Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production
Abstract
1. Introduction
2. Results and Discussion
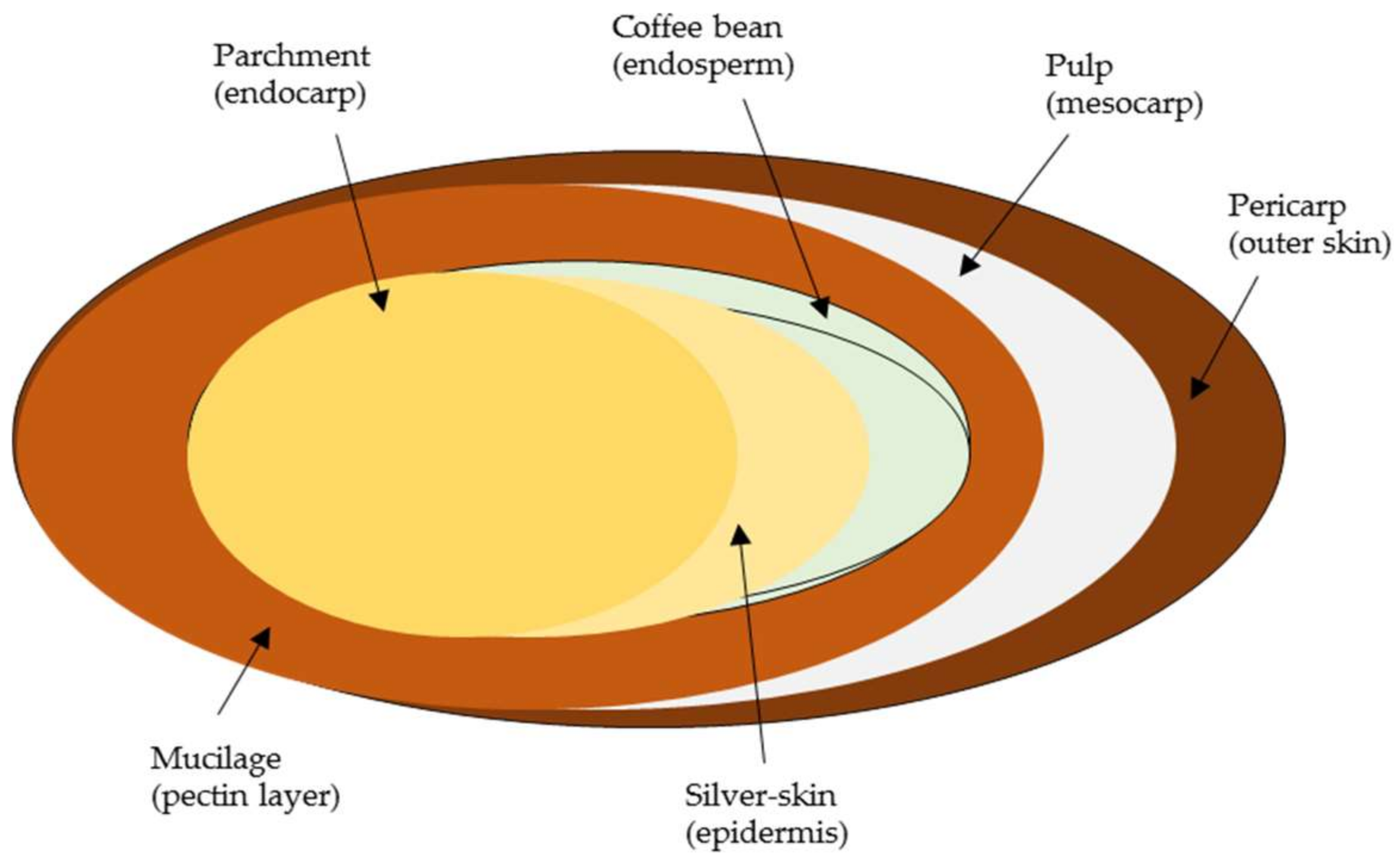
2.1. Compositional Analysis of Coffee Mucilage
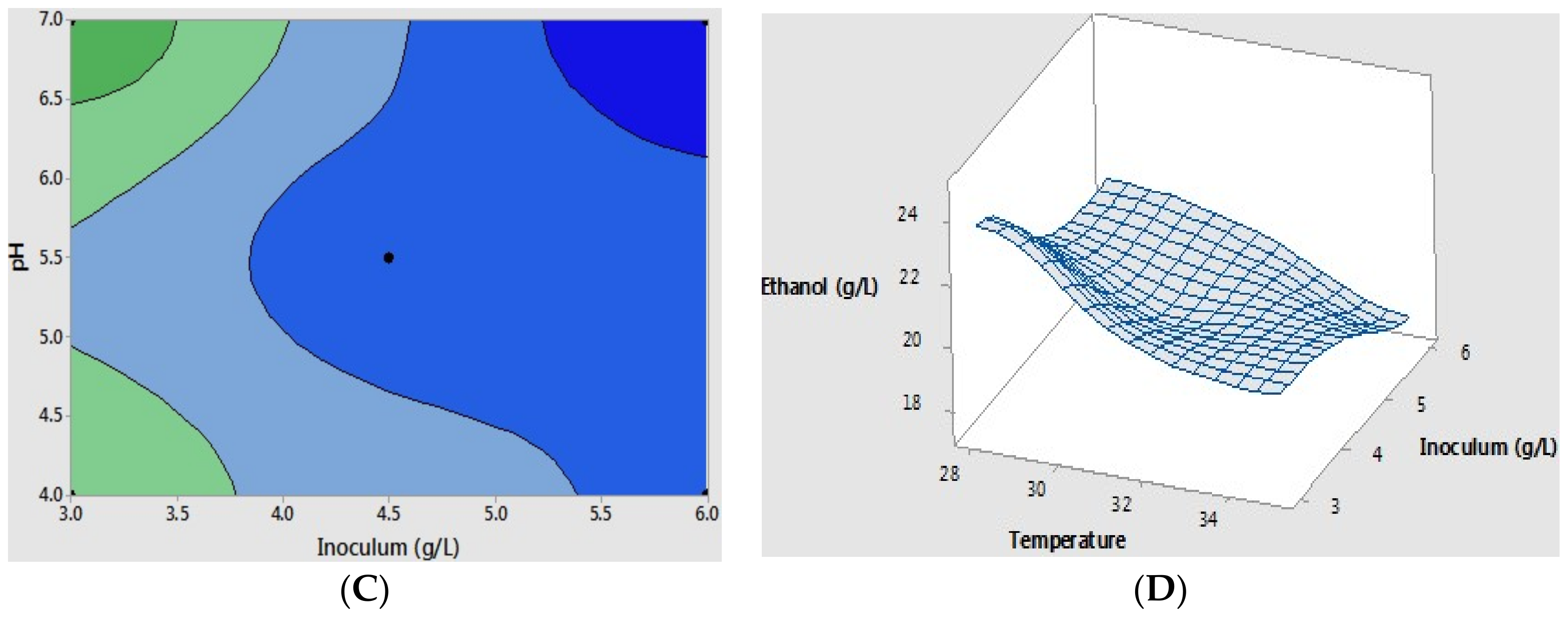
2.2. Optimization of Coffee Mucilage Fermentation
2.3. Mucilage Fermentation Both in the Flasks and 5 L Bio-Reactors
3. Materials and Methods
3.1. Coffee Mucilage
3.2. Microorganism and Fermentation Experiments
3.3. Analytic Assays
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moore, P.H.; Ming, R. (Eds.) Genomics of Tropical Crop Plants; Springer: New York, NY, USA, 2008; ISBN 9780387712185. [Google Scholar]
- Souza, R.M. (Ed.) Plant-Parasitic Nematodes of Coffee; Springer: Dordrecht, The Netherlands, 2008; ISBN 9781402087196. [Google Scholar]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Cappelletti, S.; Daria, P.; Sani, G.; Aromatario, M. Caffeine: Cognitive and Physical Performance Enhancer or Psychoactive Drug? Curr. Neuropharmacol. 2015, 13, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Food Chemistry; Springer: Berlin, Germany, 2009; ISBN 9783540699330. [Google Scholar]
- Berbert, P.A.; Queiroz, D.M.; Sousa, E.F.; Molina, M.B.; Melo, E.C.; Faroni, L.R.D. Dielectric properties of parchment coffee. J. Agric. Eng. Res. 2001, 80, 65–80. [Google Scholar] [CrossRef]
- Prata, E.R.B.A.; Oliveira, L.S. Fresh coffee husks as potential sources of anthocyanins. LWT Food Sci. Technol. 2007, 40, 1555–1560. [Google Scholar] [CrossRef]
- Kondamudi, N.; Mohapatra, S.K.; Misra, M. Spent Coffee Grounds as a Versatile Source of Green Energy. J. Agric. Food Chem. 2008, 56, 11757–11760. [Google Scholar] [CrossRef] [PubMed]
- Saenger, M.; Hartge, E.U.; Werther, J.; Ogada, T.; Siagi, Z. Combustion of coffee husks. Renew. Energy 2001, 23, 103–121. [Google Scholar] [CrossRef]
- Bressani, R.; Braham, J.E. (Eds.) Coffee Pulp: Composition, Technology, and Utilization; International Development Research Centre: Ottawa, ON, Canada, 1978. [Google Scholar]
- Bakker, R.R.C. Availability of Lignocellulosic Feedstocks for Lactic Acid Production. Feedstock Availability, Lactic Acid Production Potential and Selection Criteria; Wageningen UR Food & Biobased Research: Wageningen, The Netherlands, 2013; pp. 1–62. [Google Scholar]
- Murthy, P.S.; Madhava Naidu, M. Sustainable management of coffee industry by-products and value addition—A review. Resour. Conserv. Recycl. 2012, 66, 45–58. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S.; Nunes, A.A.; Alves, C.C.O. Microwave assisted thermal treatment of defective coffee beans press cake for the production of adsorbents. Bioresour. Technol. 2010, 101, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, W.E.; Franca, A.S.; Oliveira, L.S.; Rocha, S.D. Untreated coffee husks as biosorbents for the removal of heavy metals from aqueous solutions. J. Hazard. Mater. 2008, 152, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.S.; Franca, A.S.; Alves, T.M.; Rocha, S.D.F. Evaluation of untreated coffee husks as potential biosorbents for treatment of dye contaminated waters. J. Hazard. Mater. 2008, 155, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Gouvea, B.M.; Torres, C.; Franca, A.S.; Oliveira, L.S.; Oliveira, E.S. Feasibility of ethanol production from coffee husks. Biotechnol. Lett. 2009, 31, 1315–1319. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sariñana, B.Y.; De León-Rodriguez, A.; Saldaña-Trinidad, S.; Joseph, S.P. Optimization of bioethanol production from coffee mucilage. BioResources 2015, 10, 4326–4338. [Google Scholar] [CrossRef][Green Version]
- Pérez-Sariñana, B.Y.; Saldaña-Trinidad, S.; Guerrero-Fajardo, C.A.; Santis-Espinosa, L.F.; Sebastian, P.J. A simple method to determine bioethanol production from coffee mucilage, verified by HPLC. BioResources 2015, 10, 2691–2698. [Google Scholar] [CrossRef]
- Machado, C.M.M.; Soccol, C.R.; De Oliveira, B.H.; Pandey, A. Gibberellic acid production by solid-state fermentation in coffee husk. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 2002, 102–103, 179–191. [Google Scholar] [CrossRef]
- Murthy, P.S.; Naidu, M.M.; Srinivas, P. Production of α-amylase under solid-state fermentation utilizing coffee waste. J. Chem. Technol. Biotechnol. 2009, 84, 1246–1249. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Esposito, F.; Napolitano, A.; Ritieni, A.; Fogliano, V. Characterization of a New Potential Functional Ingredient: Coffee Silverskin. J. Agric. Food Chem. 2004, 52, 1338–1343. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, A.; Fogliano, V.; Tafuri, A.; Ritieni, A. Natural occurrence of ochratoxin A and antioxidant activities of green and roasted coffees and corresponding byproducts. J. Agric. Food Chem. 2007, 55, 10499–10504. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars metabolism and ethanol production by different yeast strains from coffee industry wastes hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Brand, D.; Pandey, A.; Rodriguez-Leon, J.A.; Roussos, S.; Brand, I.; Soccol, C.R. Packed bed column fermenter and kinetic modeling for upgrading the nutritional quality of coffee husk in solid-state fermentation. Biotechnol. Prog. 2001, 17, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Brand, D.; Pandey, A.; Roussos, S.; Soccol, C.R. Biological detoxification of coffee husk by filamentous fungi using a solid state fermentation system. Enzyme Microb. Technol. 2000, 27, 127–133. [Google Scholar] [CrossRef]
- Orozco, A.L.; Pérez, M.I.; Guevara, O.; Rodríguez, J.; Hernández, M.; González-Vila, F.J.; Polvillo, O.; Arias, M.E. Biotechnological enhancement of coffee pulp residues by solid-state fermentation with Streptomyces. Py-GC/MS analysis. J. Anal. Appl. Pyrolysis 2008, 81, 247–252. [Google Scholar] [CrossRef]
- Pandey, A.; Soccol, C.R.; Nigam, P.; Brand, D.; Mohan, R.; Roussos, S. Biotechnological potential of coffee pulp and coffee husk for bioprocesses. Biochem. Eng. J. 2000, 6, 153–162. [Google Scholar] [CrossRef]
- Ulloa Rojas, J.B.; Verreth, J.A.J.; Van Weerd, J.H.; Huisman, E.A. Effect of different chemical treatments on nutritional and antinutritional properties of coffee pulp. Anim. Feed Sci. Technol. 2002, 99, 195–204. [Google Scholar] [CrossRef]
- Heimbach, J.T.; Marone, P.A.; Hunter, J.M.; Nemzer, B.V.; Stanley, S.M.; Kennepohl, E. Safety studies on products from whole coffee fruit. Food Chem. Toxicol. 2010, 48, 2517–2525. [Google Scholar] [CrossRef] [PubMed]
- Murthy, P.S.; Naidu, M.M. Recovery of Phenolic Antioxidants and Functional Compounds from Coffee Industry By-Products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Clifford, M.N.; Willson, K.C. (Eds.) Coffee: Botany, Biochemistry and Production of Beans and Beverage; AVI Publishing Company, Inc.: Westport, CT, USA, 1985; ISBN 9781461566595. [Google Scholar]
- Avallone, S.; Guyot, B.; Brillouet, J.M.; Olguin, E.; Guiraud, J.P. Microbiological and biochemical study of coffee fermentation. Curr. Microbiol. 2001, 42, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Hernández, M.A.; Rodríguez Susa, M.; Andres, Y. Use of coffee mucilage as a new substrate for hydrogen production in anaerobic co-digestion with swine manure. Bioresour. Technol. 2014, 168, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Neu, A.K.; Pleissner, D.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative utilization of coffee mucilage using Bacillus coagulans and investigation of down-stream processing of fermentation broth for optically pure l(+)-lactic acid production. Bioresour. Technol. 2016, 211, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, A.; Bajpai, P. Effect of sugar concentration in Jerusalem artichoke extract on Kluyveromyces marxianus growth and ethanol production. Appl. Environ. Microbiol. 1983, 45, 723–725. [Google Scholar] [PubMed]
- Spencer, J.F.T.; Spencer, D.M. (Eds.) Yeasts in Natural and Artificial Haritats; Springer: Berlin/Heidelberg, Germany, 1997; ISBN 9783642081606. [Google Scholar]
- Campbell, I. Yeast and Fermentation; Elsevier Ltd.: Amsterdam, The Netherlands, 2003; ISBN 9780126692020. [Google Scholar]
- John, I.P.; Ailsa, D.H. Fungi and Food Spoilage; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 9780387922065. [Google Scholar]
- Casey, E.; Sedlak, M.; Ho, N.W.Y.; Mosier, N.S. Effect of acetic acid and pH on the cofermentation of glucose and xylose to ethanol by a genetically engineered strain of Saccharomyces cerevisiae. FEMS Yeast Res. 2010, 10, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ximenes, E.A.; Nichols, N.N.; Cao, G.; Frazer, S.E.; Ladisch, M.R. Maleic acid treatment of biologically detoxified corn stover liquor. Bioresour. Technol. 2016, 216, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Casey, E.; Mosier, N.S.; Adamec, J.; Stockdale, Z.; Ho, N.; Sedlak, M. Effect of salts on the Co-fermentation of glucose and xylose by a genetically engineered strain of Saccharomyces cerevisiae. Biotechnol. Biofuels 2013, 6, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Effects of acetic acid and lactic acid on the growth of Saccharomyces cerevisiae in a minimal medium. J. Ind. Microbiol. Biotechnol. 2001, 26, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Pampulha, M.E.; Loureiro-Dias, M.C. Combined effect of acetic acid, pH and ethanol on intracellular pH of fermenting yeast. Appl. Microbiol. Biotechnol. 1989, 31, 547–550. [Google Scholar] [CrossRef]
- Van Maris, A.J.A.; Abbott, D.A.; Bellissimi, E.; van den Brink, J.; Kuyper, M.; Luttik, M.A.H.; Wisselink, H.W.; Scheffers, W.A.; van Dijken, J.P.; Pronk, J.T. Alcoholic fermentation of carbon sources in biomass hydrolysates by Saccharomyces cerevisiae: Current status. Antonie van Leeuwenhoek 2006, 90, 391–418. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzyme Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Frazer, S.E.; Kim, D.; Cotta, M.A.; Ladisch, M. Bioabatement with hemicellulase supplementation to reduce enzymatic hydrolysis inhibitors. Bioresour. Technol. 2015, 190, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Orrego, D.; Ximenes, E.A.; Ladisch, M.R. Cellulose conversion of corn pericarp without pretreatment. Bioresour. Technol. 2017, 245, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Physico-Chemical Conversion of Lignocellulose: Inhibitor Effects and Detoxification Strategies: A Mini Review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Adeboye, P.T.; Bettiga, M.; Aldaeus, F.; Larsson, P.T.; Olsson, L. Catabolism of coniferyl aldehyde, ferulic acid and p-coumaric acid by Saccharomyces cerevisiae yields less toxic products. Microb. Cell Fact. 2015, 14, 149. [Google Scholar] [CrossRef] [PubMed]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl. Microbiol. Biotechnol. 2004, 66, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kreke, T.; Hendrickson, R.; Parenti, J.; Ladisch, M.R. Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour. Technol. 2013, 135, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ku, S. Beneficial Effects of Monascus sp. KCCM 10093 Pigments and Derivatives: A Mini Review. Molecules 2018, 23, 98. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ku, S. Bacillus Cellulase Molecular Cloning, Expression, and Surface Display on the Outer Membrane of Escherichia coli. Molecules 2018, 23, 503. [Google Scholar] [CrossRef] [PubMed]
- International Oranization for Standardization. ISO 6869 Animal Feeding Stuffs—Method Using Atomic Absorption Spectrometry; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- International Oranization for Standardization. Validating UV/Visible Spectrophotometers; ISO: Geneva, Switzerland, 1998; pp. 1–10. [Google Scholar]
- International Oranization for Standardization. International Standard; ISO: Geneva, Switzerland, 2005. [Google Scholar]
- Chen, Y.; Sharma-Shivappa, R.R.; Keshwani, D.; Chen, C. Potential of agricultural residues and hay for bioethanol production. Appl. Biochem. Biotechnol. 2007, 142, 276–290. [Google Scholar] [CrossRef] [PubMed]



| (A) Component | Concentration (g L−1) |
| Glucose | 37.1 |
| Galactose | 14.7 |
| Lactose | 0.8 |
| Acetic acid | 1.2 |
| Protein | 0.3 |
| (B) Mineral | Parts-Per-Million (ppm) |
| Calcium | 337 |
| Iron | 73 |
| Magnesium | 81 |
| Potassium | 116 (mg L−1) |
| Phosphorus | 115 |
| Sodium | 29 |
| Experiment | Temperature (°C) | pH | Inoculum (g L−1) | Ethanol Production (g L−1) | Standard Deviation (g L−1) | Percent Ethanol Yield (%) |
|---|---|---|---|---|---|---|
| 1 | 28.0 | 4.0 | 3.0 | 22.2 | 0.8 | 83.9 |
| 2 | 35.0 | 4.0 | 3.0 | 20.3 | 1.2 | 76.8 |
| 3 | 28.0 | 7.0 | 3.0 | 21.6 | 1.3 | 81.8 |
| 4 | 35.0 | 7.0 | 3.0 | 20.0 | 0.8 | 75.5 |
| 5 | 28.0 | 4.0 | 6.0 | 21.6 | 0.7 | 81.5 |
| 6 | 35.0 | 4.0 | 6.0 | 18.4 | 1.2 | 69.3 |
| 7 | 28.0 | 7.0 | 6.0 | 19.2 | 1.1 | 72.5 |
| 8 | 35.0 | 7.0 | 6.0 | 17.4 | 0.9 | 65.8 |
| 9 | 31.5 | 5.5 | 4.5 | 19.6 | 1.2 | 73.9 |
| Yeast Strain | Ethanol Yield (g ethanol/g Substrate) | Reference | ||
|---|---|---|---|---|
| Coffee Mucilage | Coffee Silver-Skin | Spent Coffee Grounds | ||
| S. cerevisiae (NRRL Y-1546) | 0.46 ± 0.02 | - | - | Current Study |
| P. stiptis (NRRL-Y-7124) | - | 0.11 ± 0.02 | 0.26 ± 0.01 | [23] |
| S. cerevisiae (RL-11) | - | 0.13 ± 0.03 | 0.26 ± 0.02 | [23] |
| K. fragilis (Kf1) | - | 0.01 ± 0.00 | 0.13 ± 0.02 | [23] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies 2018, 11, 786. https://doi.org/10.3390/en11040786
Orrego D, Zapata-Zapata AD, Kim D. Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies. 2018; 11(4):786. https://doi.org/10.3390/en11040786
Chicago/Turabian StyleOrrego, David, Arley David Zapata-Zapata, and Daehwan Kim. 2018. "Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production" Energies 11, no. 4: 786. https://doi.org/10.3390/en11040786
APA StyleOrrego, D., Zapata-Zapata, A. D., & Kim, D. (2018). Optimization and Scale-Up of Coffee Mucilage Fermentation for Ethanol Production. Energies, 11(4), 786. https://doi.org/10.3390/en11040786






