In Deep Analysis on the Behavior of Grape Marc Constituents during Hydrothermal Carbonization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock Characteristics
2.2. Physical and Chemical Characterizations
2.3. Experimental Procedure
2.4. The Severity Factor Approach
3. Results and Discussion
3.1. Solid Phase (Hydrochar)
3.2. Liquid Products
3.3. Gaseous Products
3.4. Overall Balances
3.5. Yields Prediction Using a Severity Factor
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- European Commission. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 2 May 2016).
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 89–124. [Google Scholar] [CrossRef]
- Basso, D.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of waste biomass: Progress report and prospects. In Proceedings of the 21st European Biomass Conference and Exhibition, Copenhagen, Denmark, 3–7 June 2013. [Google Scholar]
- Volpe, M.; Goldfarb, J.L.; Fiori, L. Hydrothermal carbonization of Opuntia ficus-indica cladodes: Role of process parameters on hydrochar properties. Bioresour. Technol. 2018, 247, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Volpe, M.; Volpe, R.; Fiori, L.; Dahl, O. Spatially resolved spectral determiantion of polysaccharides in hydrothermally carbonized biomass. Green Chem. 2018. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Strong, P.J.; Xu, S.; Liu, S.; Lu, K.; Sheng, K.; Guo, J.; Che, L.; He, L.; et al. Thermal Properties of Biochars Derived from Waste Biomass Generated by Agricultural and Forestry Sectors. Energies 2017, 10, 469. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoon, Y.-M. Energy Recovery Efficiency of Poultry Slaughterhouse Sludge Cake by Hydrothermal Carbonization. Energies 2017, 10, 1876. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Kong, L. Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density. Energies 2017, 10, 2094. [Google Scholar] [CrossRef]
- Zhao, S.-X.; Ta, N.; Wang, X.-D. Effect of Temperature on the Structural and Physicochemical Properties of Biochar with Apple Tree Branches as Feedstock Material. Energies 2017, 10, 1293. [Google Scholar]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Cai, J.; Li, B.; Chen, C.; Wang, J.; Zhao, M.; Zhang, K. Hydrothermal carbonization of tobacco stalk for fuel application. Bioresour. Technol. 2016, 220, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, M.; Kwong, C.W.; Broström, M.; Yoshikawa, K. Hydrothermal treatment of grape marc for solid fuel applications. Energy Convers. Manag. 2017, 145, 371–377. [Google Scholar] [CrossRef]
- Basso, D.; Pavanetto, R. Greenpeat: An innovative sustainable material recovered from waste. Procedia Environ. Sci. Eng. Manag. 2017, 4, 9–16. [Google Scholar]
- Purnomo, C.W.; Castello, D.; Fiori, L. Granular activated carbon from grape seeds hydrothermal char. Appl. Sci. 2018, 8, 331. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, E. Treatment of urban sludge by hydrothermal carbonization. Bioresour. Technol. 2017, 238, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations Statistics Division (FAO). 2015. Available online: http://faostat3.fao.org/home/E (accessed on 19 February 2015).
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Lucian, M.; Fiori, L. Hydrothermal carbonization of waste biomass: Process design, modeling, energy efficiency and cost analysis. Energies 2017, 10, 211. [Google Scholar] [CrossRef]
- Fiori, L.; Florio, L. Gasification and combustion of grape marc: Comparison among different scenarios. Waste Biomass Valorization 2010, 1, 191–200. [Google Scholar] [CrossRef]
- Jordan, R. Ecorecycle Australian Report on Grape Marc Utilization—Cold Pressed Grape Seed Oil and Meal by the Cooperative Research Centre for International Food Manufacture and Packaging Science. 2008. Available online: http://www.ecorecycle.vic.gov.au (accessed on 22 April 2018).
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; De Bolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol: Chemical composition, pre-treatment and saccharification. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.; Hortala, E.-W.; Patuzzi, F.; Castello, D.; Baratieri, M.; Fiori, L. Hydrothermal carbonization of off-specification compost: A byproduct of the organic municipal solid waste treatment. Bioresour. Technol. 2015, 182, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Fiori, L.; Basso, D.; Castello, D.; Baratieri, M. Hydrothermal carbonization of biomass: Design of a batch reactor and preliminary experimental results. Chem. Eng. Trans. 2014, 37, 55–60. [Google Scholar] [CrossRef]
- Ruyter, H.P. Coalification model. Fuel 1982, 61, 1182–1187. [Google Scholar] [CrossRef]
- Abatzoglou, N.; Chornet, E.; Belkacemi, K.; Overend, R.P. Phenomenological kinetics of complex systems: The development of a generalized severity parameter and its application to lignocellulosics fractionation. Chem. Eng. Sci. 1992, 47, 1109–1122. [Google Scholar] [CrossRef]
- Janga, K.K.; Oyaas, K.; Hertzberg, T.; Moe, S.T. Application of a pseudo-kinetic generalized severity model to the concentrated sulfuric acid hydrolysis of pinewood and aspenwood. BioResources 2012, 7, 2728–2741. [Google Scholar]
- Kieseler, S.; Neubauer, Y.; Zobel, N. Ultimate and proximate correlations for estimating the higher heating value of hydrothermal solids. Energy Fuels 2013, 27, 908–918. [Google Scholar] [CrossRef]
- Reza, M.T.; Yan, W.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J.; Vàsquez, V.R. Reaction kinetics of hydrothermal carbonization of loblolly pine. Bioresour. Technol. 2013, 139, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Forchheim, D.; Hornung, U.; Kruse, A.; Sutter, T. Kinetic modelling of hydrothermal lignin depolymerisation. Waste Biomass Valorization 2014, 5, 985–994. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Graphical-statistical method for the study of structure and reaction processes of coal. Fuel 1950, 29, 269–284. [Google Scholar]
- Funke, A.; Ziegler, F. Heat of reaction measurements for hydrothermal carbonization of biomass. Bioresour. Technol. 2011, 102, 7595–7598. [Google Scholar] [CrossRef] [PubMed]
- Schafer, H.N.S. Factors affecting the equilibrium moisture content of low rank coals. Fuel 1972, 51, 4–9. [Google Scholar] [CrossRef]
- Blaszò, M.; Jakab, E.; Vargha, A.; Székely, T.; Zoebel, H.; Klare, H.; Keil, G. The effect of hydrothermal treatment on Merseburg lignite. Fuel 1986, 65, 337–341. [Google Scholar]
- Lau, F.S.; Roberts, M.J.; Rue, D.M.; Punwani, D.V.; Wen, W.W.; Johnson, P.B. Peat beneficiation by wet carbonization. Int. J. Coal Geol. 1987, 8, 111–121. [Google Scholar] [CrossRef]
- Pala, M.; Kantarli, I.C.; Buyukisik, H.B.; Yanik, J. Hydrothermal carbonization and torrefaction of grape pomace: A comparative evaluation. Bioresour. Technol. 2014, 161, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Namioka, T.; Yoshikawa, K. Effects of hydrothermal treatment on characteristics and combustion behaviors of municipal solid wastes. Appl. Energy 2011, 88, 3659–3664. [Google Scholar] [CrossRef]
- Müller, J.B.; Vogel, F. Tar and coke formation during hydrothermal processing of glycerol and glucose. Influence of temperature, residence time and feed concentration. J. Supercrit. Fluids 2012, 70, 126–136. [Google Scholar]
- Hoekman, S.K.; Broch, A.; Felix, L.; Farthing, W. Hydrothermal carbonization (HTC) of loblolly pine using a continuous, reactive twin-screw extruder. Energy Convers. Manag. 2017, 134, 247–259. [Google Scholar] [CrossRef]
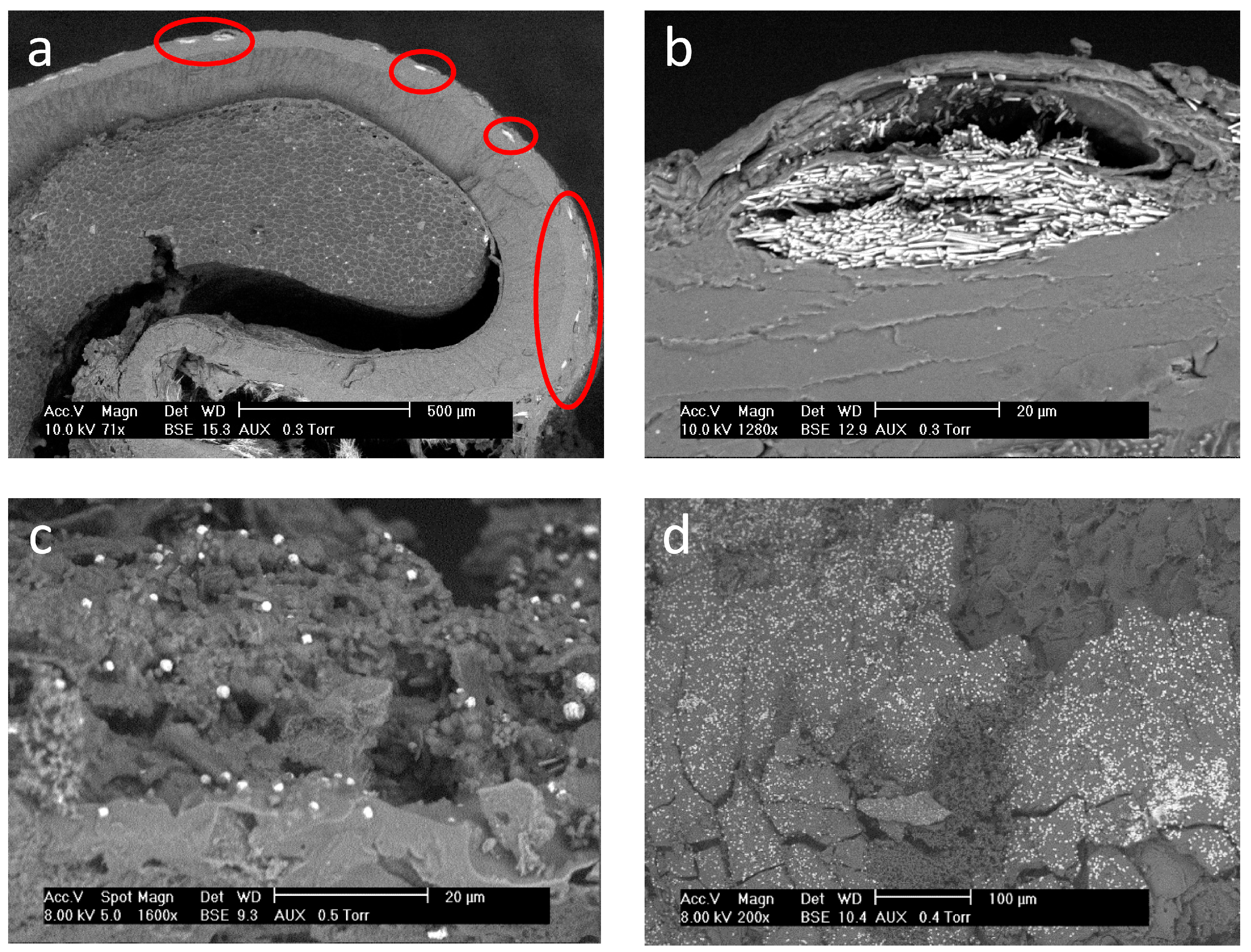
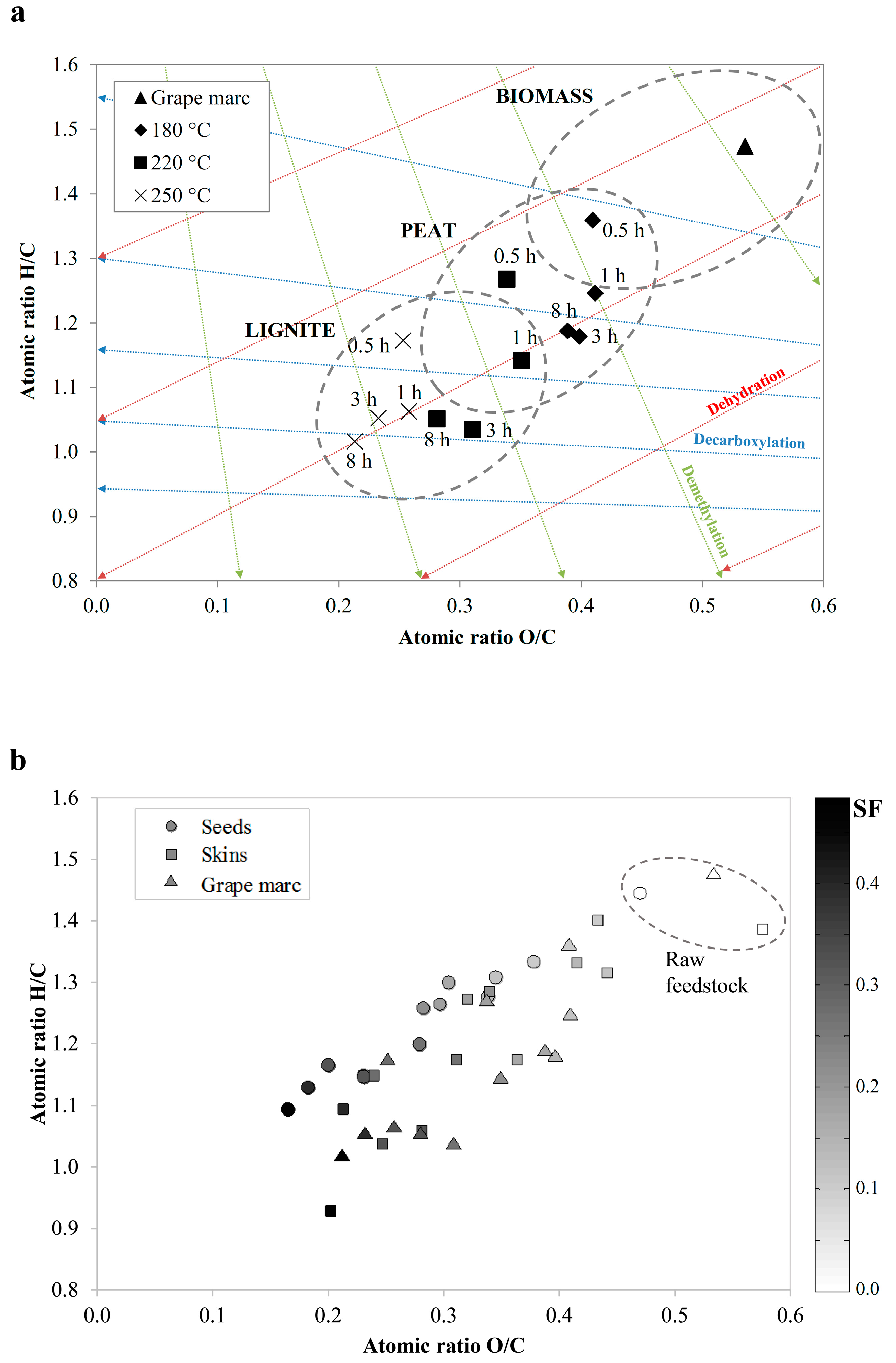
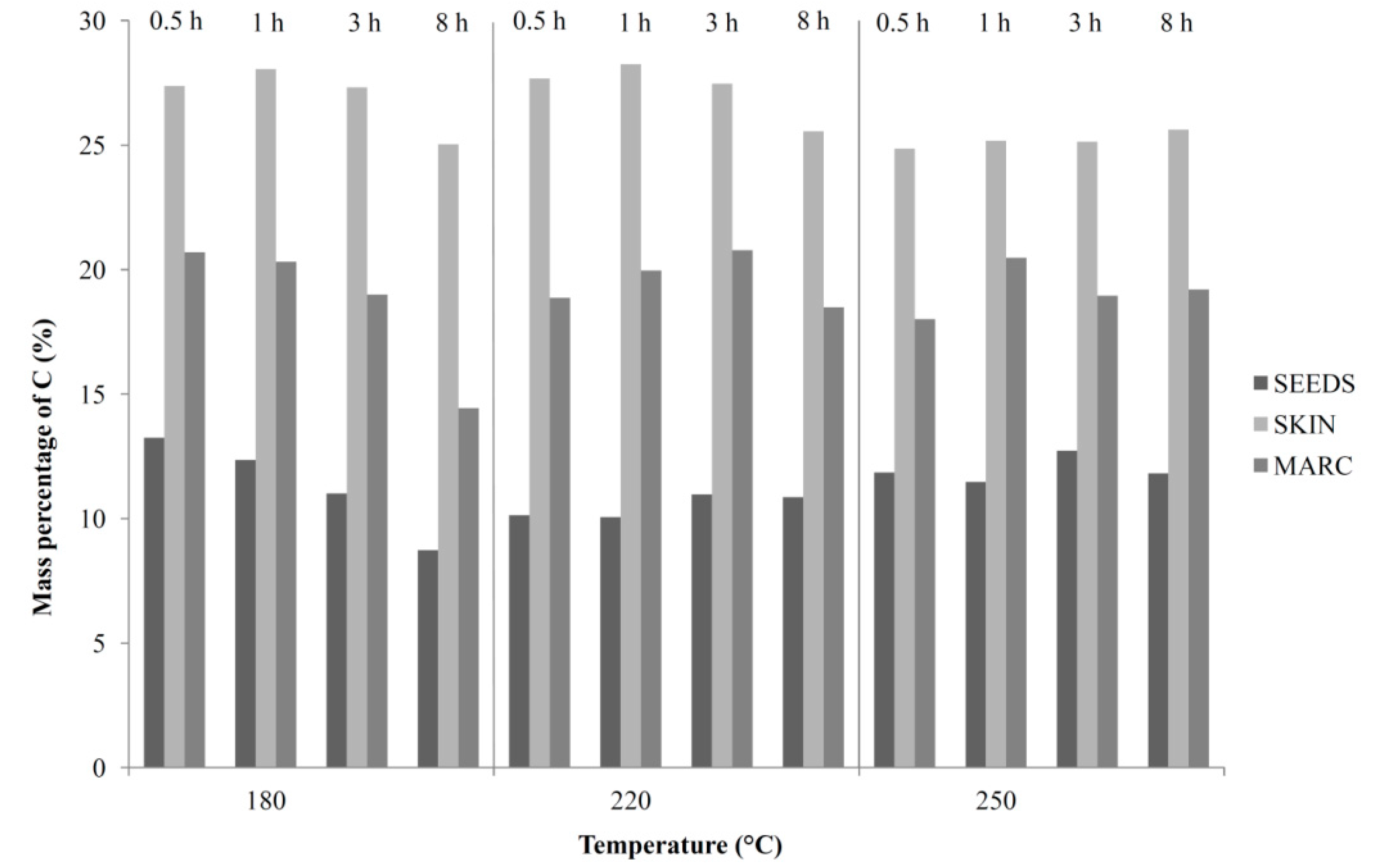
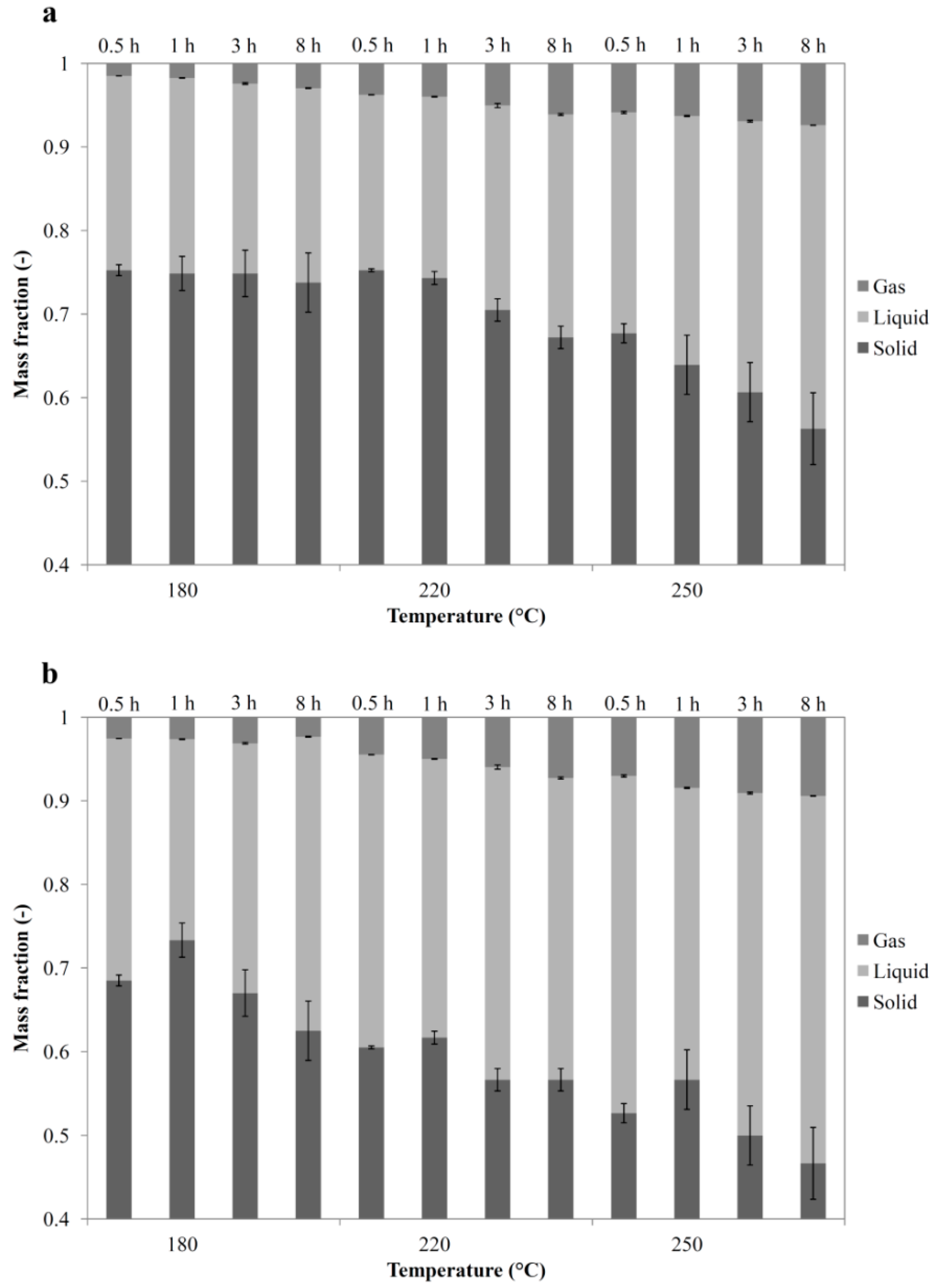
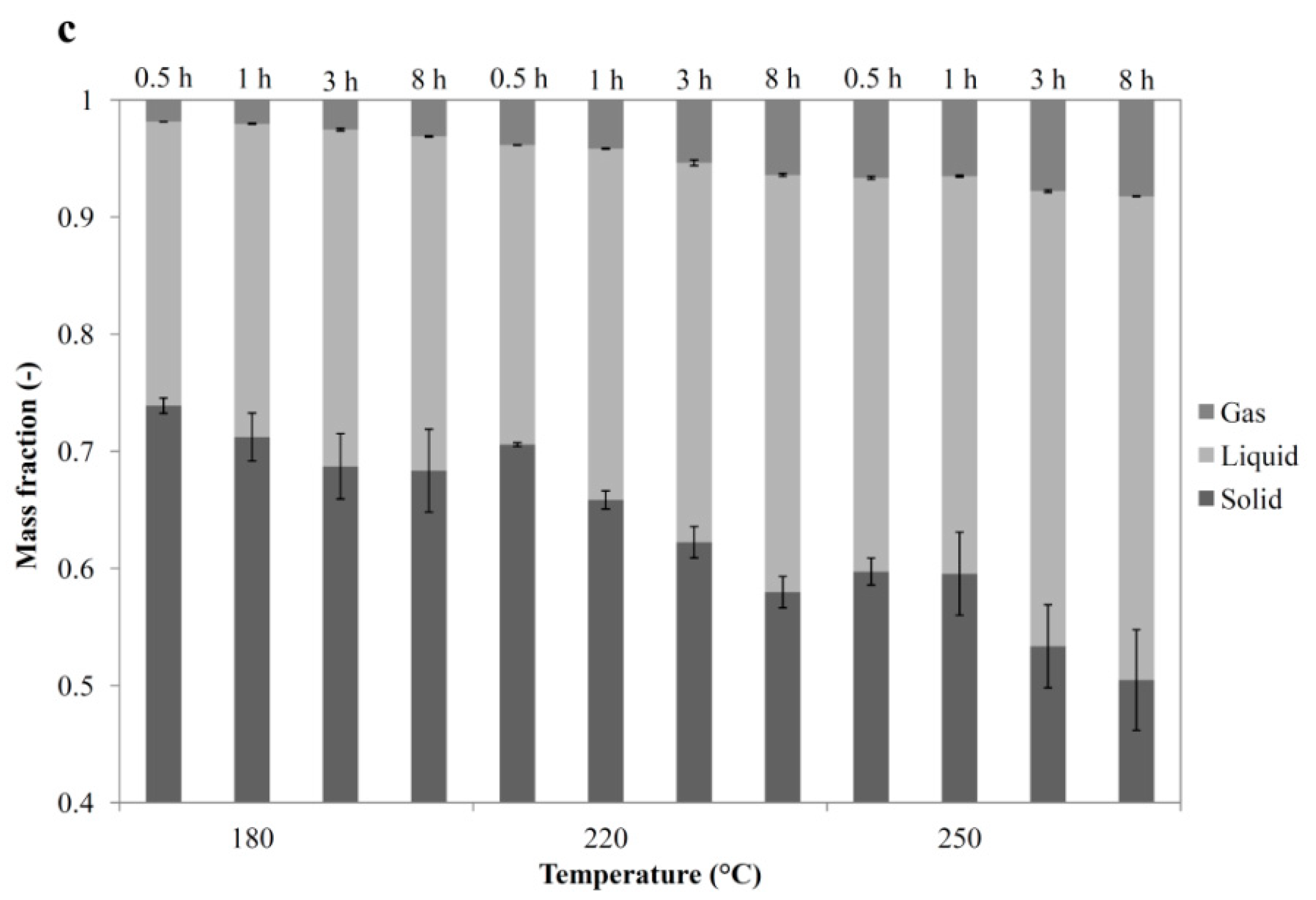
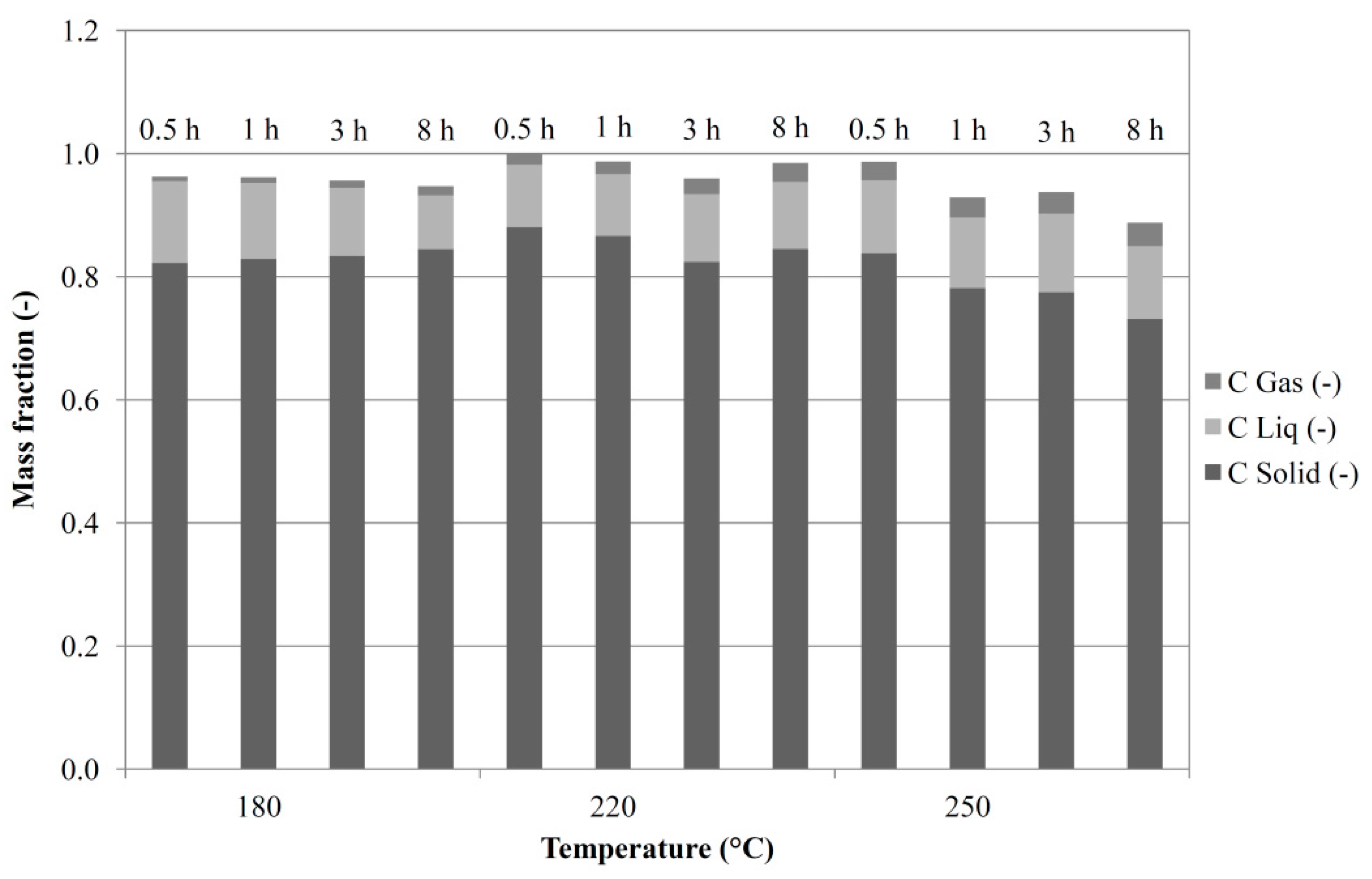
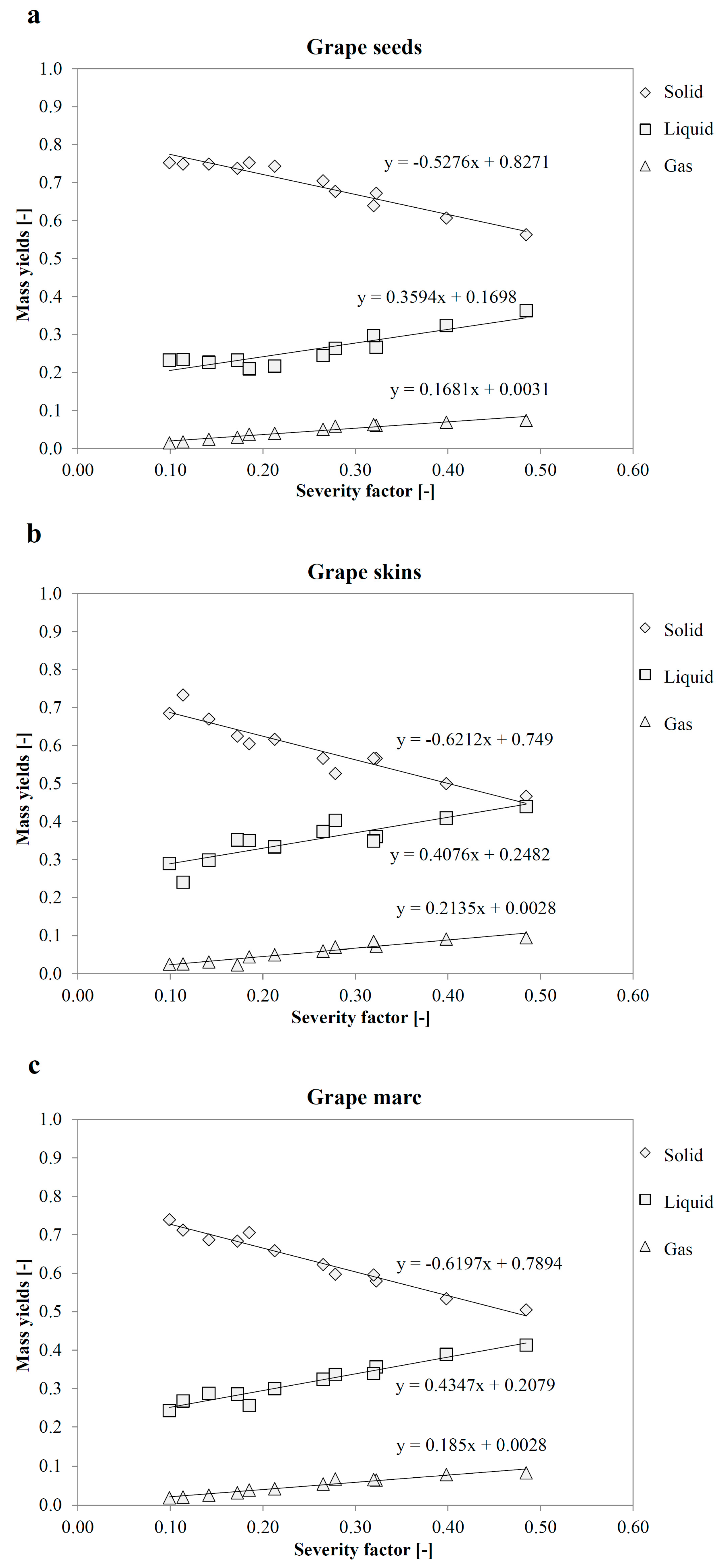
| Grape Seeds | Grape Skins | Grape Marc | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Time (h) | SF | C (%) | H (%) | N (%) | O (%) | Ash (%) | C (%) | H (%) | N (%) | O (%) | Ash (%) | C (%) | H (%) | N (%) | O (%) | Ash (%) |
| Raw feedstock | - | 0 | 54.4 | 6.6 | 1.6 | 34.2 | 3.2 | 46.8 | 5.4 | 2.6 | 36.2 | 9.0 | 49.7 | 6.2 | 2.4 | 35.5 | 6.1 |
| 180 | 0.5 | 0.099 | 59.5 | 6.7 | 1.4 | 30.1 | 2.2 | 54.9 | 6.5 | 2.5 | 31.9 | 3.9 | 56.9 | 6.5 | 2.0 | 31.1 | 3.3 |
| 1 | 0.114 | 60.2 | 6.6 | 1.3 | 27.8 | 2.5 | 54.5 | 6.0 | 2.6 | 32.2 | 4.6 | 56.2 | 5.9 | 2.5 | 30.8 | 4.5 | |
| 3 | 0.142 | 60.6 | 6.5 | 1.4 | 27.4 | 2.7 | 55.4 | 6.2 | 2.3 | 30.8 | 5.1 | 57.0 | 5.6 | 2.6 | 30.3 | 4.5 | |
| 8 | 0.172 | 62.3 | 6.8 | 1.4 | 25.4 | 3.5 | 58.2 | 5.7 | 2.1 | 28.3 | 5.6 | 57.2 | 5.7 | 2.8 | 29.7 | 3.7 | |
| 220 | 0.5 | 0.185 | 63.7 | 6.8 | 1.5 | 25.3 | 2.6 | 59.7 | 6.4 | 2.3 | 25.6 | 5.6 | 60.4 | 6.4 | 2.0 | 27.3 | 3.7 |
| 1 | 0.213 | 63.4 | 6.7 | 1.6 | 24.0 | 2.8 | 58.8 | 6.3 | 2.3 | 26.7 | 5.4 | 59.8 | 5.7 | 2.6 | 28.0 | 3.8 | |
| 3 | 0.265 | 63.6 | 6.4 | 1.6 | 23.8 | 2.9 | 61.0 | 6.0 | 2.3 | 25.4 | 5.2 | 62.5 | 5.4 | 2.7 | 25.9 | 3.5 | |
| 8 | 0.323 | 68.4 | 6.7 | 1.9 | 18.3 | 3.1 | 62.3 | 5.5 | 2.7 | 23.4 | 5.9 | 64.1 | 5.7 | 2.2 | 24.0 | 4.0 | |
| 250 | 0.5 | 0.278 | 67.3 | 6.5 | 1.7 | 20.8 | 3.6 | 64.7 | 6.2 | 2.3 | 20.7 | 5.8 | 64.7 | 6.4 | 2.2 | 21.8 | 4.8 |
| 1 | 0.320 | 66.5 | 6.4 | 1.8 | 20.5 | 3.0 | 63.9 | 5.6 | 2.7 | 21.2 | 6.6 | 64.9 | 5.8 | 2.8 | 22.3 | 3.9 | |
| 3 | 0.398 | 69.5 | 6.6 | 1.9 | 17.0 | 3.1 | 66.3 | 6.1 | 3.0 | 18.9 | 5.6 | 65.6 | 6.0 | 2.8 | 20.3 | 5.3 | |
| 8 | 0.485 | 70.7 | 6.5 | 2.0 | 15.7 | 3.9 | 68.2 | 5.3 | 3.1 | 18.4 | 4.3 | 68.1 | 5.8 | 2.6 | 19.4 | 4.2 | |
| Temperature (°C) | Time (h) | HHV Seeds (MJ/kg) | HHV Skins (MJ/kg) | HHV Marc (MJ/kg) |
|---|---|---|---|---|
| Raw feedstock | - | 22.52 | 18.66 | 20.59 |
| 180 | 0.5 | 22.84 | 19.16 | 21.51 |
| 1 | 24.92 | 19.38 | 22.46 | |
| 3 | 24.36 | 20.69 | 22.72 | |
| 8 | 24.36 | 23.84 | 23.52 | |
| 220 | 0.5 | 22.70 | 21.84 | 21.03 |
| 1 | 22.91 | 24.36 | 20.95 | |
| 3 | 26.35 | 23.11 | 21.71 | |
| 8 | 26.09 | 26.72 | 24.01 | |
| 250 | 0.5 | 22.97 | 21.88 | 23.24 |
| 1 | 23.33 | 24.56 | 24.86 | |
| 3 | 27.81 | 24.85 | 26.09 | |
| 8 | 28.79 | 27.54 | 26.19 |
| Feedstock | Temp. (°C) | Res. Time (h) | Al | Ca | Cu | Fe | K | Mg | P | S | Si | Zn | Total (μg/L) | Total (μg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grape seeds | 180 | 0.5 | 0.0 | 65.6 | 0.3 | 7.2 | 1716.9 | 63.5 | 141.8 | 2.0 | 22.5 | 1.4 | 2021.2 | 41.2 |
| 1 | 0.0 | 54.6 | 0.4 | 31.8 | 2223.6 | 87.5 | 344.4 | 46.8 | 69.0 | 3.3 | 2861.3 | 58.4 | ||
| 3 | 0.0 | 15.9 | 1.6 | 17.7 | 2247.2 | 83.3 | 279.7 | 42.6 | 51.1 | 2.4 | 2741.6 | 55.9 | ||
| 8 | 0.0 | 6.1 | 0.2 | 21.8 | 2151.4 | 73.5 | 224.6 | 27.0 | 46.1 | 2.8 | 2553.5 | 52.1 | ||
| 220 | 0.5 | 0.0 | 37.2 | 0.4 | 8.5 | 1788.6 | 42.3 | 41.6 | 3.2 | 21.9 | 0.5 | 1944.3 | 39.7 | |
| 1 | 0.0 | 3.8 | 0.4 | 4.4 | 2031.4 | 47.9 | 74.7 | 38.5 | 59.4 | 1.1 | 2261.6 | 46.1 | ||
| 3 | 0.0 | 3.3 | 0.1 | 3.5 | 1945.0 | 44.2 | 20.7 | 28.7 | 45.4 | 0.9 | 2091.8 | 42.7 | ||
| 8 | 0.0 | 89.2 | 0.4 | 2.0 | 2036.4 | 43.6 | 6.3 | 20.1 | 46.9 | 1.2 | 2245.9 | 45.8 | ||
| 250 | 0.5 | 0.0 | 49.1 | 0.0 | 11.1 | 2197.2 | 17.1 | 32.8 | 6.4 | 43.9 | 0.9 | 2358.4 | 48.1 | |
| 1 | 0.0 | 127.3 | 0.3 | 3.3 | 2099.1 | 37.4 | 19.6 | 38.6 | 62.6 | 1.8 | 2389.9 | 48.8 | ||
| 3 | 0.0 | 131.8 | 0.2 | 1.0 | 2233.1 | 37.4 | 9.7 | 16.0 | 57.5 | 0.9 | 2487.7 | 50.7 | ||
| 8 | 0.0 | 165.6 | 0.4 | 0.4 | 2255.2 | 36.2 | 3.4 | 11.8 | 44.6 | 1.0 | 2518.6 | 51.4 | ||
| Grape skins | 180 | 0.5 | 0.0 | 126.5 | 0.0 | 0.5 | 2006.4 | 33.7 | 78.2 | 6.4 | 14.1 | 0.3 | 2266.1 | 68.0 |
| 1 | 0.0 | 126.8 | 0.0 | 1.1 | 2144.8 | 36.5 | 129.9 | 56.6 | 18.4 | 2.9 | 2517.0 | 75.5 | ||
| 3 | 0.0 | 69.8 | 0.0 | 1.2 | 2433.3 | 37.6 | 126.2 | 62.2 | 16.7 | 2.7 | 2749.6 | 82.5 | ||
| 8 | 0.0 | 12.4 | 0.0 | 4.2 | 2541.4 | 38.7 | 102.9 | 56.8 | 19.3 | 2.0 | 2777.8 | 83.3 | ||
| 220 | 0.5 | 0.0 | 40.8 | 0.0 | 0.8 | 2612.5 | 23.8 | 42.4 | 9.5 | 12.4 | 0.2 | 2742.5 | 82.3 | |
| 1 | 0.0 | 3.9 | 0.0 | 1.5 | 2887.0 | 30.3 | 75.8 | 73.7 | 16.7 | 2.2 | 3091.2 | 92.7 | ||
| 3 | 0.0 | 5.6 | 0.0 | 0.0 | 2936.0 | 27.2 | 51.7 | 64.5 | 18.4 | 2.1 | 3105.5 | 93.2 | ||
| 8 | 0.0 | 2.6 | 0.0 | 0.0 | 2897.0 | 24.6 | 37.5 | 63.9 | 22.3 | 3.4 | 3051.3 | 91.5 | ||
| 250 | 0.5 | 0.0 | 30.3 | 0.0 | 0.2 | 2381.1 | 19.5 | 15.4 | 12.9 | 11.8 | 0.1 | 2471.2 | 74.1 | |
| 1 | 0.0 | 3.6 | 0.0 | 0.0 | 3000.8 | 19.9 | 39.3 | 71.1 | 22.9 | 1.7 | 3159.3 | 94.8 | ||
| 3 | 0.0 | 19.8 | 0.0 | 0.0 | 3077.1 | 17.4 | 14.9 | 59.3 | 34.7 | 1.3 | 3224.5 | 96.7 | ||
| 8 | 0.0 | 27.0 | 0.0 | 0.0 | 3012.5 | 15.7 | 23.8 | 54.3 | 27.5 | 0.9 | 3161.7 | 94.9 | ||
| Grape marc | 180 | 0.5 | 0.0 | 153.8 | 0.0 | 3.3 | 2495.9 | 57.7 | 129.0 | 10.9 | 24.2 | 0.9 | 2875.7 | 77.6 |
| 1 | 0.0 | 133.6 | 0.0 | 6.2 | 2522.2 | 65.2 | 217.4 | 96.7 | 25.1 | 3.3 | 3069.8 | 82.9 | ||
| 3 | 0.0 | 38.9 | 0.0 | 7.1 | 3014.0 | 68.6 | 199.6 | 98.0 | 30.6 | 1.3 | 3458.1 | 93.4 | ||
| 8 | 0.0 | 12.1 | 0.0 | 6.1 | 3116.4 | 52.9 | 144.5 | 70.2 | 29.3 | 1.2 | 3432.7 | 92.7 | ||
| 220 | 0.5 | 0.0 | 34.0 | 0.0 | 0.7 | 2974.5 | 38.7 | 54.4 | 7.2 | 20.7 | 0.4 | 3130.6 | 84.5 | |
| 1 | 0.0 | 11.4 | 0.0 | 2.8 | 3806.0 | 49.8 | 114.1 | 92.7 | 30.5 | 1.9 | 4109.2 | 110.9 | ||
| 3 | 0.0 | 7.9 | 0.0 | 1.8 | 4159.5 | 45.9 | 73.1 | 86.1 | 36.6 | 3.3 | 4414.2 | 119.2 | ||
| 8 | 0.0 | 7.9 | 0.0 | 0.1 | 3402.4 | 39.6 | 49.8 | 65.6 | 31.9 | 1.6 | 3598.7 | 97.2 | ||
| 250 | 0.5 | 0.0 | 19.9 | 0.0 | 0.4 | 2870.5 | 29.3 | 5.1 | 12.0 | 25.6 | 0.4 | 2963.2 | 80.0 | |
| 1 | 0.0 | 8.5 | 0.0 | 0.0 | 3616.1 | 35.6 | 45.0 | 69.5 | 34.5 | 1.6 | 3810.6 | 102.9 | ||
| 3 | 0.0 | 41.8 | 0.0 | 0.0 | 3697.2 | 29.0 | 27.5 | 55.9 | 34.9 | 1.5 | 3887.8 | 105.0 | ||
| 8 | 0.0 | 47.3 | 0.0 | 0.0 | 3577.2 | 28.8 | 14.2 | 47.8 | 41.3 | 1.0 | 3757.6 | 101.5 |
| Temperature (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 180 | 220 | 250 | |||||||
| Residence Time (h) | Seeds | Skins | Marc | Seeds | Skins | Marc | Seeds | Skins | Marc |
| 0.5 | 1.5 | 2.5 | 1.9 | 3.8 | 4.5 | 3.9 | 5.9 | 7.0 | 6.7 |
| 1 | 1.7 | 2.6 | 2.0 | 4.0 | 5.0 | 4.2 | 6.3 | 8.5 | 6.5 |
| 3 | 2.4 | 3.1 | 2.5 | 5.0 | 6.0 | 5.4 | 6.9 | 9.1 | 7.8 |
| 8 | 3.0 | 3.6 | 3.1 | 6.1 | 7.3 | 6.4 | 7.4 | 9.4 | 8.2 |
| Temperature (°C) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 180 | 220 | 250 | |||||||||||
| Feedstock | Res. Time (h) | CO2 | CO | CH4 | H2 | CO2 | CO | CH4 | H2 | CO2 | CO | CH4 | H2 |
| Grape seeds | 0.5 | 97.65 | 2.09 | 0.00 | 0.26 | 96.79 | 3.06 | 0.00 | 0.15 | 94.91 | 4.85 | 0.03 | 0.20 |
| 1 | 99.08 | 0.80 | 0.00 | 0.12 | 95.76 | 3.91 | 0.01 | 0.32 | 94.43 | 5.25 | 0.06 | 0.27 | |
| 3 | 98.37 | 1.16 | 0.00 | 0.47 | 95.41 | 4.27 | 0.03 | 0.29 | 94.60 | 4.87 | 0.08 | 0.45 | |
| 8 | 97.19 | 2.11 | 0.00 | 0.70 | 94.86 | 4.45 | 0.02 | 0.66 | 92.59 | 5.93 | 0.38 | 1.11 | |
| Grape skins | 0.5 | 99.42 | 0.46 | 0.00 | 0.11 | 97.27 | 2.51 | 0.01 | 0.20 | 95.17 | 4.59 | 0.03 | 0.21 |
| 1 | 99.34 | 0.49 | 0.00 | 0.16 | 96.65 | 3.09 | 0.00 | 0.26 | 94.21 | 5.54 | 0.00 | 0.25 | |
| 3 | 98.90 | 0.78 | 0.00 | 0.32 | 96.30 | 3.37 | 0.00 | 0.33 | 93.78 | 5.80 | 0.00 | 0.42 | |
| 8 | 96.55 | 2.28 | 0.00 | 1.17 | 95.73 | 3.79 | 0.00 | 0.48 | 95.85 | 3.56 | 0.00 | 0.59 | |
| Grape marc | 0.5 | 99.43 | 0.52 | 0.00 | 0.05 | 96.89 | 2.96 | 0.02 | 0.12 | 94.93 | 4.86 | 0.04 | 0.17 |
| 1 | 99.10 | 0.71 | 0.05 | 0.14 | 95.97 | 3.72 | 0.03 | 0.29 | 93.74 | 5.89 | 0.07 | 0.29 | |
| 3 | 98.75 | 1.01 | 0.02 | 0.22 | 96.02 | 3.63 | 0.04 | 0.30 | 94.82 | 4.71 | 0.11 | 0.35 | |
| 8 | 98.17 | 1.44 | 0.03 | 0.36 | 96.02 | 3.64 | 0.10 | 0.24 | 96.32 | 2.95 | 0.17 | 0.55 | |
| Yield | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Solid (Hydrochar) | Liquid | Gas | |||||||
| SF | Exp | Mean | Err % | Exp | Mean | Err % | Exp | Mean | Err % |
| 0.1 | 0.727 | 0.731 | −0.4 | 0.251 | 0.247 | 1.6 | 0.021 | 0.022 | −3.3 |
| 0.2 | 0.665 | 0.673 | −1.1 | 0.295 | 0.286 | 3.2 | 0.040 | 0.041 | −3.2 |
| 0.3 | 0.603 | 0.616 | −2.0 | 0.338 | 0.324 | 4.4 | 0.058 | 0.060 | −3.1 |
| 0.4 | 0.542 | 0.558 | −3.0 | 0.382 | 0.362 | 5.3 | 0.077 | 0.079 | −3.1 |
| 0.5 | 0.480 | 0.501 | −4.3 | 0.425 | 0.401 | 6.1 | 0.095 | 0.098 | −3.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, D.; Weiss-Hortala, E.; Patuzzi, F.; Baratieri, M.; Fiori, L. In Deep Analysis on the Behavior of Grape Marc Constituents during Hydrothermal Carbonization. Energies 2018, 11, 1379. https://doi.org/10.3390/en11061379
Basso D, Weiss-Hortala E, Patuzzi F, Baratieri M, Fiori L. In Deep Analysis on the Behavior of Grape Marc Constituents during Hydrothermal Carbonization. Energies. 2018; 11(6):1379. https://doi.org/10.3390/en11061379
Chicago/Turabian StyleBasso, Daniele, Elsa Weiss-Hortala, Francesco Patuzzi, Marco Baratieri, and Luca Fiori. 2018. "In Deep Analysis on the Behavior of Grape Marc Constituents during Hydrothermal Carbonization" Energies 11, no. 6: 1379. https://doi.org/10.3390/en11061379
APA StyleBasso, D., Weiss-Hortala, E., Patuzzi, F., Baratieri, M., & Fiori, L. (2018). In Deep Analysis on the Behavior of Grape Marc Constituents during Hydrothermal Carbonization. Energies, 11(6), 1379. https://doi.org/10.3390/en11061379







