Simulation for the Effects of Well Pressure and Initial Temperature on Methane Hydrate Dissociation
Abstract
:1. Introduction
2. Methods
2.1. Mathematical Model
2.2. Mathematical Model Verification
3. Results and Analysis
3.1. Effects on Pressure Distribution
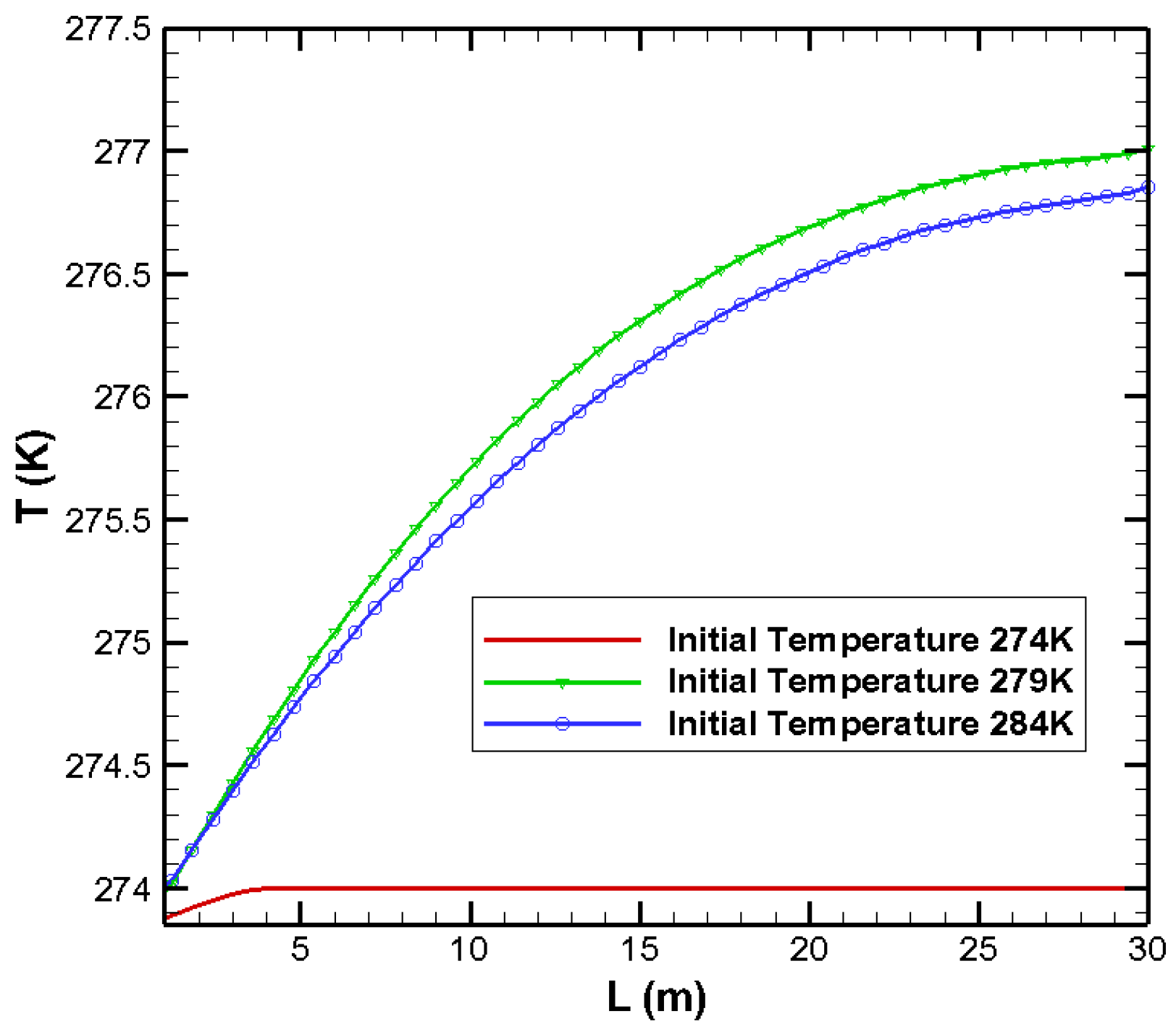
3.2. Effects on Temperature Distribution
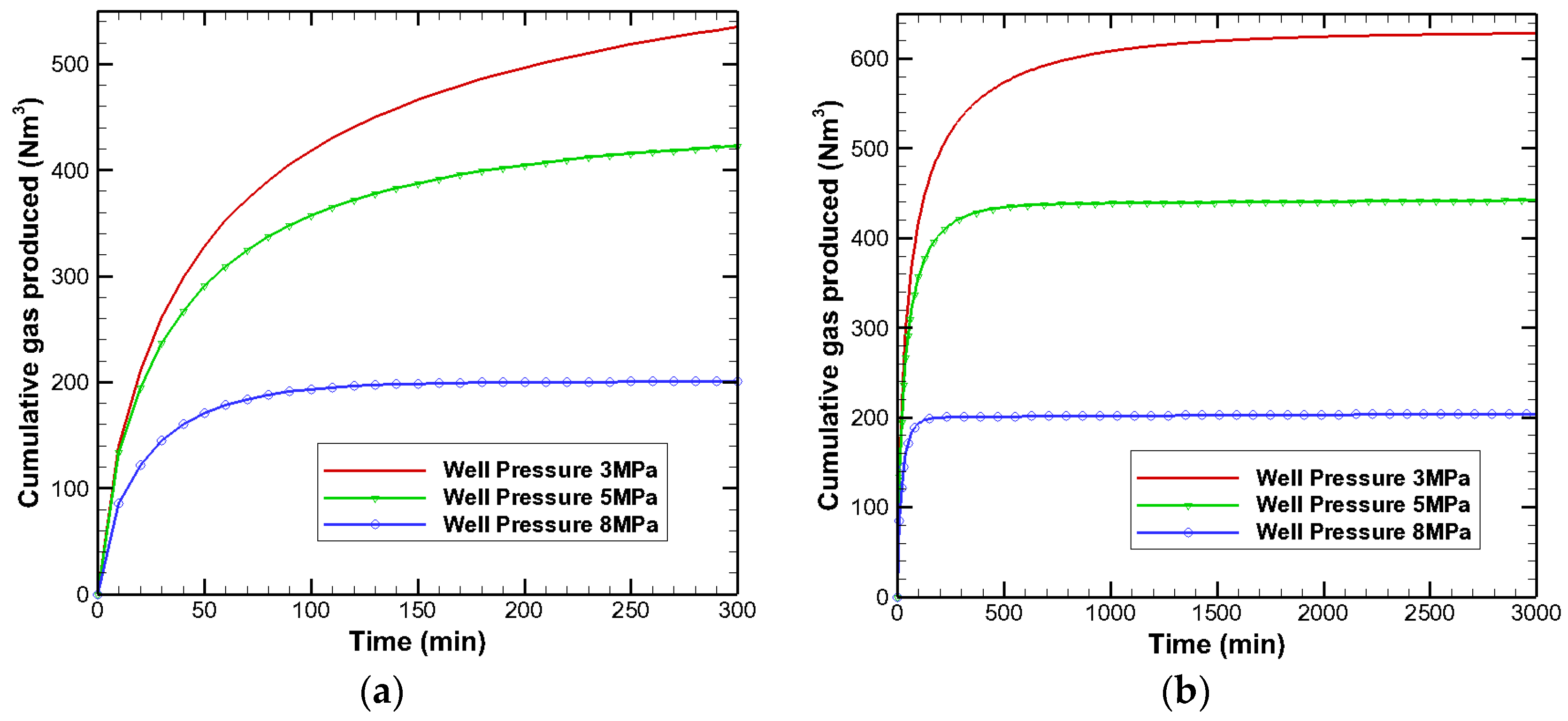
3.3. Effects on Gas Production
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Van der Waals, J.; Platteeuw, J. Clathrate solutions. Adv. Chem. Phys. 2007, 2, 1–57. [Google Scholar]
- Clarke, M.A.; Pooladi-Darvish, M.; Bishnoi, P.R. A method to predict equilibrium conditions of gas hydrate formation in porous media. Ind. Eng. Chem. Res. 1999, 38, 2485–2490. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Modeling gas hydrate phase equilibria in laboratory and natural porous media. Ind. Eng. Chem. Res. 2001, 40, 4197–4208. [Google Scholar] [CrossRef]
- Kim, H.; Bishnoi, P.; Heidemann, R.; Rizvi, S. Kinetics of methane hydrate decomposition. Chem. Eng. Sci. 1987, 42, 1645–1653. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Ishizaki, T. Dissociation condition measurements of methane hydrate in confined small pores of porous glass. J. Phys. Chem. B 1999, 103, 3659–3662. [Google Scholar] [CrossRef]
- Seshadri, K.; Wilder, J.W.; Smith, D.H. Measurements of equilibrium pressures and temperatures for propane hydrate in silica gels with different pore-size distributions. J. Phys. Chem. B 2001, 105, 2627–2631. [Google Scholar] [CrossRef]
- Smith, D.H.; Wilder, J.W.; Seshadri, K. Methane hydrate equilibria in silica gels with broad pore-size distributions. AIChE J. 2002, 48, 393–400. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.; Uchida, T. Methane and carbon dioxide hydrate phase behavior in small porous silica gels: Three-phase equilibrium determination and thermodynamic modeling. Langmuir 2002, 18, 9164–9170. [Google Scholar] [CrossRef]
- Sloan, E.D., Jr.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kwon, T.-H.; Cho, G.-C.; Santamarina, J.C. Gas hydrate dissociation in sediments: Pressure-temperature evolution. Geochem. Geophys. Geosyst. 2008, 9. [Google Scholar] [CrossRef]
- Song, Y.; Cheng, C.; Zhao, J.; Zhu, Z.; Liu, W.; Yang, M.; Xue, K. Evaluation of gas production from methane hydrates using depressurization, thermal stimulation and combined methods. Appl. Energy 2015, 145, 265–277. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Z.; Song, Y.; Liu, W.; Zhang, Y.; Wang, D. Analyzing the process of gas production for natural gas hydrate using depressurization. Appl. Energy 2015, 142, 125–134. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, D.; Yang, M.; Song, Y. Analysis of heat transfer effects on gas production from methane hydrate by depressurization. Int. J. Heat Mass Trans. 2014, 77, 529–541. [Google Scholar] [CrossRef]
- Jiang, L.; Wu, B.; Song, Y.; Yang, M.; Wang, D.; Liu, Y.; Xue, Z. Mass transfer coefficient measurement during brine flush in a CO2-filled packed bed by X-ray CT scanning. Int. J. Heat Mass Transf. 2017, 115, 615–624. [Google Scholar] [CrossRef]
- De Roo, J.; Peters, C.; Lichtenthaler, R.; Diepen, G. Occurrence of methane hydrate in saturated and unsaturated solutions of sodium chloride and water in dependence of temperature and pressure. AIChE J. 1983, 29, 651–657. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Effect of temperature and pressure on the solubility of carbon dioxide in water in the presence of gas hydrate. Fluid Phase Equilib. 2001, 190, 127–134. [Google Scholar] [CrossRef]
- Maekawa, T.; Itoh, S.; Sakata, S.; Igari, S.-I.; Imai, N. Pressure and temperature conditions for methane hydrate dissociation in sodium chloride solutions. Geochem. J. 1995, 29, 325–329. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, B. Memory effect on the pressure-temperature condition and induction time of gas hydrate nucleation. J. Nat. Gas Chem. 2010, 19, 446–451. [Google Scholar] [CrossRef]
- Holder, G.D.; Angert, P.F. Simulation of gas production from a reservoir containing both gas hydrates and free natural gas. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26–29 September 1982; Society of Petroleum Engineers: Richardson, TX, USA, 1982. [Google Scholar]
- McGuire, P.L. Recovery of gas from hydrate deposits using conventional technology. In Proceedings of the SPE Unconventional Gas Recovery Symposium, Pittsburgh, PA, USA, 16–18 May 1982; Society of Petroleum Engineers: Richardson, TX, USA, 1982. [Google Scholar]
- Burshears, M.; O’brien, T.; Malone, R. A multi-phase, multi-dimensional, variable composition simulation of gas production from a conventional gas reservoir in contact with hydrates. In Proceedings of the SPE Unconventional Gas Technology Symposium, Louisville, KY, USA, 18–21 May 1986; Society of Petroleum Engineers: Richardson, TX, USA, 1986. [Google Scholar]
- Zhao, J.; Yu, T.; Song, Y.; Liu, D.; Liu, W.; Liu, Y.; Yang, M.; Ruan, X.; Li, Y. Numerical simulation of gas production from hydrate deposits using a single vertical well by depressurization in the Qilian Mountain permafrost, Qinghai-Tibet Plateau, China. Energy 2013, 52, 308–319. [Google Scholar] [CrossRef]
- Jamaluddin, A.; Kalogerakis, N.; Bishnoi, P. Modelling of decomposition of a synthetic core of methane gas hydrate by coupling intrinsic kinetics with heat transfer rates. Can. J. Chem. Eng. 1989, 67, 948–954. [Google Scholar] [CrossRef]
- Makogon, I.F. Hydrates of Hydrocarbons; Pennwell Books: Houston, TX, USA, 1997. [Google Scholar]
- Masuda, Y.; Fujinaga, Y.; Naganawa, S.; Fujita, K.; Sato, K.; Hayashi, Y. Modeling and experimental studies on dissociation of methane gas hydrates in Berea sandstone cores. In Proceedings of the 3rd International Conference on Gas Hydrates, Salt Lake City, UT, USA, 18–22 July 1999; pp. 18–22. [Google Scholar]
- Masuda, Y.; Kurihara, M.; Ohuchi, H.; Sato, T. A field-scale simulation study on gas productivity of formations containing gas hydrates. In Proceedings of the 4th International Conference on Gas Hydrate, Yokohama, Japan, 19–23 May 2002; pp. 40–46. [Google Scholar]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Moridis, G.; Apps, J.; Pruess, K.; Myer, L. EOSHYDR: A TOUGH2 Module for CH4-Hydrate Release and Flow in the Subsurface; Report LBNL-42386; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 1998. [Google Scholar]
- Moridisd, G.J.; Collett, T.S. Preliminary Studies of Gas Production from Methane Hydrates in the Eileen Area, Alaska; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2002. [Google Scholar]
- Moridis, G.J. TOUGH + HYDRATE v1.2 User’s Manual: A Code for the Simulation of System Behavior in Hydrate-Bearing Geologic Media; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2014. [Google Scholar]
- Bai, Y.; Li, Q.; Li, X.; Du, Y. The simulation of nature gas production from ocean gas hydrate reservoir by depressurization. Sci. China Ser. E Technol. Sci. 2008, 51, 1272–1282. [Google Scholar] [CrossRef]
- Oyama, H.; Konno, Y.; Masuda, Y.; Narita, H. Dependence of depressurization-induced dissociation of methane hydrate bearing laboratory cores on heat transfer. Energy Fuels 2009, 23, 4995–5002. [Google Scholar] [CrossRef]
- Uchida, S.; Soga, K.; Yamamoto, K. Critical state soil constitutive model for methane hydrate soil. J. Geophys. Res. Solid Earth 2012, 117. [Google Scholar] [CrossRef]
- Daigle, H.; Dugan, B. Capillary controls on methane hydrate distribution and fracturing in advective systems. Geochem. Geophys. Geosyst. 2013, 12. [Google Scholar] [CrossRef]
- Mahabadi, N.; Dai, S.; Seol, Y.; Sup Yun, T.; Jang, J. The water retention curve and relative permeability for gas production from hydrate-bearing sediments: Pore-network model simulation. Geochem. Geophys. Geosyst. 2016, 17, 3099–3110. [Google Scholar] [CrossRef]
- Anderson, R.; Tohidi, B.; Webber, J.B.W. Gas hydrate growth and dissociation in narrow pore networks: Capillary inhibition and hysteresis phenomena, in Sediment- Hosted Gas Hydrates: New Insights on Natural and Synthetic Systems. Geol. Soc. Spec. Publ. 2009, 319, 145–159. [Google Scholar] [CrossRef]
- Selim, M.; Sloan, E. Hydrate dissociation in sediment. SPE Res. Eng. 1990, 5, 245–251. [Google Scholar] [CrossRef]
- Van Genuchten, M.T. A closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
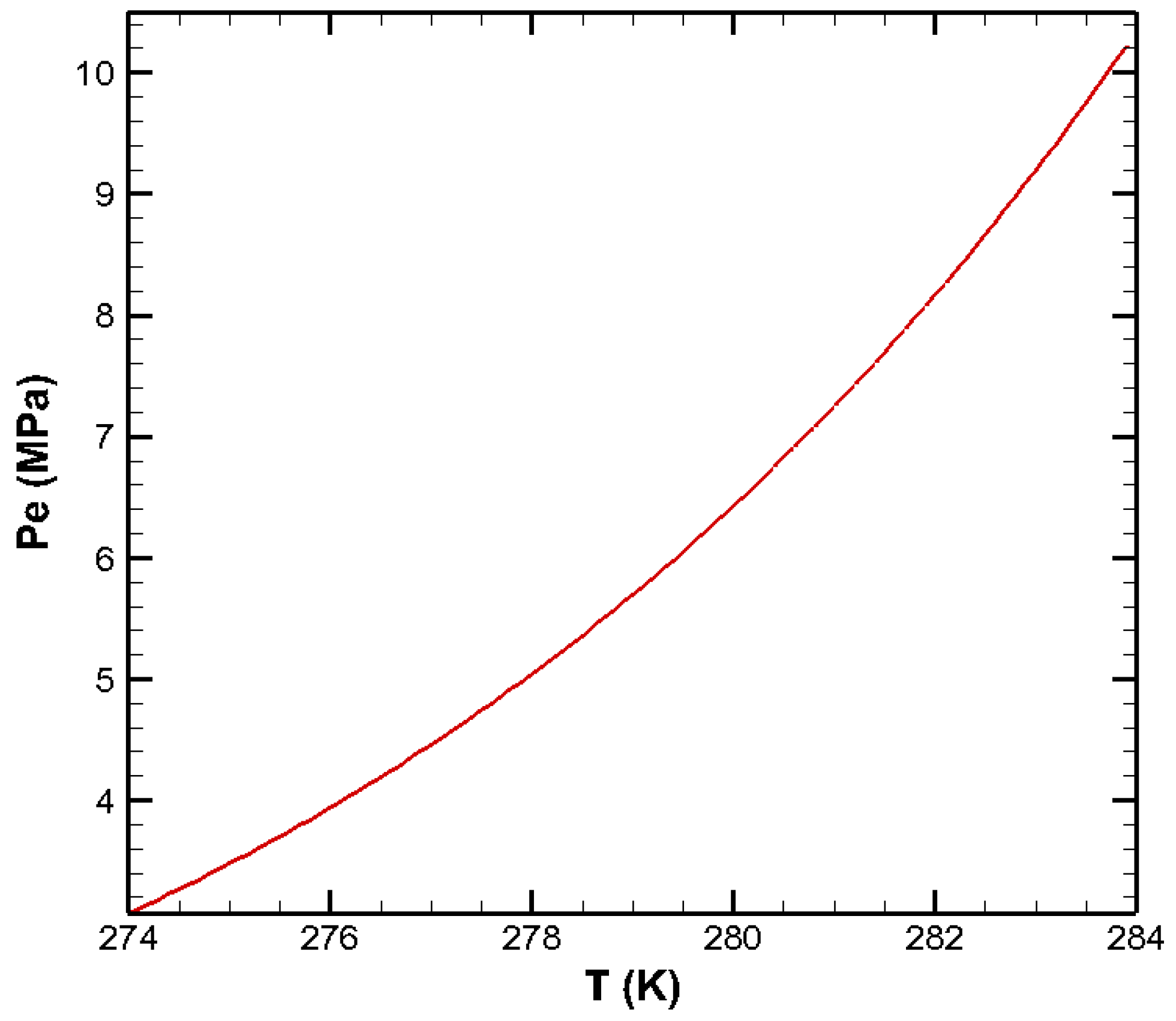
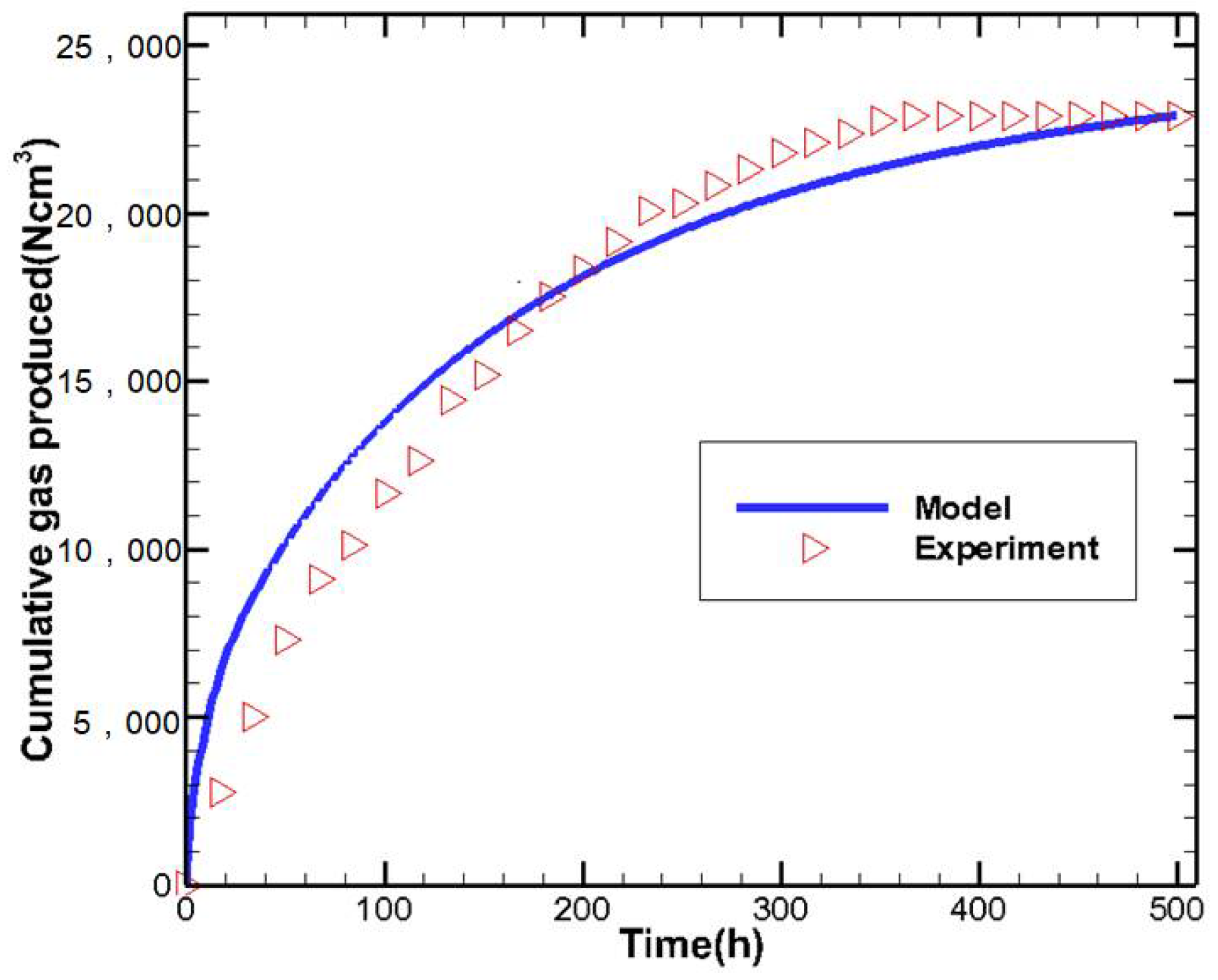
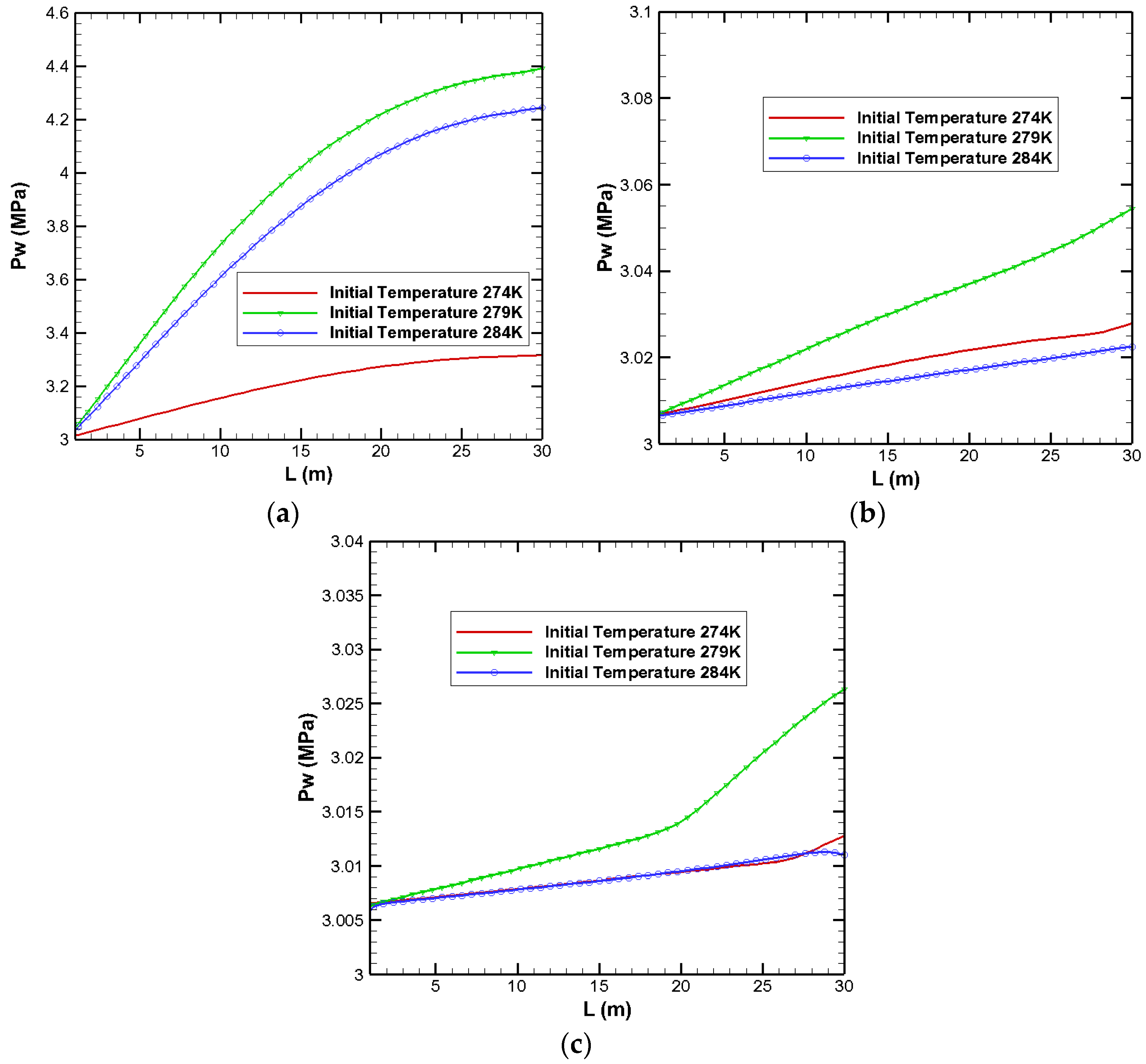
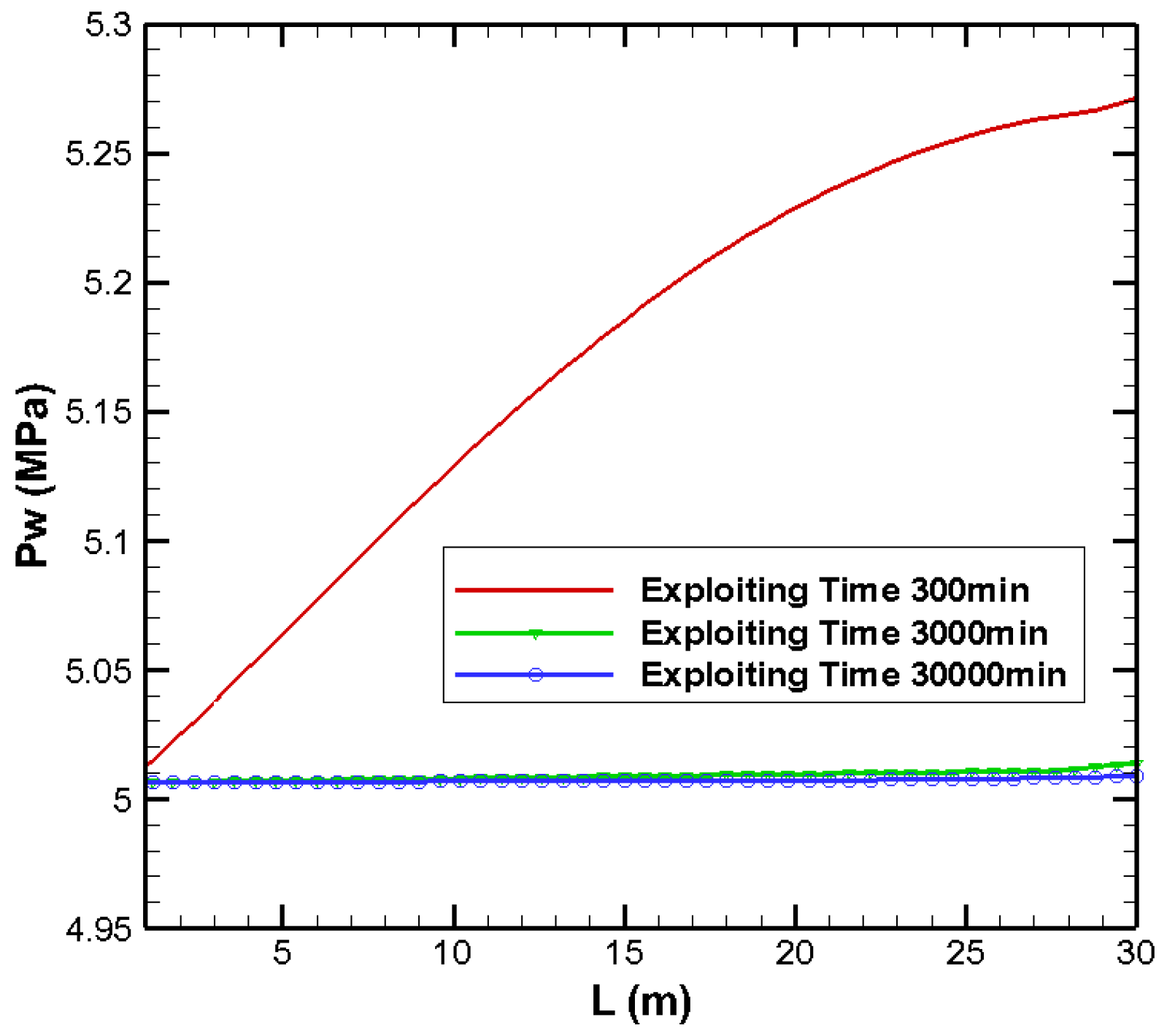


| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Reservoir length, L (cm) | 30 | Initial hydrate saturation, Sh (%) | 45 |
| Initial permeability, K0 (md) | 97.98 | Initial water saturation, Sw (%) | 20 |
| Porosity, Φ (%) | 18.2 | Initial gas saturation, Sg (%) | 35 |
| Initial reservoir pressure, P0 (MPa) | 3.75 | Permeability reduction index, N | 4 |
| Initial reservoir temperature, T0 (K) | 275.45 | - | - |
| Parameters | Values | Parameters | Values |
|---|---|---|---|
| Reservoir length, L (m) | 30 | Initial hydrate saturation, Sh (%) | 45 |
| Initial permeability, K0 (md) | 97.98 | Initial water saturation, Sw (%) | 20 |
| Porosity, Φ (%) | 20 | Initial gas saturation, Sg (%) | 35 |
| Initial reservoir pressure, P0 (MPa) | 10.4 | Permeability reduction index, N | 4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, M.; Li, W.; Dong, B.; Chen, C.; Wang, X. Simulation for the Effects of Well Pressure and Initial Temperature on Methane Hydrate Dissociation. Energies 2018, 11, 1179. https://doi.org/10.3390/en11051179
Yu M, Li W, Dong B, Chen C, Wang X. Simulation for the Effects of Well Pressure and Initial Temperature on Methane Hydrate Dissociation. Energies. 2018; 11(5):1179. https://doi.org/10.3390/en11051179
Chicago/Turabian StyleYu, Minghao, Weizhong Li, Bo Dong, Cong Chen, and Xin Wang. 2018. "Simulation for the Effects of Well Pressure and Initial Temperature on Methane Hydrate Dissociation" Energies 11, no. 5: 1179. https://doi.org/10.3390/en11051179




