. Sci. Technol. 2012, 46, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Eggersdorfer, M.L.; Kadau, D.; Herrmann, H.J.; Pratsinis, S.E. Multiparticle Sintering Dynamics: From Fractal-Like Aggregates to Compact Structures. Langmuir 2011, 27, 6358–6367. [Google Scholar] [CrossRef] [PubMed]
- Price, P. Direct Injection Gasoline Engine Particulate Emissions. Ph.D. Thesis, Engineering Science, University of Oxford, Oxford, UK, 2009. [Google Scholar]
- Kittelson, D.B. Engines and nanoparticles. J. Aerosol. Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Sementa, P.; Maria Vaglieco, B.; Catapano, F. Thermodynamic and optical characterizations of a high performance GDI engine operating in homogeneous and stratified charge mixture conditions fueled with gasoline and bio-ethanol. Fuel 2012, 96, 204–219. [Google Scholar] [CrossRef]
- Karjalainen, P.; Pirjola, L.; Heikkila, J.; Lahnde, T.; Tzamkiozis, T.; Ntziachristos, L.; Keskinen, J.; Ronkko, T. Exhaust particles of modern gasoline vehicles: A laboratory and an on-road study. Atmos. Environ. 2014, 97, 262–270. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Vanhanen, J.; Väkevä, M.; Martini, G. Investigation of vehicle exhaust sub-23 nm particle emissions. Aerosol. Sci. Technol. 2017, 51, 626–641. [Google Scholar] [CrossRef]
- Lobo, P.; Durdina, L.; Smallwood, G.J.; Rindlisbacher, T.; Siegerist, F.; Black, E.A.; Yu, Z.; Mensah, A.A.; Hagen, D.E.; Miake-Lye, R.C.; et al. Measurement of Aircraft Engine Non-Volatile PM Emissions: Results of the Aviation-Particle Regulatory Instrumentation Demonstration Experiment (A-PRIDE) 4 Campaign. Aerosol. Sci. Technol. 2015, 49, 472–484. [Google Scholar] [CrossRef]
- Zhao, L.; Yu, X.; Qian, D.; Dong, W.; Sun, P.; He, L.; Yang, S. The effects of EGR and ignition timing on emissions of GDI engine. Sci. China Technol. Sci. 2013, 56, 3144–3150. [Google Scholar] [CrossRef]
- Szybist, J.P.; Youngquist, A.D.; Barone, T.L.; Storey, J.M.; Moore, W.R.; Foster, M.; Confer, K. Ethanol Blends and Engine Operating Strategy Effects on Light-Duty Spark-Ignition Engine Particle Emissions. Energy Fuel 2011, 25, 4977–4985. [Google Scholar] [CrossRef]
- Qin, J.; Li, X.; Pei, Y. Effects of Combustion Parameters and Lubricating Oil on Particulate Matter Emissions from a Turbo-Charged GDI Engine Fueled with Methanol/Gasoline Blends; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Price, P.; Twiney, B.; Stone, R.; Kar, K.; Walmsley, H. Particulate and Hydrocarbon Emissions from a Spray Guided Direct Injection Spark Ignition Engine with Oxygenate Fuel Blends; SAE Technical Paper 2007-01-0472; 2007. [Google Scholar]
- Pei, Y.Q.; Qin, J.; Pan, S.Z. Experimental study on the particulate matter emission characteristics for a direct-injection gasoline engine. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 228, 604–616. [Google Scholar] [CrossRef]
- Cho, J.; Si, W.; Jang, W.; Jin, D.; Myung, C.-L.; Park, S. Impact of intermediate ethanol blends on particulate matter emission from a spark ignition direct injection (SIDI) engine. Appl. Energy 2015, 160, 592–602. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Herreros, J.M.; Wang, J.; Cracknell, R. Impact of fuel and injection system on particle emissions from a GDI engine. Appl. Energy 2014, 132, 178–191. [Google Scholar] [CrossRef]
- Whitaker, P.; Kapus, P.; Ogris, M.; Hollerer, P. Measures to Reduce Particulate Emissions from Gasoline DI engines. SAE Int. J. Engines 2011, 3, 1498–1512. [Google Scholar] [CrossRef]
- Chen, L.; Stone, R.; Richardson, D. Effect of the valve timing and the coolant temperature on particulate emissions from a gasoline direct-injection engine fuelled with gasoline and with a gasoline–ethanol blend. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2012, 226, 1419–1430. [Google Scholar] [CrossRef]
- Drake, M.C.; Fansler, T.D.; Solomon, A.S.; Szekely, G.A. Piston Fuel Films as a Source of Smoke and Hydrocarbon Emissions from a Wall-Controlled Spark-Ignited Direct-Injection Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2003. [Google Scholar]
- Stevens, E.; Steeper, R. Piston Wetting in an Optical DISI Engine: Fuel Films, Pool Fires, and Soot Generation; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Mehta, D.; Alger, T.; Hall, M.; Matthews, R.D.; Ng, H. Particulate Characterization of a DISI Research Engine using a Nephelometer and In-Cylinder Visualization; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2001. [Google Scholar]
- Karlsson, R.B.; Heywood, J.B. Piston Fuel Film Observations in an Optical Access GDI Engine; SAE Technical Paper; 2001. [Google Scholar]
- Short, D.; Vu, D.; Chen, V.; Espinoza, C.; Berte, T.; Karavalakis, G.; Durbin, T.D.; Asa-Awuku, A. Understanding particles emitted from spray and wall-guided gasoline direct injection and flex fuel vehicles operating on ethanol and iso-butanol gasoline blends. Aerosol. Sci. Technol. 2017, 51, 330–341. [Google Scholar] [CrossRef][Green Version]
- Braisher, M.; Stone, R.; Price, P. Particle Number Emissions from a Range of European Vehicles; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Rodriguez, J.F.; Cheng, W.K. Effect of Operation Strategy on First Cycle CO, HC, and PM/PN Emissions in a GDI Engine. SAE Int. J. Engines 2015, 8, 1098–1106. [Google Scholar] [CrossRef]
- Zhao, F.; Lai, M.C.; Harrington, D.L. Automotive spark-ignited direct-injection gasoline engines. Prog. Energy Combust. Sci. 1999, 25, 437–562. [Google Scholar] [CrossRef]
- Stone, R.; Chen, L.; Hinton, N.; Leach, F.; Xu, F. GDI Engine Operation with Ethanol/Gasoline Blends and Aqueous Ethanol. J Automot. Saf. Energy 2012, 3, 257–264. [Google Scholar]
- Karavalakis, G.; Short, D.; Vu, D.; Villela, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the regulated emissions, air toxics, ultrafine particles, and black carbon from SI-PFI and SI-DI vehicles operating on different ethanol and iso-butanol blends. Fuel 2014, 128, 410–421. [Google Scholar] [CrossRef]
- Whitaker, P.; Kapus, P.; Ogris, M.; Hollerer, P. Measures to Reduce Particulate Emissions from Gasoline DI engines. SAE Int. J. Engines 2011, 4, 1498–1512. [Google Scholar] [CrossRef]
- Sabathil, D.; Koenigstein, A.; Schaffner, P.; Fritzsche, J.; Doehler, A. The Influence of DISI Engine Operating Parameters on Particle Number Emissions; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2011. [Google Scholar]
- De Francqueville, L. Effects of Ethanol Addition in RON 95 Gasoline on GDI Stratified Combustion; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2011. [Google Scholar]
- Su, J.; Xu, M.; Yin, P.; Gao, Y.; Hung, D. Particle Number Emissions Reduction Using Multiple Injection Strategies in a Boosted Spark-Ignition Direct-Injection (SIDI) Gasoline Engine. SAE Int. J. Engines 2014, 8, 20–29. [Google Scholar] [CrossRef]
- Bertsch, M.; Weidenlener, A.; Dörnhöfer, J.; Koch, T.; Velji, A. Effect of implementing large-scale charge motion, reducing hydraulic flow of the injector and increasing injection pressure on particle emissions of a GDI engine at WOT and boosted operation. Int. J. Engine Res. 2016, 18, 467–489. [Google Scholar] [CrossRef]
- Piock, W.; Hoffmann, G.; Berndorfer, A.; Salemi, P.; Fusshoeller, B. Strategies towards Meeting Future Particulate Matter Emission Requirements in Homogeneous Gasoline Direct Injection Engines. SAE Int. J. Engines 2011, 4, 1455–1468. [Google Scholar] [CrossRef]
- Stone, R. Introduction to Internal Combustion Engines; Macmillan Press Ltd.: London, UK, 1999. [Google Scholar]
- Efthymiou, P.; Davy, M.H.; Garner, C.P.; Hargrave, G.K.; Rimmer, J.E.T.; Richardson, D. Insights into Cold-Start DISI Combustion in an Optical Engine Operating at −7 °C. SAE Int. J. Engines 2013, 6, 1059–1074. [Google Scholar] [CrossRef]
- Hedge, M.; Weber, P.; Gingrich, J.; Alger, T.; Khalek, I.A. Effect of EGR on Particle Emissions from a GDI Engine. SAE Int. J. Engines 2011, 4, 650–666. [Google Scholar] [CrossRef]
- Alger, T.; Gingrich, J.; Khalek, I.A.; Mangold, B. The Role of EGR in PM Emissions from Gasoline Engines. SAE Int. J. Fuels Lubr. 2010, 3, 85–98. [Google Scholar] [CrossRef]
- Leach, F.; Stone, R.; Richardson, D.; Lewis, A.; Akehurst, S.; Turner, J.; Remmert, S.; Campbell, R.; Cracknell, R. Particulate emissions from a highly boosted GDI engine. Int. J. Engine Res. 2017. [Google Scholar] [CrossRef]
- Stępień, Z.; Czerwinski, J.; Comte, P.; Oleksiak, S. Nanoparticle and Non-legislated Gaseous Emissions from a Gasoline Direct-Injection Car with Ethanol Blend Fuels and Detergent Additives. Energy Fuels 2016, 30, 7268–7276. [Google Scholar] [CrossRef]
- Zhu, R.; Hu, J.; Bao, X.; He, L.; Zu, L. Effects of aromatics, olefins and distillation temperatures (T50 & T90) on particle mass and number emissions from gasoline direct injection (GDI) vehicles. Energy Policy 2017, 101, 185–193. [Google Scholar]
- Czerwinski, J.; Comte, P.; Stepien, Z.; Oleksiak, S. Effects of Ethanol Blend Fuels E10 and E85 on the Non-Legislated Emissions of a Flex Fuel Passenger Car; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2016. [Google Scholar]
- Price, P.; Stone, R.; OudeNijeweme, D.; Chen, X. Cold Start Particulate Emissions from a Second Generation DI Gasoline Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2007. [Google Scholar]
- Fu, H.; Wang, Y.; Li, X.; Shuai, S.-J. Impacts of Cold-Start and Gasoline RON on Particulate Emission from Vehicles Powered by GDI and PFI Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Ramadhas, A.S.; Singh, P.K.; Mathai, R.; Sehgal, A.K. Impact of Ambient Temperature Conditions on Cold Start Combustion, Gaseous and Particle Emissions from Gasoline Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2017. [Google Scholar]
- Mamakos, A.; Martini, G.; Marotta, A.; Manfredi, U. Assessment of different technical options in reducing particle emissions from gasoline direct injection vehicles. J. Aerosol. Sci. 2013, 63, 115–125. [Google Scholar] [CrossRef]
- Mathis, U.; Mohr, M.; Forss, A.-M. Comprehensive particle characterization of modern gasoline and diesel passenger cars at low ambient temperatures. Atmos. Environ. 2005, 39, 107–117. [Google Scholar] [CrossRef]
- Seong, H.; Lee, K.; Choi, S. Effects of Engine Operating Parameters on Morphology of Particulates from a Gasoline Direct Injection (GDI) Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Lee, K.O.; Cole, R.; Sekar, R.; Choi, M.Y.; Kang, J.S.; Bae, C.S.; Shin, H.D. Morphological investigation of the microstructure, dimensions, and fractal geometry of diesel particulates. Proc. Combust. Inst. 2002, 29, 647–653. [Google Scholar] [CrossRef]
- Lapuerta, M.; Martos, F.J.; Herreros, J.M. Effect of engine operating conditions on the size of primary particles composing diesel soot agglomerates. J. Aerosol. Sci. 2007, 38, 455–466. [Google Scholar] [CrossRef]
- Lee, K.; Seong, H.; Church, W.; McConnell, S. IExamination of Particulate Emissions from Alcohol Blended Fuel Combustion in a GDI Engine. In Proceedings of the 8th International Symposium on Diagnostics and Modeling of Combustion in Internal Combustion Engines (COMODIA), Fukuoka, Japan, 24–28 July 2012. [Google Scholar]
- Lee, K.O.; Seong, H.; Sakai, S.; Hageman, M.; Rothamer, D. Detailed Morphological Properties of Nanoparticles from Gasoline Direct Injection Engine Combustion of Ethanol Blends; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Hancock, D.; Fraser, N.; Jeremy, M.; Sykes, R.; Blaxill, H. A New 3 Cylinder 1.2l Advanced Downsizing Technology Demonstrator Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2008. [Google Scholar]
- Martin, S.; Beidl, C.; Mueller, R. Responsiveness of a 30 Bar BMEP 3-Cylinder Engine: Opportunities and Limits of Turbocharged Downsizing; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Friedfeldt, R.; Zenner, T.; Ernst, R.; Fraser, A. Three-Cylinder Gasoline Engine with Direct Injection. Auto Tech. Rev. 2013, 2, 32–37. [Google Scholar] [CrossRef]
- Li, T.; Yin, T.; Wang, B. Anatomy of the cooled EGR effects on soot emission reduction in boosted spark-ignited direct-injection engines. Appl. Energy 2017, 190, 43–56. [Google Scholar] [CrossRef]
- Leach, F.C.P.; Stone, R.; Richardson, D.; Turner, J.W.G.; Lewis, A.; Akehurst, S.; Remmert, S.; Campbell, S.; Cracknell, R. The effect of oxygenate fuels on PN emissions from a highly boosted GDI engine. Fuel 2018, 225, 277–286. [Google Scholar] [CrossRef]
- Di Iorio, S.; Catapano, F.; Sementa, P.; Vaglieco, B.M.; Florio, S.; Rebesco, E.; Terna, D. Effect of Octane Number Obtained with Different Oxygenated Components on the Engine Performance and Emissions of a Small GDI Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2014. [Google Scholar]
- Yinhui, W.; Rong, Z.; Yanhong, Q.; Jianfei, P.; Mengren, L.; Jianrong, L.; Yusheng, W.; Min, H.; Shijin, S. The impact of fuel compositions on the particulate emissions of direct injection gasoline engine. Fuel 2016, 166, 543–552. [Google Scholar] [CrossRef]
- Barrientos, E.J.; Anderson, J.E.; Maricq, M.M.; Boehman, A.L. Particulate matter indices using fuel smoke point for vehicle emissions with gasoline, ethanol blends, and butanol blends. Combust. Flame 2016, 167, 308–319. [Google Scholar] [CrossRef]
- Oh, C.; Cha, G. Influence of oxygenate content on particulate matter emission in gasoline direct injection engine. Int. J. Automot. Technol. 2013, 14, 829–836. [Google Scholar] [CrossRef]
- Aikawa, K.; Sakurai, T.; Jetter, J.J. Development of a Predictive Model for Gasoline Vehicle Particulate Matter Emissions. SAE Int. J. Fuels Lubr. 2010, 3, 610–622. [Google Scholar] [CrossRef]
- Leach, F.; Stone, R.; Richardson, D. The Influence of Fuel Properties on Particulate Number Emissions from a Direct Injection Spark Ignition Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Aikawa, K.; Jetter, J.J. Impact of gasoline composition on particulate matter emissions from a direct-injection gasoline engine: Applicability of the particulate matter index. Int. J. Engine Res. 2013, 15, 298–306. [Google Scholar] [CrossRef]
- Graham, J.H.; Dixon, R.; Gregory, K. Predicting the Thermal State of Generators On-Board UAVs; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Sobotowski, R.A.; Butler, A.D.; Guerra, Z. A Pilot Study of Fuel Impacts on PM Emissions from Light-Duty Gasoline Vehicles. SAE Int. J. Fuels Lubr. 2015, 8, 214–233. [Google Scholar] [CrossRef]
- Karavalakis, G.; Short, D.; Vu, D.; Russell, R.; Hajbabaei, M.; Asa-Awuku, A.; Durbin, T.D. Evaluating the Effects of Aromatics Content in Gasoline on Gaseous and Particulate Matter Emissions from SI-PFI and SIDI Vehicles. Environ. Sci. Technol. 2015, 49, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Leach, F.; Stone, R.; Fennell, D.; Hayden, D.; Richardson, D.; Wicks, N. Predicting the particulate matter emissions from spray-guided gasoline direct-injection spark ignition engines. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2016, 231, 717–730. [Google Scholar] [CrossRef]
- Chapman, E.; Winston-Galant, M.; Geng, P.; Latigo, R.; Boehman, A. Alternative Fuel Property Correlations to the Honda Particulate Matter Index (PMI); SAE Technical Paper 2016-01-2250; 2016. [Google Scholar]
- Chen, L.; Zhang, Z.; Gong, W.; Liang, Z. Quantifying the effects of fuel compositions on GDI-derived particle emissions using the optimal mixture design of experiments. Fuel 2015, 154, 252–260. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Kang, J.; Jun, S.; Rew, S.; Lee, D.; Park, S. Fuel Effect on Particle Emissions of a Direct Injection Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Chen, L.; Stone, R. Measurement of Enthalpies of Vaporization of Isooctane and Ethanol Blends and Their Effects on PM Emissions from a GDI Engine. Energy Fuels 2011, 25, 1254–1259. [Google Scholar] [CrossRef]
- Wittmann, J.-H.; Menger, L. Novel Index for Evaluation of Particle Formation Tendencies of Fuels with Different Chemical Compositions. SAE Int. J. Fuels Lubr. 2017, 10. [Google Scholar] [CrossRef]
- Karavalakis, G.; Durbin, T.D.; Yang, J.; Ventura, L.; Xu, K. Fuel Effects on PM Emissions from Different Vehicle/Engine Configurations: A Literature Review; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Shi, Y.; Cortes-Morales, A.; Taylor, B. Study of Gasoline Particulate Matter Index with Refinery Blends; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Concawe Environmental Science for the European Refining Industry. Available online: https://circabc.europa.eu/webdav/CircaBC/GROW/wltp/Library/RDE-LDV/RDE%204/Meeting%20RDE4%204-5%20May%202017/Concawe%20presentation%20for%20RDE-LDV%20WG.pdf (accessed on 5 May 2017).
- Leach, F. A Review of the Requirements for Injection Systems and the Effects of Fuel Quality on Particulate Emissions from GDI Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Liu, H.; Ma, X.; Li, B.; Chen, L.; Wang, Z.; Wang, J. Combustion and emission characteristics of a direct injection diesel engine fueled with biodiesel and PODE/biodiesel fuel blends. Fuel 2017, 209, 62–68. [Google Scholar] [CrossRef]
- Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. Available online: https://infostore.saiglobal.com/preview/is/en/2012/i.s.en228-2012.pdf?sku=1600459 (accessed on 12 May 2016).
- 42 U.S.Code §7546-Renewable fuel. Available online: https://www.law.cornell.edu/uscode/text/42/7546 (accessed on 12 May 2016).
- Wang, X.; Ge, Y.; Liu, L.; Peng, Z.; Hao, L.; Yin, H.; Ding, Y.; Wang, J. Evaluation on toxic reduction and fuel economy of a gasoline direct injection-(GDI-)powered passenger car fueled with methanol–gasoline blends with various substitution ratios. Appl. Energy 2015, 157, 134–143. [Google Scholar] [CrossRef]
- Maricq, M.M.; Szente, J.J.; Jahr, K. The Impact of Ethanol Fuel Blends on PM Emissions from a Light-Duty GDI Vehicle. Aerosol. Sci. Technol. 2012, 46, 576–583. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, T.; Jia, M.; Wei, Q.; Meng, X.; Shu, G. Combustion and particle number emissions of a direct injection spark ignition engine operating on ethanol/gasoline and n-butanol/gasoline blends with exhaust gas recirculation. Fuel 2014, 130, 177–188. [Google Scholar] [CrossRef]
- Turner, J.W.; Lewis, A.G.; Akehurst, S.; Brace, C.J.; Verhelst, S.; Vancoillie, J.; Sileghem, L.; Leach, F.; Edwards, P.P. Alcohol fuels for spark-ignition engines: Performance, efficiency and emission effects at mid to high blend rates for binary mixtures and pure components. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2018, 232, 36–56. [Google Scholar] [CrossRef]
- Leach, F.; Stone, R.; Davy, M.; Richardson, D. Richardson, Comparing the effect of different oxygenate components on PN emissions from GDI engines. In Internal Combustion Engines; Royal College of Physicians: London, UK, 2015. [Google Scholar]
- Mulawa, P.A.; Cadle, S.H.; Knapp, K.; Zweidinger, R.; Snow, R.; Lucas, R.; Goldbach, J. Effect of Ambient Temperature and E-10 Fuel on Primary Exhaust Particulate Matter Emissions from Light-Duty Vehicles. Environ. Sci. Technol. 1997, 31, 1302–1307. [Google Scholar] [CrossRef]
- Storey, J.; Barone, T.; Norman, K.; Lewis, S. Ethanol Blend Effects on Direct Injection Spark-Ignition Gasoline Vehicle Particulate Matter Emissions. SAE Int. J. Fuels Lubr. 2010, 3, 650–659. [Google Scholar] [CrossRef]
- Catapano, F.; Di Iorio, S.; Lazzaro, M.; Sementa, P.; Vaglieco, B.M. Characterization of Ethanol Blends Combustion Processes and Soot Formation in a GDI Optical Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2013. [Google Scholar]
- Chen, L.; Braisher, M.; Crossley, A.; Stone, R.; Richardson, D. The Influence of Ethanol Blends on Particulate Matter Emissions from Gasoline Direct Injection Engines; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2010. [Google Scholar]
- Burke, S.C.; Ratcliff, M.; McCormick, R.; Rhoads, R.; Windom, B. Distillation-based Droplet Modeling of Non-Ideal Oxygenated Gasoline Blends: Investigating the Role of Droplet Evaporation on PM Emissions. SAE Int. J. Fuels Lubr. 2017, 10, 69–81. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Chen, R.; Nishida, K. Spray evaporation of ethanol–gasoline-like blend and combustion of ethanol–gasoline blend injected by hole-type nozzle for direct-injection spark ignition engines. Fuel 2014, 134, 263–273. [Google Scholar] [CrossRef]
- Rodríguez-Antón, L.M.; Gutiérrez-Martín, F.; Martinez-Arevalo, C. Experimental determination of some physical properties of gasoline, ethanol and ETBE ternary blends. Fuel 2015, 156, 81–86. [Google Scholar] [CrossRef]
- Luo, Y.Q.; Zhu, L.; Fang, J.H.; Zhuang, Z.Y.; Guan, C.; Xia, C.; Xie, X.M.; Huang, Z. Size distribution, chemical composition and oxidation reactivity of particulate matter from gasoline direct injection (GDI) engine fueled with ethanol-gasoline fuel. Appl. Therm. Eng. 2015, 89, 647–655. [Google Scholar] [CrossRef]
- Geng, P.; Zhang, H.; Yang, S. Experimental investigation on the combustion and particulate matter (PM) emissions from a port-fuel injection (PFI) gasoline engine fueled with methanol–ultralow sulfur gasoline blends. Fuel 2015, 145, 221–227. [Google Scholar] [CrossRef]
- Mohd Murad, S.H.; Camm, J.; Stone, C.R.; Davy, M.H.; Richardson, D. Spray Behaviour and Particulate Matter Emissions with M15 Methanol/Gasoline Blends in a GDI Engine; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2016. [Google Scholar]
- Liang, B.; Ge, Y.; Tan, J.; Han, X.; Gao, L.; Hao, L.; Ye, W.; Dai, P. Comparison of PM emissions from a gasoline direct injected (GDI) vehicle and a port fuel injected (PFI) vehicle measured by electrical low pressure impactor (ELPI) with two fuels: Gasoline and M15 methanol gasoline. J. Aerosol. Sci. 2013, 57, 22–31. [Google Scholar] [CrossRef]
- Turner, J.; Pearson, R.; Purvis, R.; Dekker, E. GEM Ternary Blends: Removing the Biomass Limit by using Iso-Stoichiometric Mixtures of Gasoline, Ethanol and Methanol; SAE Technical Paper 2011-24-0113; 2011. [Google Scholar]
- Pearson, R.; Turner, J.; Bell, A.; de Goede, S.; Woolard, C.; Davy, M. Iso-Stoichiometric Fuel Blends: Characterization of Physico-Chemical Properties for Mixtures of Gasoline, Ethanol, Methanol, and Water. Proc. Inst. Mech. Eng. Part D J. Automob. Eng. 2014, 229, 111–139. [Google Scholar] [CrossRef]
- Sileghem, L.; Coppens, A.; Casier, B.; Vancoillie, J.; Verhelst, S. Performance and emissions of iso-stoichiometric ternary GEM blends on a production SI engine. Fuel 2014, 117, 286–293. [Google Scholar] [CrossRef]
- Turner, J.W.G.; Pearson, R.J.; Dekker, E.; Iosefa, B.; Johansson, K.; Ac Bergström, K. Extending the role of alcohols as transport fuels using iso-stoichiometric ternary blends of gasoline, ethanol and methanol. Appl. Energy 2013, 102, 72–86. [Google Scholar] [CrossRef]
- Jin, C.; Yao, M.; Liu, H.; Chia-fon, F.L.; Ji, J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011, 15, 4080–4106. [Google Scholar] [CrossRef]
- Tao, L.; Tan, E.C.D.; McCormick, R.; Zhang, M.; Aden, A.; He, X.; Zigler, B.T. Techno-economic analysis and life-cycle assessment of cellulosic isobutanol and comparison with cellulosic ethanol and n-butanol. Biofuels Bioprod. Biorefin. 2014, 8, 30–48. [Google Scholar] [CrossRef]
| Emissions | Units | Euro 5a | Euro 5b | Euro 6b | Euro 6c |
|---|---|---|---|---|---|
| September 2009 | September 2011 | September 2014 | September 2017 | ||
| PM | mg/km | 5 | 4.5 | 4.5 | 4.5 |
| PN | #/km | - | - | 6.0 × 1012 | 6.0 × 1011 |
| Fuels | (kJ/kg) | (kJ/kg Stoichiometric Mixture) † | LHV (MJ/L) | RON |
|---|---|---|---|---|
| Gasoline | 310 * | 20 * | 32.3 | 97.0 |
| Methanol | 1191 | 159 | 17.9 | 127–136 |
| Ethanol | 922 | 92 | 21.2 | 120–135 |
| n-Butanol | 688 | 57 | 26.8 | 96 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, M.; Chen, L.; Leach, F.; Ding, S. A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques. Energies 2018, 11, 1417. https://doi.org/10.3390/en11061417
Raza M, Chen L, Leach F, Ding S. A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques. Energies. 2018; 11(6):1417. https://doi.org/10.3390/en11061417
Chicago/Turabian StyleRaza, Mohsin, Longfei Chen, Felix Leach, and Shiting Ding. 2018. "A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques" Energies 11, no. 6: 1417. https://doi.org/10.3390/en11061417
APA StyleRaza, M., Chen, L., Leach, F., & Ding, S. (2018). A Review of Particulate Number (PN) Emissions from Gasoline Direct Injection (GDI) Engines and Their Control Techniques. Energies, 11(6), 1417. https://doi.org/10.3390/en11061417