Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
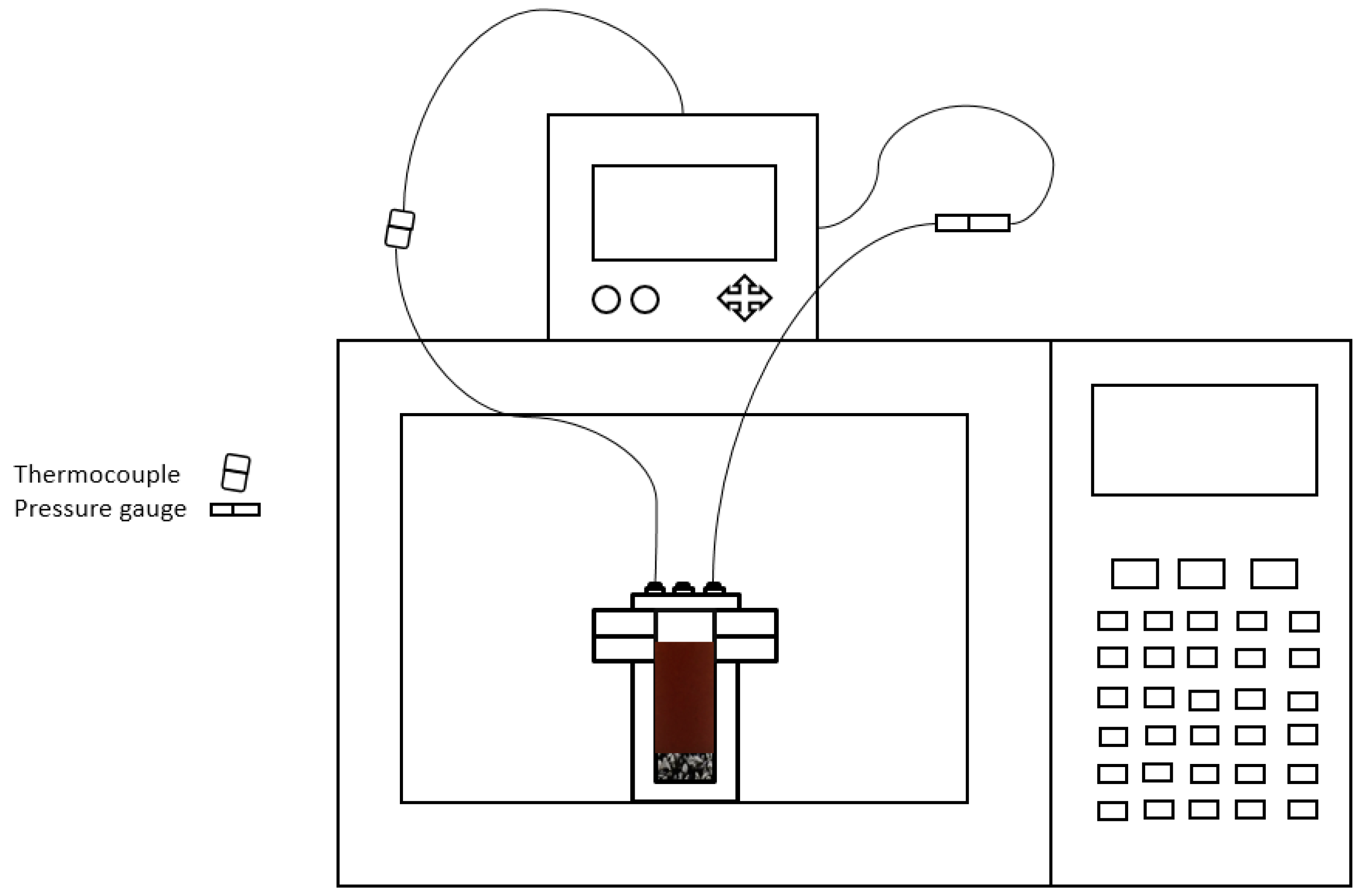
2.2. Experimental Procedure
2.3. Characterization of Biomass and Products
2.3.1. Solid Fraction
2.3.2. Liquid Fraction
2.3.3. Fuel Analysis
3. Results and Discussion
3.1. Carbon Balance of Brewer’s Spent Grains during Hydrothermal Carbonization
3.2. Characteristics of Hydrochars
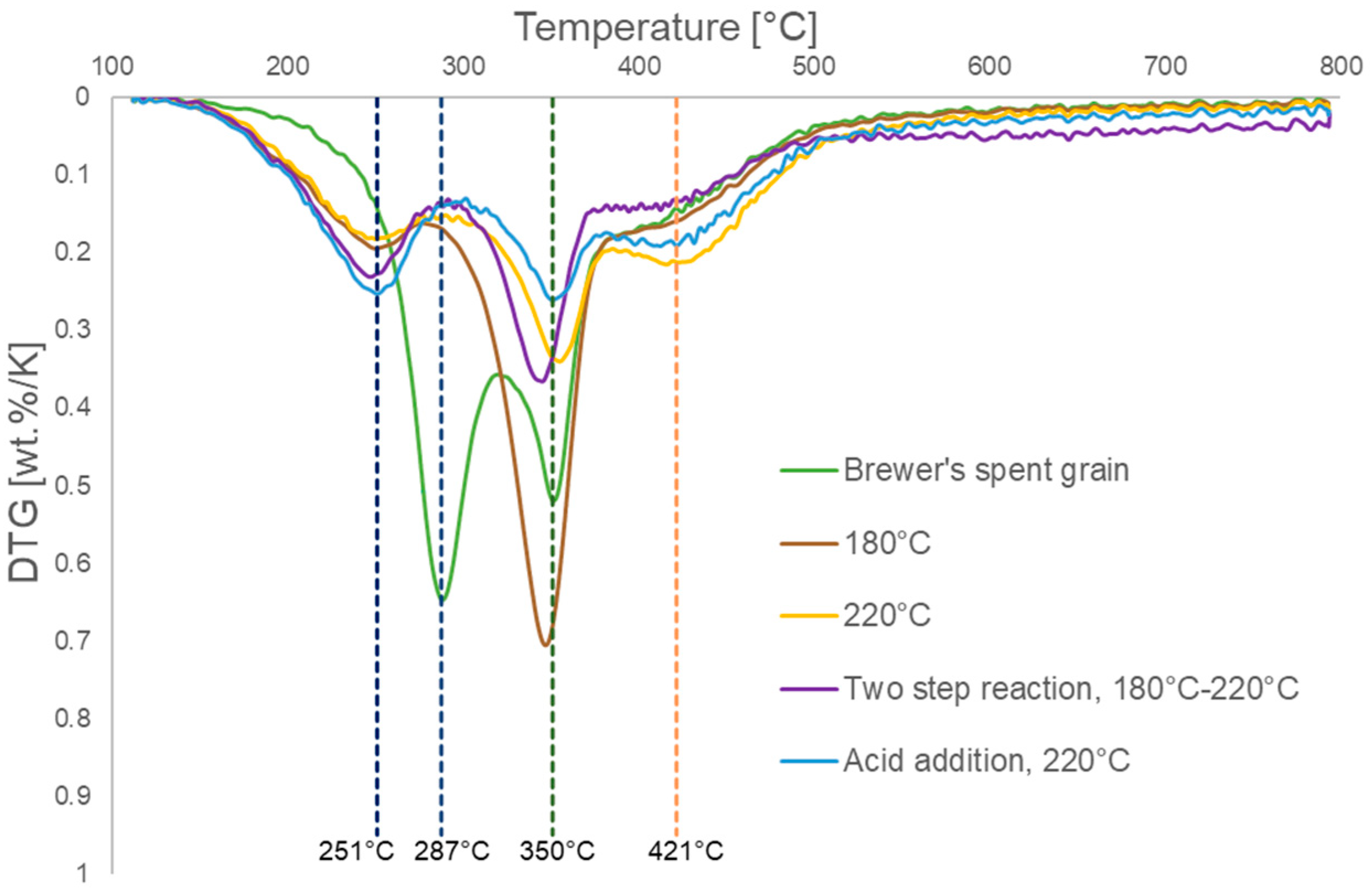
3.3. Thermogravimetric Analysis
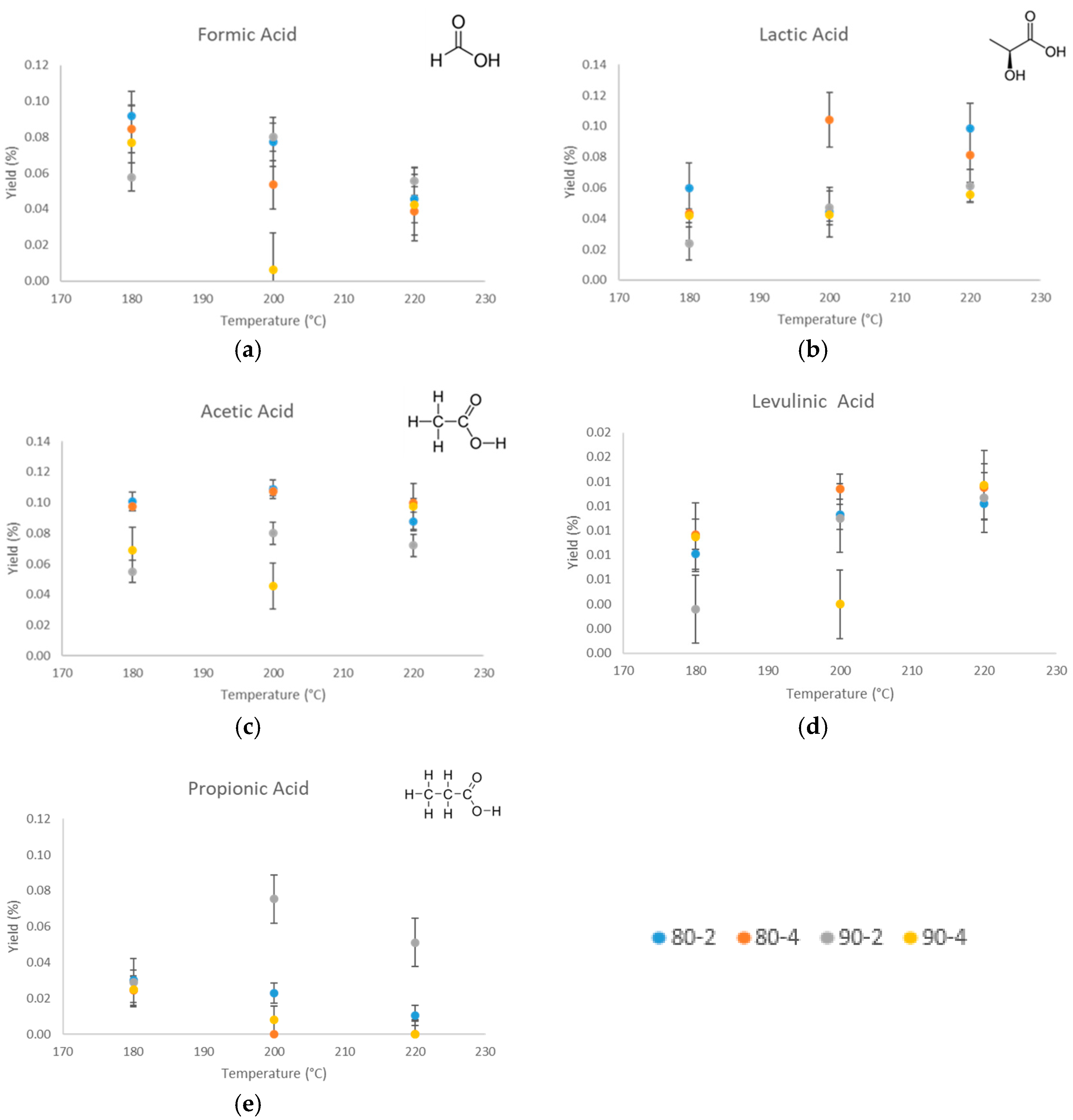
3.4. Characteristics of Liquid Phase
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Basso, D.; Patuzzi, F.; Castello, D.; Baratieri, M.; Rada, E.C.; Weiss-Hortala, E.; Fiori, L. Agro-industrial waste to solid biofuel through hydrothermal carbonization. Waste Manag. 2016, 47, 114–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub- and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2014, 2, 71–106. [Google Scholar] [CrossRef]
- Gao, P.; Zhou, Y.; Meng, F.; Zhang, Y.; Liu, Z.; Zhang, W.; Xue, G. Preparation and characterization of hydrochar from waste eucalyptus bark by hydrothermal carbonization. Energy 2016, 97, 238–245. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Effects of hydrolysis and carbonization reactions on hydrochar production. Bioresour. Technol. 2015, 192, 328–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukashima, H. The Infrared Spectra of Artificial Coal made from Submerged Wood at Uozu, Toyama Prefecture, Japan. Bull. Chem. Soc. Jpn. 1966, 39, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, M.; Benavente, V.; Fullana, A. Hydrothermal carbonization of lignocellulosic biomass: Effect of process conditions on hydrochar properties. Appl. Energy 2015, 155, 576–584. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A review on hydrothermal liquefaction of biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- Yanik, J.; Ebale, S.; Kruse, A.; Saglam, M.; Yuskel, M. Biomass gasification in supercritical water: Part 1. Effect of the nature of biomass. Fuel 2007, 86, 2410–2415. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Bioref. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Prawisudha, P. Hydrothermal Treatment of Municipal Solid Waste for Producing Solid Fuel. In Application of Hydrothermal Reactions to Biomass Conversion; Jin, F., Ed.; Springer: Heidelberg, Germany; New York, NY, USA, 2014; pp. 355–383. [Google Scholar]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2011, 51, 9023–9031. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Koch, F.; Stelzl, K.; Wüst, D.; Zeller, M. Fate of Nitrogen during Hydrothermal Carbonization. Energy Fuels 2016, 30, 8037–8042. [Google Scholar] [CrossRef]
- Karayıldırım, T.; Sınağ, A.; Kruse, A. Char and Coke Formation as Unwanted Side Reaction of the Hydrothermal Biomass Gasification. Chem. Eng. Technol. 2008, 31, 1561–1568. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Fractionation and characterization of brewers’ spent grain protein hydrolysates. J. Agric. Food Chem. 2009, 57, 5563–5570. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Jiménez, J.J.; Bartolomé, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of brewer’s spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Celus, I.; Brijs, K.; Delcour, J.A. Enzymatic hydrolysis of brewers’ spent grain proteins and technofunctional properties of the resulting hydrolysates. J. Agric. Food Chem. 2007, 55, 8703–8710. [Google Scholar] [CrossRef] [PubMed]
- Pruksakit, W.; Patumsawad, S. Hydrothermal Carbonization (HTC) of Sugarcane Stranded: Effect of Operation Condition to Hydrochar Production. Energy Procedia 2016, 100, 223–226. [Google Scholar] [CrossRef]
- Ulbrich, M.; Preßl, D.; Fendt, S.; Gaderer, M.; Spliethoff, H. Impact of HTC reaction conditions on the hydrochar properties and CO2 gasification properties of spent grains. Fuel Process. Technol. 2017, 167, 663–669. [Google Scholar] [CrossRef]
- Saba, A.; Saha, P.; Reza, M.T. Co-Hydrothermal Carbonization of coal-biomass blend: Influence of temperature on solid fuel properties. Fuel Process. Technol. 2017, 167, 711–720. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Pecha, B.; Arauzo, P.; Garcia-Perez, M. Impact of combined acid washing and acid impregnation on the pyrolysis of Douglas fir wood. J. Anal. Appl. Pyrolysis 2015, 114, 127–137. [Google Scholar] [CrossRef]
- D02 Committee. Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants; ASTM D-5291; American Society for Testing and Materials (ASTM) International: West Conshohocken, PA, USA, 1996. [Google Scholar]
- D07 Committee. Test Method for Chemical Analysis of Wood Charcoal; ASTM D1762-84; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Reza, M.T.; Uddin, M.H.; Lynam, J.G.; Hoekman, S.K.; Coronella, C.J. Hydrothermal carbonization of loblolly pine: Reaction chemistry and water balance. Biomass Conv. Bioref. 2014, 4, 311–321. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Poerschmann, J.; Weiner, B.; Wedwitschka, H.; Baskyr, I.; Koehler, R.; Kopinke, F.-D. Characterization of biocoals and dissolved organic matter phases obtained upon hydrothermal carbonization of brewer’s spent grain. Bioresour. Technol. 2014, 164, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.F.; da Silva, D.D.; Carmo, H.; Ferreira, I.M. Protective ability against oxidative stress of brewers’ spent grain protein hydrolysates. Food Chem. 2017, 228, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Tompsett, G.A.; Guerra, P.; Timko, M.T.; Rostagno, M.A.; Martínez, J.; Forster-Carneiro, T. Sugars and char formation on subcritical water hydrolysis of sugarcane straw. Bioresour. Technol. 2017, 243, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.; Funke, A.; Titirici, M.-M. Hydrothermal conversion of biomass to fuels and energetic materials. Curr. Opin. Chem. Biol. 2013, 17, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Ma, X.; Lin, Y. Effects of hydrothermal treatment temperature and residence time on characteristics and combustion behaviors of green waste. Appl. Therm. Eng. 2016, 104, 678–686. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Chen, X.; Ma, X.; Peng, X.; Lin, Y.; Yao, Z. Conversion of sweet potato waste to solid fuel via hydrothermal carbonization. Bioresour. Technol. 2018, 249, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Zhao, X.; Becker, G.C.; Faweya, N.; Rodriguez Correa, C.; Yang, S.; Xie, X.; KRUSE, A. Fertilizer and activated carbon production by hydrothermal carbonization of digestate. Biomass Conv. Bioref. 2018, 8, 423–436. [Google Scholar] [CrossRef]
- Volpe, M.; Fiori, L. From olive waste to solid biofuel through hydrothermal carbonisation: The role of temperature and solid load on secondary char formation and hydrochar energy properties. J. Anal. Appl. Pyrolysis 2017, 124, 63–72. [Google Scholar] [CrossRef]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Yoon, Y.-M. Energy Recovery Efficiency of Poultry Slaughterhouse Sludge Cake by Hydrothermal Carbonization. Energies 2017, 10, 1876. [Google Scholar] [CrossRef]
- Körner, P.; Jung, D.; Kruse, A. The effect of different Brønsted acids on the hydrothermal conversion of fructose to HMF. Green Chem. 2018, 20, 2231–2241. [Google Scholar] [CrossRef]
- Wikberg, H.; Grönqvist, S.; Niemi, P.; Mikkelson, A.; Siika-Aho, M.; Kanerva, H.; Käsper, A.; Tamminen, T. Hydrothermal treatment followed by enzymatic hydrolysis and hydrothermal carbonization as means to valorise agro- and forest-based biomass residues. Bioresour. Technol. 2017, 235, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Licursi, D.; Antonetti, C.; Mattonai, M.; Pérez-Armada, L.; Rivas, S.; Ribechini, E.; Raspolli Galletti, A.M. Multi-valorisation of giant reed (Arundo Donax L.) to give levulinic acid and valuable phenolic antioxidants. Ind. Crops Prod. 2018, 112, 6–17. [Google Scholar] [CrossRef]
- Brunner, G. Near critical and supercritical water. Part I. Hydrolytic and hydrothermal processes. J. Supercrit. Fluids 2009, 47, 373–381. [Google Scholar] [CrossRef]
- Hodge, J.E. Dehydrated Foods, Chemistry of Browning Reactions in Model Systems. J. Agric. Food Chem. 1953, 1, 928–943. [Google Scholar] [CrossRef]
- Moreschi, S.R.M.; Petenate, A.J.; Meireles, M.A.A. Hydrolysis of ginger bagasse starch in subcritical water and carbon dioxide. J. Agric. Food Chem. 2004, 52, 1753–1758. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E. Wood Chemistry. Fundamentals and Applications; Academic Press, Inc.: San Diego, CA, USA; London, UK, 1993. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Kruse, A.; Zevaco, T. Properties of Hydrochar as Function of Feedstock, Reaction Conditions and Post-Treatment. Energies 2018, 11, 674. [Google Scholar] [CrossRef]
- Liu, C.; Huang, X.; Kong, L. Efficient Low Temperature Hydrothermal Carbonization of Chinese Reed for Biochar with High Energy Density. Energies 2017, 10, 2094. [Google Scholar] [CrossRef]
- Ekpo, U.; Ross, A.B.; Camargo-Valero, M.A.; Williams, P.T. A comparison of product yields and inorganic content in process streams following thermal hydrolysis and hydrothermal processing of microalgae, manure and digestate. Bioresour. Technol. 2016, 200, 951–960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucian, M.; Volpe, M.; Gao, L.; Piro, G.; Goldfarb, J.L.; Fiori, L. Impact of hydrothermal carbonization conditions on the formation of hydrochars and secondary chars from the organic fraction of municipal solid waste. Fuel 2018, 233, 257–268. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Bicker, M.; Endres, S.; Ott, L.; Vogel, H. Catalytical conversion of carbohydrates in subcritical water: A new chemical process for lactic acid production. J. Mol. Catal. A Chem. 2005, 239, 151–157. [Google Scholar] [CrossRef]
- Rasrendra, C.B.; Makertihartha, I.G.B.N.; Adisasmito, S.; Heeres, H.J. Green Chemicals from d-Glucose: Systematic Studies on Catalytic Effects of Inorganic Salts on the Chemo-Selectivity and Yield in Aqueous Solutions. Top. Catal. 2010, 53, 1241–1247. [Google Scholar] [CrossRef]
- Mäki-Arvela, P.; Simakova, I.L.; Salmi, T.; Murzin, D.Y. Production of lactic acid/lactates from biomass and their catalytic transformations to commodities. Chem. Rev. 2014, 114, 1909–1971. [Google Scholar] [CrossRef] [PubMed]
- Zan, Y.; Sun, Y.; Kong, L.; Miao, G.; Bao, L.; Wang, H.; Li, S.; Sun, Y. Formic Acid-Induced Controlled-Release Hydrolysis of Microalgae (Scenedesmus) to Lactic Acid over Sn-Beta Catalyst. ChemSusChem 2018. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.M.; Ikarashi, A.; Saito, Y.; Watanabe, M.; Smith, R.L.; Arai, K. Dehydration of lactic acid to acrylic acid in high temperature water at high pressures. J. Supercrit. Fluids 2009, 50, 257–264. [Google Scholar] [CrossRef]
- Steinbach, D.; Kruse, A.; Sauer, J.; Vetter, P. Sucrose Is a Promising Feedstock for the Synthesis of the Platform Chemical Hydroxymethylfurfural. Energies 2018, 11, 645. [Google Scholar] [CrossRef]
- Licursi, D.; Antonetti, C.; Fulignati, S.; Vitolo, S.; Puccini, M.; Ribechini, E.; Bernazzani, L.; Raspolli Galletti, A.M. In-depth characterization of valuable char obtained from hydrothermal conversion of hazelnut shells to levulinic acid. Bioresour. Technol. 2017, 244, 880–888. [Google Scholar] [CrossRef] [PubMed]


| Parameters | Units | Values |
|---|---|---|
| Dry solid content (105 °C) | wt.% | 3.75 |
| Volatile matter (VM, 950 °C) | wt.% (db) | 76.25 |
| Ash content (750 °C) | wt.% (db) | 4.01 |
| Fixed carbon (FC) content | wt.% (db) | 16.00 |
| Higher heating value (HHV) | MJ kg−1 (db) | 22.25 |
| C | wt.% (db) | 51.27 |
| H | wt.% (db) | 6.97 |
| N | wt.% (db) | 4.68 |
| S | wt.% (db) | 0.29 |
| Hemicellulose content | wt.% (db) | 43.03 |
| Cellulose content | wt.% (db) | 23.69 |
| Lignin content | wt.% (db) | 5.78 |
| Extractives | wt.% (db) | 15.56 |
| Proteins | wt.% (db) 1 | 29.26 |
| Samples | Proximate Analysis (wt.% Dry Basis) | Fuel Ratio | Hy (%) | Ey (%) | Ed | ||
|---|---|---|---|---|---|---|---|
| VM | Ash | FC | |||||
| Raw material | 77.87 | 4.30 | 17.83 | 0.23 | - | - | - |
| HTC-80-180-2 | 74.92 | 4.29 | 20.79 | 0.28 | 68.00 | 80.81 | 1.19 |
| HTC-80-200-2 | 71.96 | 4.29 | 23.75 | 0.33 | 64.07 | 78.09 | 1.22 |
| HTC-80-220-2 | 69.03 | 4.26 | 26.71 | 0.39 | 57.99 | 76.10 | 1.31 |
| HTC-80-180-4 | 72.79 | 4.18 | 23.03 | 0.32 | 67.52 | 83.54 | 1.24 |
| HTC-80-200-4 | 70.50 | 4.15 | 25.35 | 0.36 | 63.48 | 76.60 | 1.21 |
| HTC-80-220-4 | 66.17 | 4.22 | 29.61 | 0.45 | 55.04 | 72.43 | 1.32 |
| HTC-90-180-2 | 74.22 | 3.39 | 22.39 | 0.30 | 66.17 | 79.49 | 1.20 |
| HTC-90-200-2 | 71.69 | 3.24 | 25.07 | 0.35 | 62.12 | 76.84 | 1.24 |
| HTC-90-220-2 | 68.04 | 3.22 | 28.74 | 0.42 | 52.04 | 69.14 | 1.33 |
| HTC-90-180-4 | 73.15 | 3.12 | 23.73 | 0.32 | 65.26 | 76.79 | 1.18 |
| HTC-90-200-4 | 70.33 | 3.16 | 26.50 | 0.38 | 60.53 | 76.21 | 1.26 |
| HTC-90-220-4 | 64.86 | 3.27 | 31.88 | 0.49 | 50.59 | 66.02 | 1.31 |
| HTC-80-180/220-4 | 65.84 | 4.11 | 30.05 | 0.46 | 67.05 | 85.51 | 1.28 |
| HTC-90-180/220-4 | 65.09 | 3.12 | 31.79 | 0.49 | 70.30 | 90.64 | 1.29 |
| HTC-80-220-acid | 61.54 | 3.67 | 34.79 | 0.57 | 60.36 | 79.11 | 1.31 |
| HTC-90-220-acid | 59.35 | 2.81 | 37.84 | 0.64 | 52.56 | 71.28 | 1.36 |
| Samples | Solid wt.% | Liquid wt.% |
|---|---|---|
| HTC-80-180-2 | 0.86 | 0.14 |
| HTC-80-200-2 | 0.86 | 0.14 |
| HTC-80-220-2 | 0.84 | 0.16 |
| HTC-80-180-4 | 0.86 | 0.14 |
| HTC-80-200-4 | 0.84 | 0.16 |
| HTC-80-220-4 | 0.84 | 0.16 |
| HTC-90-180-2 | 0.83 | 0.17 |
| HTC-90-200-2 | 0.82 | 0.18 |
| HTC-90-220-2 | 0.78 | 0.22 |
| HTC-90-180-4 | 0.82 | 0.18 |
| HTC-90-200-4 | 0.81 | 0.19 |
| HTC-90-220-4 | 0.82 | 0.18 |
| HTC-80-180/220-4 | 0.85 | 0.15 |
| HTC-90-180/220-4 | 0.84 | 0.16 |
| HTC-80-220-acid | 0.71 | 0.29 |
| HTC-90-220-acid | 0.66 | 0.34 |
| Samples | Elemental Analysis (% wt. Dry Basis) | O/C | H/C | HHV (MJ/kg) | ||||
|---|---|---|---|---|---|---|---|---|
| N | C | H | S | O | ||||
| Raw material | 4.68 | 51.27 | 6.97 | 0.29 | 36.52 | 0.53 | 1.63 | 22.28 |
| HTC-80-180-2 | 4.37 | 60.32 | 7.07 | 0.45 | 27.80 | 0.35 | 1.41 | 26.48 |
| HTC-80-200-2 | 4.12 | 62.02 | 7.02 | 0.42 | 26.42 | 0.32 | 1.36 | 27.16 |
| HTC-80-220-2 | 4.41 | 65.81 | 7.28 | 0.43 | 22.07 | 0.25 | 1.33 | 29.24 |
| HTC-80-180-4 | 4.30 | 62.89 | 7.02 | 0.43 | 25.36 | 0.30 | 1.34 | 27.57 |
| HTC-80-200-4 | 4.09 | 61.19 | 7.09 | 0.46 | 27.17 | 0.33 | 1.39 | 26.89 |
| HTC-80-220-4 | 4.46 | 67.08 | 6.89 | 0.43 | 21.14 | 0.24 | 1.23 | 29.32 |
| HTC-90-180-2 | 3.63 | 60.18 | 7.39 | 0.44 | 28.36 | 0.35 | 1.47 | 26.76 |
| HTC-90-200-2 | 3.80 | 62.29 | 7.25 | 0.45 | 26.21 | 0.32 | 1.40 | 27.56 |
| HTC-90-220-2 | 3.75 | 66.55 | 7.35 | 0.41 | 21.94 | 0.25 | 1.33 | 29.60 |
| HTC-90-180-4 | 3.49 | 59.96 | 7.05 | 0.42 | 29.07 | 0.36 | 1.41 | 26.22 |
| HTC-90-200-4 | 3.86 | 63.54 | 7.19 | 0.45 | 24.96 | 0.29 | 1.36 | 28.05 |
| HTC-90-220-4 | 4.25 | 66.60 | 6.89 | 0.43 | 21.83 | 0.25 | 1.24 | 29.08 |
| HTC-80-180/220-4 | 4.40 | 64.84 | 6.97 | 0.49 | 23.30 | 0.27 | 1.29 | 28.42 |
| HTC-90-80/220-4 | 4.37 | 65.46 | 6.99 | 0.53 | 22.66 | 0.26 | 1.28 | 28.73 |
| HTC-80-220-acid | 4.55 | 67.29 | 6.71 | 0.51 | 20.94 | 0.23 | 1.20 | 29.20 |
| HTC-90-220-acid | 4.20 | 68.67 | 7.03 | 0.50 | 19.60 | 0.21 | 1.23 | 30.22 |
| Samples | pH | Lactic Acid | Formic Acid | Acetic Acid | Propionic Acid | HMF | Furfural | LA |
|---|---|---|---|---|---|---|---|---|
| HTC-80-180-2 | 4.00 | 0.06 | 0.09 | 0.10 | 0.03 | 0.01 | 0.01 | 0.01 |
| HTC-80-200-2 | 4.38 | 0.04 | 0.08 | 0.11 | 0.02 | 0.00 | 0.00 | 0.01 |
| HTC-80-220-2 | 4.53 | 0.10 | 0.05 | 0.09 | 0.01 | 0.00 | 0.00 | 0.01 |
| HTC-80-180-4 | 4.16 | 0.04 | 0.08 | 0.10 | 0.02 | 0.00 | 0.00 | 0.01 |
| HTC-80-200-4 | 4.53 | 0.10 | 0.05 | 0.11 | 0.00 | 0.00 | 0.00 | 0.01 |
| HTC-80-220-4 | 4.53 | 0.08 | 0.04 | 0.10 | 0.00 | 0.00 | 0.00 | 0.01 |
| HTC-90-180-2 | 3.91 | 0.02 | 0.06 | 0.05 | 0.03 | 0.01 | 0.02 | 0.00 |
| HTC-90-200-2 | 4.42 | 0.05 | 0.08 | 0.08 | 0.08 | 0.00 | 0.00 | 0.01 |
| HTC-90-220-2 | 4.56 | 0.06 | 0.06 | 0.07 | 0.05 | 0.00 | 0.00 | 0.01 |
| HTC-90-180-4 | 4.04 | 0.04 | 0.08 | 0.07 | 0.02 | 0.00 | 0.00 | 0.01 |
| HTC-90-200-4 | 4.59 | 0.04 | 0.01 | 0.05 | 0.01 | 0.00 | 0.00 | 0.00 |
| HTC-90-220-4 | 4.66 | 0.06 | 0.04 | 0.10 | 0.00 | 0.00 | 0.00 | 0.01 |
| HTC-80-180/220-4 | 4.64 | 0.08 | 0.04 | 0.10 | 0.10 | 0.00 | 0.00 | 0.01 |
| HTC-90-180/220-4 | 4.54 | 0.09 | 0.05 | 0.09 | 0.17 | 0.00 | 0.00 | 0.01 |
| HTC-80-220-acid | 3.67 | 0.07 | 0.03 | 106.40 | 0.15 | 0.00 | 0.00 | 0.02 |
| HTC-90-220-acid | 3.57 | 0.05 | 0.03 | 100.52 | 0.11 | 0.00 | 0.00 | 0.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arauzo, P.J.; Olszewski, M.P.; Kruse, A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies 2018, 11, 3226. https://doi.org/10.3390/en11113226
Arauzo PJ, Olszewski MP, Kruse A. Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies. 2018; 11(11):3226. https://doi.org/10.3390/en11113226
Chicago/Turabian StyleArauzo, Pablo J., Maciej P. Olszewski, and Andrea Kruse. 2018. "Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock" Energies 11, no. 11: 3226. https://doi.org/10.3390/en11113226
APA StyleArauzo, P. J., Olszewski, M. P., & Kruse, A. (2018). Hydrothermal Carbonization Brewer’s Spent Grains with the Focus on Improving the Degradation of the Feedstock. Energies, 11(11), 3226. https://doi.org/10.3390/en11113226






