Technology Evolution in Membrane-Based CCS
Abstract
:1. Introduction
2. Methodology
3. Results and Discussion
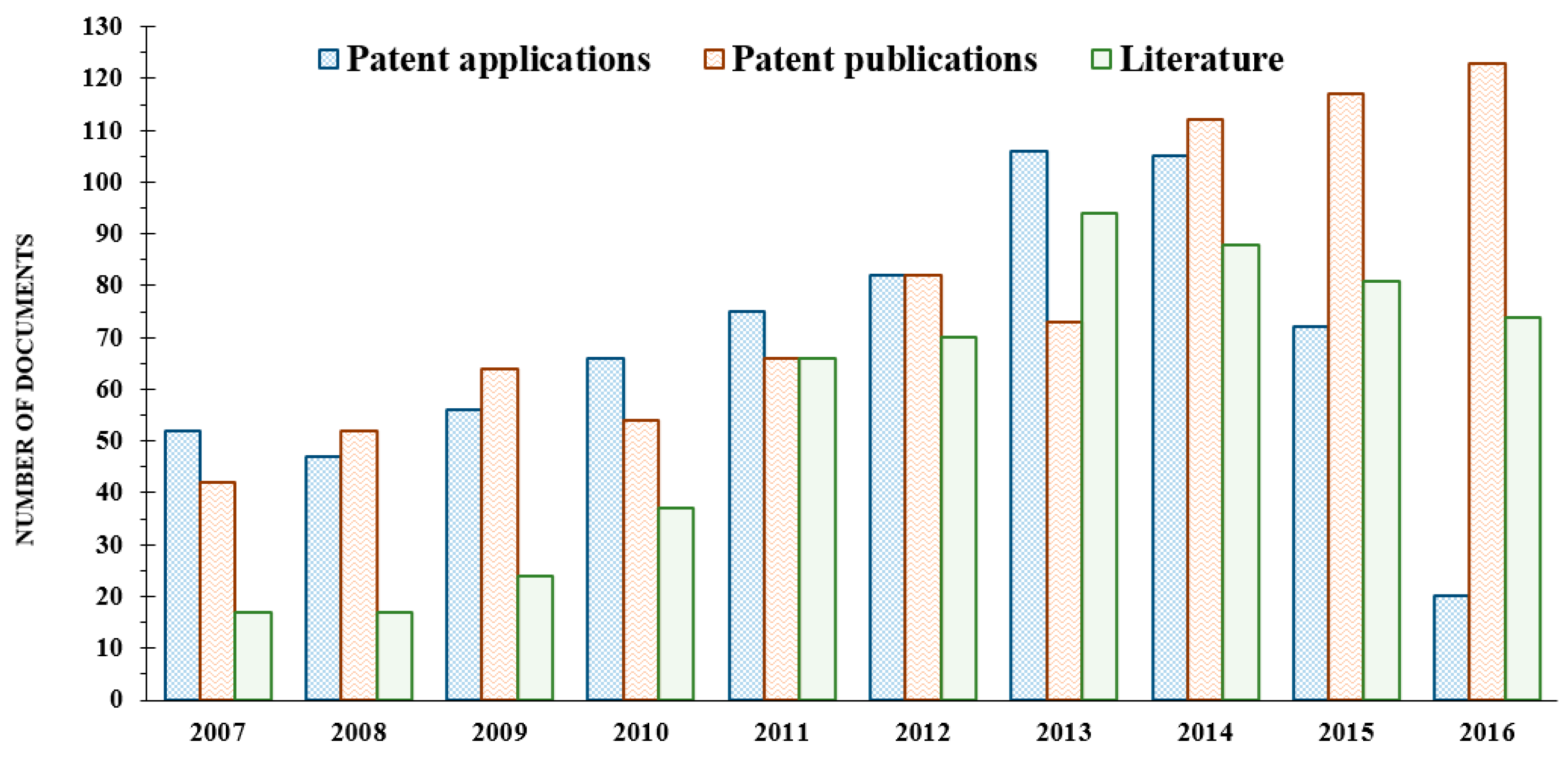
3.1. Analysis of Scientific Literature
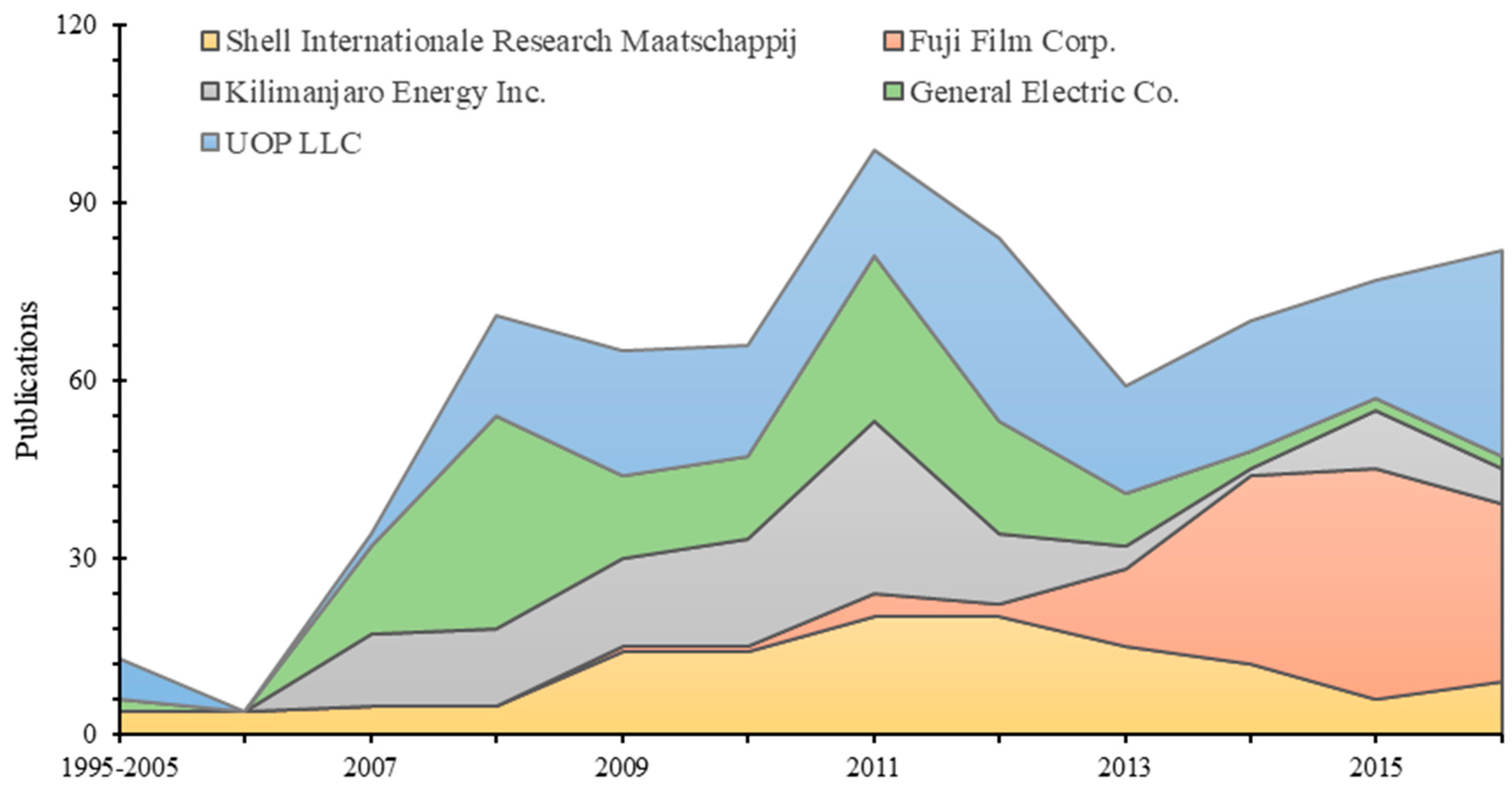
3.2. Number of Patents and Assignees
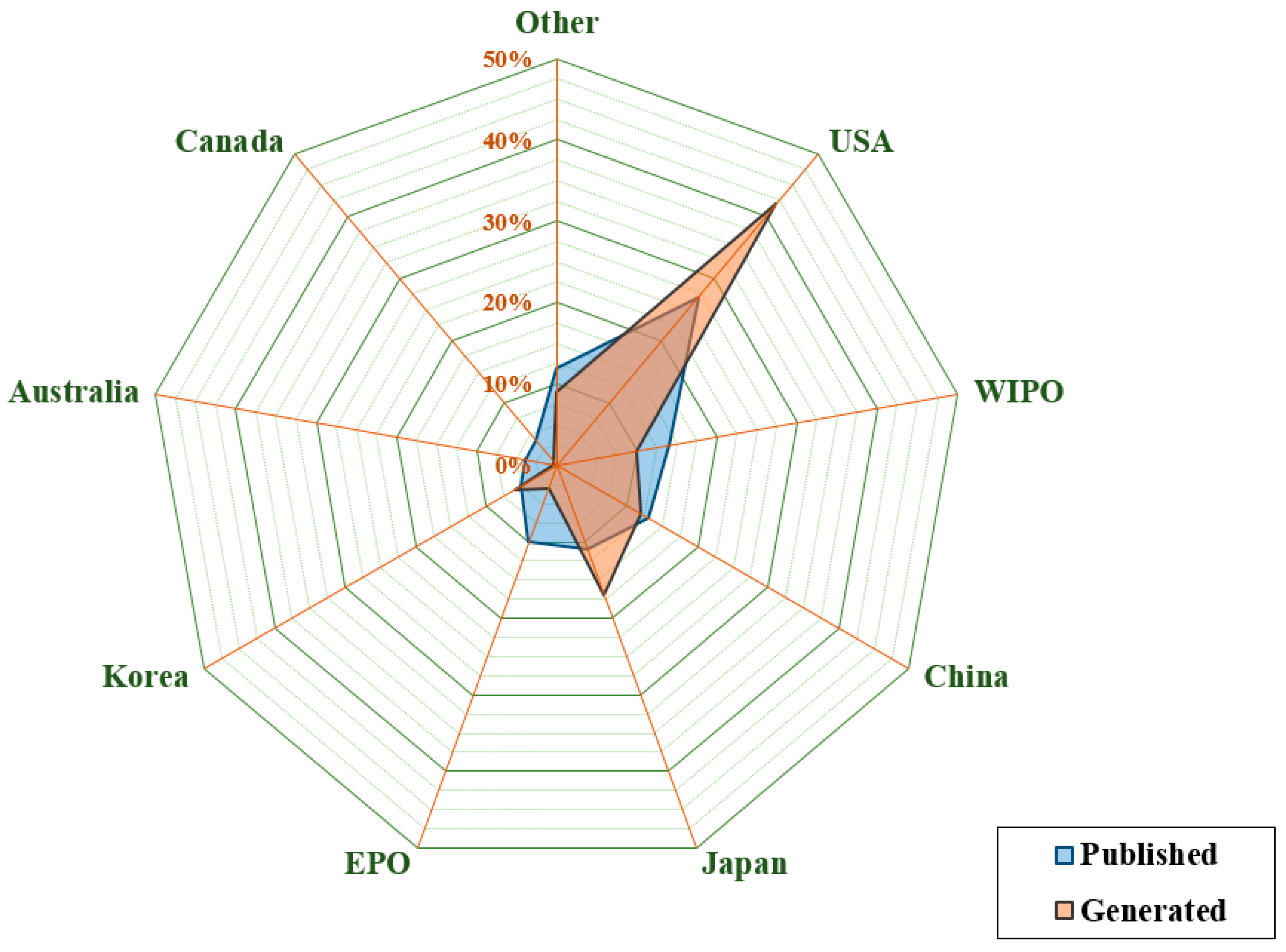
3.3. Analysis by Country/Office and Assignee Nationality
3.4. Analysis by Innovation Index
4. Summary
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CCS | carbon capture and storage |
| CNRS | Centre National de la Recherche Scientifique |
| Co. | company |
| CO2CRC | Cooperative Research Centre for Greenhouse Gas Technologies |
| Corp. | corporation |
| CPC | Cooperative Patent Classification |
| DEA | diethanolamine |
| DWPI | Derwent World Patents Index |
| EPO | European Patent Office |
| GHG | greeenhouse gas |
| GO | graphene oxide |
| IEA | International Energy Agency |
| Inc. | incorporation |
| LCA | Life Cycle Assessment |
| LLC | limited liability company |
| MMMs | mixed matrix membranes |
| MOF | metal–organic frameworks |
| NTNU | Norwegian University of Science & Technology |
| PEBAX | polyether block amide |
| PEO | polyethilene oxide |
| SCI | Science Citation Index |
| US/A | United States of America |
| WIPO | World Intellectual Property Office |
| ZIF | zeolitic imidazolate framework |
References
- International Energy Agency. World Energy Outlook: 2016; OECD/IEA: Paris, France, 2016. [Google Scholar]
- Aaron-Morrison, A.P.; Ackerman, S.A.; Adams, N.G. State of the climate in 2015. Bull. Am. Meteorol. Soc. 2016, 97, S12–S75. [Google Scholar] [CrossRef]
- Oeschger, H. CO2 and the greenhouse effect: Present assessment and perspectives. Ciba Found. Symp. 1993, 175, 2–17, discussion 17. [Google Scholar] [PubMed]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in carbon dioxide separation and capture: A review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Brown, M.A.; Kim, G.; Smith, A.M.; Southworth, K. Exploring the impact of energy efficiency as a carbon mitigation strategy in the U.S. Energy Policy 2017, 109, 249–259. [Google Scholar] [CrossRef]
- Herring, H. Energy efficiency—A critical view. Energy 2006, 31, 10–20. [Google Scholar] [CrossRef]
- Palm, J.; Thollander, P. An interdisciplinary perspective on industrial energy efficiency. Appl. Energy 2010, 87, 3255–3261. [Google Scholar] [CrossRef] [Green Version]
- Alfonsin, V.; Suarez, A.; Urrejola, S.; Miguez, J.; Sanchez, A. Integration of several renewable energies for internal combustion engine substitution in a commercial sailboat. Int. J. Hydrogen Energy 2015, 40, 6689–6701. [Google Scholar] [CrossRef]
- Cai, Y.P.; Huang, G.H.; Yeh, S.C.; Liu, L.; Li, G.C. A modeling approach for investigating climate change impacts on renewable energy utilization. Int. J. Energy Res. 2012, 36, 764–777. [Google Scholar] [CrossRef]
- de Vries, B.J.M.; van Vuuren, D.P.; Hoogwijk, M.M. Renewable energy sources: Their global potential for the first-half of the 21st century at a global level: An integrated approach. Energy Policy 2007, 35, 2590–2610. [Google Scholar] [CrossRef] [Green Version]
- Krozer, Y. Energy markets: Changes toward decarbonization and valorization. Curr. Opin. Chem. Eng. 2017, 17, 61–67. [Google Scholar] [CrossRef]
- Murillo, S.; Míguez, J.L.; Porteiro, J.; Hernández, J.J.; López-González, L.M. Viability of LPG use in low-power outboard engines for reduction in consumption and pollutant emissions. Int. J. Energy Res. 2003, 27, 467–480. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef] [Green Version]
- Rubin, E.; De Coninck, H. IPCC Special Report on Carbon Dioxide Capture and Storage; TNO (2004): Cost Curves for CO2 Storage, Part 2; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The U.S. Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control. 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef] [Green Version]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Zheng, B.; Xu, J. Carbon capture and storage development trends from a techno-paradigm perspective. Energies 2014, 7, 5221–5250. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Strazza, C.; Del Borghi, A.; Gallo, M. Development of specific rules for the application of life cycle assessment to carbon capture and storage. Energies 2013, 6, 1250–1265. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Q.M.; Wang, P.B. Evaluation of post-combustion CO2 capture technologies. Adv. Mater. Res. 2013, 734–737, 1881–1886. [Google Scholar]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Jansen, D.; Gazzani, M.; Manzolini, G.; Dijk, E.V.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Li, H.; Yan, J. Preliminary study on CO2 processing in CO2 capture from oxy-fuel combustion. In Proceedings of the ASME Turbo Expo, Montreal, QC, Canada, 14–17 May 2007; pp. 353–361. [Google Scholar]
- Cormos, C.-C. Chemical Looping with Oxygen Uncoupling (CLOU) concepts for high energy efficient power generation with near total fuel decarbonisation. Appl. Therm. Eng. 2017, 112, 924–931. [Google Scholar] [CrossRef]
- Alie, C.; Backham, L.; Croiset, E.; Douglas, P.L. Simulation of CO2 capture using MEA scrubbing: A flowsheet decomposition method. Energy Convers. Manag. 2005, 46, 475–487. [Google Scholar] [CrossRef]
- Gomes, J.; Santos, S.; Bordado, J. Choosing amine-based absorbents for CO2 capture. Environ. Technol. 2015, 36, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Kwak, N.S.; Lee, J.H.; Lee, I.Y.; Jang, K.R.; Shim, J.G. A study of the CO2 capture pilot plant by amine absorption. Energy 2012, 47, 41–46. [Google Scholar] [CrossRef]
- Vega, F.; Sanna, A.; Navarrete, B.; Maroto-Valer, M.M.; Cortés, V.J. Degradation of amine-based solvents in CO2 capture process by chemical absorption. Greenh. Gases Sci. Technol. 2014, 4, 707–733. [Google Scholar] [CrossRef]
- Pellerano, M.; Pré, P.; Kacem, M.; Delebarre, A. CO2 capture by adsorption on activated carbons using pressure modulation. Energy Procedia 2009, 1, 647–653. [Google Scholar] [CrossRef]
- Siriwardane, R.V.; Shen, M.S.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Hu, H.; Li, X.; Fang, Z.; Wei, N.; Li, Q. Small-molecule gas sorption and diffusion in coal: Molecular simulation. Energy 2010, 35, 2939–2944. [Google Scholar] [CrossRef]
- Duan, Y.; Pfeiffer, H.; Li, B.; Romero-Ibarra, I.C.; Sorescu, D.C.; Luebke, D.R.; Halley, J.W. CO2 capture properties of lithium silicates with different ratios of Li2O/SiO2: An ab initio thermodynamic and experimental approach. Phys. Chem. Chem. Phys. 2013, 15, 13538–13558. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, X.; Gray, M.L.; Duan, Y.; Luebke, D.; Li, B. Development of amino acid and amino acid-complex based solid sorbents for CO2 capture. Appl. Energy 2013, 109, 112–118. [Google Scholar] [CrossRef]
- Singh, R.; Ram Reddy, M.K.; Wilson, S.; Joshi, K.; Diniz da Costa, J.C.; Webley, P. High temperature materials for CO2 capture. Energy Procedia 2009, 1, 623–630. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.r.E. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- Ben-Mansour, R.; Qasem, N.A.A. An efficient temperature swing adsorption (TSA) process for separating CO2 from CO2/N2 mixture using Mg-MOF-74. Energy Convers. Manag. 2018, 156, 10–24. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, A.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total. Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Kuppler, R.J.; Zhou, H.C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Reali, R.S.; Smith, D.A.; Trachtenberg, M.C.; Li, J. Highly selective CO2 capture by a flexible microporous metal-organic framework (MMOF) material. Chem. A Eur. J. 2010, 16, 13951–13954. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Bonalumi, D.; Lillia, S.; Manzolini, G.; Grande, C. Innovative Process Cycle with Zeolite (MS13X) for Post Combustion Adsorption. Energy Procedia 2017, 114, 2211–2218. [Google Scholar] [CrossRef]
- Chen, C.; Park, D.W.; Ahn, W.S. CO2 capture using zeolite 13X prepared from bentonite. Appl. Surf. Sci. 2014, 292, 63–67. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. CO2 capture onto zeolite 13X and zeolite 4A by pressure swing adsorption in a fixed bed. Appl. Mech. Mater. 2014, 592–594, 1456–1460. [Google Scholar] [CrossRef]
- Yu, L.; Gong, J.; Zeng, C.; Zhang, L. Synthesis of binderless zeolite X microspheres and their CO2 adsorption properties. Sep. Purif. Technol. 2013, 118, 188–195. [Google Scholar] [CrossRef]
- Delgado, M.R.; Arean, C.O. Carbon monoxide, dinitrogen and carbon dioxide adsorption on zeolite H-Beta: IR spectroscopic and thermodynamic studies. Energy 2011, 36, 5286–5291. [Google Scholar] [CrossRef]
- Xu, G.; Liang, F.; Yang, Y.; Hu, Y.; Zhang, K.; Liu, W. An improved CO2 separation and purification system based on cryogenic separation and distillation theory. Energies 2014, 7, 3484–3502. [Google Scholar] [CrossRef]
- Tuinier, M.J.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J.A.M. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Tuinier, M.J.; van Sint Annaland, M.; Kuipers, J.A.M. A novel process for cryogenic CO2 capture using dynamically operated packed beds—An experimental and numerical study. Int. J. Greenh. Gas Control 2011, 5, 694–701. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Hamers, H.P.; Van Sint Annaland, M. Techno-economic evaluation of cryogenic CO2 capture-A comparison with absorption and membrane technology. Int. J. Greenh. Gas Control 2011, 5, 1559–1565. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Evaluation of viscosity and thermal conductivity models for CO2 mixtures applied in CO2 cryogenic process in carbon capture and storage (CCS). Appl. Therm. Eng. 2017, 123, 721–733. [Google Scholar] [CrossRef]
- Goli, A.; Shamiri, A.; Talaiekhozani, A.; Eshtiaghi, N.; Aghamohammadi, N.; Aroua, M.K. An overview of biological processes and their potential for CO2 capture. J. Environ. Manag. 2016, 183, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Yadav, G.; Sen, R. Microalgal green refinery concept for biosequestration of carbon-dioxide vis-à-vis wastewater remediation and bioenergy production: Recent technological advances in climate research. J. CO2 Util. 2017, 17, 188–206. [Google Scholar] [CrossRef]
- Van Duc Long, N.; Lee, J.; Koo, K.K.; Luis, P.; Lee, M. Recent progress and novel applications in enzymatic conversion of carbon dioxide. Energies 2017, 10, 473. [Google Scholar] [CrossRef]
- Bond, G.M.; Stringer, J.; Brandvold, D.K.; Simsek, F.A.; Medina, M.-G.; Egeland, G. Development of integrated system for biomimetic CO2 sequestration using the enzyme carbonic anhydrase. Energy Fuels 2001, 15, 309–316. [Google Scholar] [CrossRef]
- Cowan, R.M.; Ge, J.J.; Qin, Y.J.; McGregor, M.L.; Trachtenberg, M.C. CO2 capture by means of an enzyme-based reactor. Ann. N. Y. Acad. Sci. 2003, 984, 453–469. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, N.T. The technology of microalgal culturing. Biotechnol. Lett. 2008, 30, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Sadeghizadeh, A.; Farhad dad, F.; Moghaddasi, L.; Rahimi, R. CO2 capture from air by Chlorella vulgaris microalgae in an airlift photobioreactor. Bioresour. Technol. 2017, 243, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, I.; Vaidhiswaran, R.; Kamani, B.M.; Venugopal, A. Process and engineering trends in membrane based carbon capture. Renew. Sustain. Energy Rev. 2017, 68, 659–684. [Google Scholar] [CrossRef]
- Ebner, A.D.; Ritter, J.A. State-of-the-art adsorption and membrane separation processes for carbon dioxide production from carbon dioxide emitting industries. Sep. Sci. Technol. 2009, 44, 1273–1421. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Kanniche, M.; Gros-Bonnivard, R.; Jaud, P.; Valle-Marcos, J.; Amann, J.-M.; Bouallou, C. Pre-combustion, post-combustion and oxy-combustion in thermal power plant for CO2 capture. Appl. Therm. Eng. 2010, 30, 53–62. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-based carbon capture from flue gas: A review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Kotowicz, J.; Balicki, A. Enhancing the overall efficiency of a lignite-fired oxyfuel power plant with CFB boiler and membrane-based air separation unit. Energy Convers. Manag. 2014, 80, 20–31. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Doukelis, A.; Koumanakos, A.; Kakaras, E. Exergy analysis of a hydrogen fired combined cycle with natural gas reforming and membrane assisted shift reactors for CO2 capture. Energy Convers. Manag. 2012, 60, 196–203. [Google Scholar] [CrossRef]
- Park, H.S.; Lee, J.S.; Han, J.; Park, S.; Park, J.; Min, B.R. CO2 fixation by membrane separated NaCl electrolysis. Energies 2015, 8, 8704–8715. [Google Scholar] [CrossRef] [Green Version]
- Kotowicz, J.; Chmielniak, T.; Janusz-Szymańska, K. The influence of membrane CO2 separation on the efficiency of a coal-fired power plant. Energy 2010, 35, 841–850. [Google Scholar] [CrossRef]
- Kotowicz, J.; Bartela, T. Optimisation of the connection of membrane CCS installation with a supercritical coal-fired power plant. Energy 2012, 38, 118–127. [Google Scholar] [CrossRef]
- Burdyny, T.; Struchtrup, H. Hybrid membrane/cryogenic separation of oxygen from air for use in the oxy-fuel process. Energy 2010, 35, 1884–1897. [Google Scholar] [CrossRef]
- Luis, P.; Van Gerven, T.; Van der Bruggen, B. Recent developments in membrane-based technologies for CO2 capture. Prog. Energy Combust. Sci. 2012, 38, 419–448. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D. Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes. Macromolecules 1999, 32, 375–380. [Google Scholar] [CrossRef]
- Wang, S.; Han, X. Application of Polymeric Membrane in CO2 Capture from Post Combustion. Adv. Chem. Eng. Sci. 2012, 2, 7. [Google Scholar] [CrossRef]
- Kazarian, S.G.; Vincent, M.F.; Bright, F.V.; Liotta, C.L.; Eckert, C.A. Specific Intermolecular Interaction of Carbon Dioxide with Polymers. J. Am. Chem. Soc. 1996, 118, 1729–1736. [Google Scholar] [CrossRef]
- Liu, S.L.; Shao, L.; Chua, M.L.; Lau, C.H.; Wang, H.; Quan, S. Recent progress in the design of advanced PEO-containing membranes for CO2 removal. Prog. Polym. Sci. 2013, 38, 1089–1120. [Google Scholar] [CrossRef]
- Reijerkerk, S.R.; Arun, A.; Gaymans, R.J.; Nijmeijer, K.; Wessling, M. Tuning of mass transport properties of multi-block copolymers for CO2 capture applications. J. Membr. Sci. 2010, 359, 54–63. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 61–106. [Google Scholar] [CrossRef]
- Dindore, V.Y.; Brilman, D.W.F.; Versteeg, G.F. Modelling of cross-flow membrane contactors: Physical mass transfer processes. J. Membr. Sci. 2005, 251, 209–222. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, H.Y.; Feron, P.H.M.; Liang, D.T. Influence of membrane wetting on CO2 capture in microporous hollow fiber membrane contactors. Sep. Purif. Technol. 2005, 46, 33–40. [Google Scholar] [CrossRef]
- Basu, A.; Akhtar, J.; Rahman, M.H.; Islam, M.R. A Review of Separation of Gases Using Membrane Systems. Pet. Sci. Technol. 2004, 22, 1343–1368. [Google Scholar] [CrossRef]
- Pera-Titus, M. Porous inorganic membranes for CO2 capture: Present and prospects. Chem. Rev. 2014, 114, 1413–1492. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Chi, C.; Wang, X.; Peng, Y.; Qian, Y.; Hu, Z.; Dong, J.; Zhao, D. Facile Preparation of Graphene Oxide Membranes for Gas Separation. Chem. Mater. 2016, 28, 2921–2927. [Google Scholar] [CrossRef]
- Liu, C.; Kulprathipanja, S.; Hillock, A.M.W.; Husain, S.; Koros, W.J. Recent Progress in Mixed-Matrix Membranes. In Advanced Membrane Technology and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 787–819. [Google Scholar]
- Rezakazemi, M.; Ebadi Amooghin, A.; Mehdi Montazer-Rahmati, M.; Ismail, A.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Nafisi, V.; Hägg, M.-B. Development of dual layer of ZIF-8/PEBAX-2533 mixed matrix membrane for CO2 capture. J. Membr. Sci. 2014, 459, 244–255. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Huang, S.; Wu, H.; Li, Y.; Tian, Z.; Jiang, Z. Pebax–PEG–MWCNT hybrid membranes with enhanced CO2 capture properties. J. Membr. Sci. 2014, 460, 62–70. [Google Scholar] [CrossRef]
- Li, X.; Cheng, Y.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H. Efficient CO2 capture by functionalized graphene oxide nanosheets as fillers to fabricate multi-permselective mixed matrix membranes. ACS Appl. Mater. Interfaces 2015, 7, 5528–5537. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, D.; Luebke, D.R.; Pennline, H.W. A Review of Carbon Dioxide Selective Membranes: A Topical Report; National Energy Technology Lab.: Pittsburgh, PA, USA; Morgantown, WV, USA, 2003. [Google Scholar]
- Sandru, M.; Kim, T.J.; HÄGG, M.B. Gas Separation Membrane. US20120067209A1, 22 March 2012. [Google Scholar]
- Kim, T.-J.; Vrålstad, H.; Sandru, M.; Hägg, M.-B. Separation performance of PVAm composite membrane for CO2 capture at various pH levels. J. Membr. Sci. 2013, 428, 218–224. [Google Scholar] [CrossRef]
- Kasahara, S.; Kamio, E.; Ishigami, T.; Matsuyama, H. Effect of water in ionic liquids on CO2 permeability in amino acid ionic liquid-based facilitated transport membranes. J. Membr. Sci. 2012, 415–416, 168–175. [Google Scholar] [CrossRef]
- Lockwood, T. A Compararitive Review of Next-generation Carbon Capture Technologies for Coal-fired Power Plant. Energy Procedia 2017, 114, 2658–2670. [Google Scholar] [CrossRef]
- Markusson, N.; Kern, F.; Watson, J. Assessing CCS viability—A socio-technical framework. Energy Procedia 2011, 4, 5744–5751. [Google Scholar] [CrossRef]
- Noureldin, M.; Allinson, W.G.; Cinar, Y.; Baz, H. Coupling risk of storage and economic metrics for CCS projects. Int. J. Greenh. Gas Control. 2017, 60, 59–73. [Google Scholar] [CrossRef]
- Melien, T. Economic and Cost Analysis for CO2 Capture Costs in the CO2 Capture Project Scenarios. In Carbon Dioxide Capture for Storage in Deep Geologic Formations; Elsevier Science: Amsterdam, The Netherlands, 2005; pp. 47–87. [Google Scholar]
- Viebahn, P.; Vallentin, D.; Höller, S. Integrated assessment of carbon capture and storage (CCS) in South Africa's power sector. Energies 2015, 8, 14380–14406. [Google Scholar] [CrossRef]
- Valentić, V.; Žiković, S.; Višković, A. Can CCS save coal fired power plants—The European perspective. Int. J. Greenh. Gas Control 2016, 47, 266–278. [Google Scholar] [CrossRef]
- Abanades, J.C.; Rubin, E.S.; Anthony, E.J. Sorbent Cost and Performance in CO2 Capture Systems. Ind. Eng. Chem. Res. 2004, 43, 3462–3466. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2008, 47, 4883–4890. [Google Scholar] [CrossRef]
- Ho, M.T.; Allinson, G.W.; Wiley, D.E. Reducing the Cost of CO2 Capture from Flue Gases Using Membrane Technology. Ind. Eng. Chem. Res. 2008, 47, 1562–1568. [Google Scholar] [CrossRef]
- Fu, B.-R.; Hsu, S.-W.; Liu, C.-H.; Liu, Y.-C. Statistical analysis of patent data relating to the organic Rankine cycle. Renew. Sustain. Energy Rev. 2014, 39, 986–994. [Google Scholar] [CrossRef]
- Mogee, M.E. Using Patent Data for Technology Analysis and Planning. Res. Technol. Manag. 1991, 34, 43–49. [Google Scholar] [CrossRef]
- Mogee, M.E. Patent analysis for strategic advantage: Using international patent records. Compet. Intell. Rev. 1994, 5, 27–35. [Google Scholar] [CrossRef]
- Hall, B.H.; Jaffe, A.; Trajtenberg, M. Market Value and Patent Citations. RAND J. Econ. 2005, 36, 16–38. [Google Scholar]
- Linton, K. The Importance of Trade Secrets: New Directions in International Trade Policy Making and Empirical Research. J. Int. Commer. Econ. 2015, 7, 1. [Google Scholar]
- Glänzel, W.; Meyer, M. Patents cited in the scientific literature: An exploratory study of ‘reverse citation’ relations. Scientometrics 2003, 58, 415–428. [Google Scholar] [CrossRef]
- Jaffe, A.B.; de Rassenfosse, G. Patent citation data in social science research: Overview and best practices. J. Assoc. Inf. Sci. Technol. 2017, 68, 1360–1374. [Google Scholar] [CrossRef]
- Luis Míguez, J.; Porteiro, J.; Pérez-Orozco, R.; Patiño, D.; Rodríguez, S. Evolution of CO2 capture technology between 2007 and 2017 through the study of patent activity. Appl. Energy 2018, 211, 1282–1296. [Google Scholar] [CrossRef]
- Qiu, H.H.; Liu, L.G. A study on the evolution of carbon capture and storage technology based on knowledge mapping. Energies 2018, 11. [Google Scholar] [CrossRef]
- Harhoff, D.; Narin, F.; Scherer, F.M.; Vopel, K. Citation Frequency and the Value of Patented Inventions. Rev. Econ. Stat. 1999, 81, 511–515. [Google Scholar] [CrossRef]
- Choe, H.; Lee, D.H.; Seo, I.W.; Kim, H.D. Patent citation network analysis for the domain of organic photovoltaic cells: Country, institution, and technology field. Renew. Sustain. Energy Rev. 2013, 26, 492–505. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- WIPO. Terms and Abbreviations. In Handbook on Industrial Property Information and Documentation; WIPO: Geneva, Switzerland, 2013; pp. 1–41. [Google Scholar]
- PRISM® Membranes. Available online: http://www.airproducts.com/Products/Gases/supply-options/prism-membranes.aspx (accessed on 15 October 2018).
- Baker, R.W. Membrane Technology and Applications; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Drioli, E.; Barbieri, G. Membrane Engineering for the Treatment of Gases: Gas-Separation Problems with Membranes; Royal Society of Chemistry: London, UK, 2011. [Google Scholar]
- Finkenrath, M.; Bartlett, M.A.; Hoffmann, S.M.-N.; Ruud, J.A. Carbon Dioxide Capture Systems and Methods. U.S. Patent 20080127632A1, 5 June 2008. [Google Scholar]
- Ruud, J.; Molaison, J.; Schick, L.; Ku, A.Y.-C.; Liu, K.; Kulkarni, P.; Rizeq, R. Methods and Apparatus for Hydrogen Gas Production. U.S. Patent Application 11/263,269, 31 October 2005. [Google Scholar]
- Wright, A.; Lackner, K. Method and Apparatus for Extracting Carbon Dioxide from Air. US Patent 20080087165A1, 17 April 2008. [Google Scholar]
- Wright, A.B.; Lackner, K.S.; Wright, B.; Wallen, M.; Ginster, U.; Peters, E.J. Removal of Carbon Dioxide from Air. U.S. Patent WO2007016271A2, 8 February 2007. [Google Scholar]
- Hiraki, D.; Mochizuki, Y.; Yamazaki, K. Composition for Formation of Carbon Dioxide Separation Membrane, Carbon Dioxide Separation Membrane and Process for Production Thereof, and Carbon Dioxide Separation Apparatus. U.S. Patent WO2012096055A1, 20 October 2015. [Google Scholar]
- Aburaya, Y.; Yamazaki, K. Carbon Dioxide Separation Member, Method for Producing Same, and Carbon Dioxide Separation Module. WO2013018538A1, 20 February 2013. [Google Scholar]
- Kuzushita, K.; Matsuyama, H.; Okada, O.; Shimada, K.; Teramoto, M.; Yegani, R. Carbon Dioxide Separation Apparatus. JP2009195900A, 3 September 2009. [Google Scholar]
- Jain, R. Carbon Dioxide Recovery. U.S. Patent 20100251887A1, 7 October 2010. [Google Scholar]
- Trachtenberg, M. Methods, Apparatuses, and Reactors for Gas Separation. U.S. Patent 20070004023A1, 19 May 2004. [Google Scholar]
- Marand, E.; Kim, S. Ordered Mesopore Silica Mixed Matrix Membranes, and Production Methods for Making Ordered Mesopore Silica Mixed Matric Membranes. U.S. Patent 20070022877A1, 5 July 2006. [Google Scholar]
| ID | Organization | Documents | % Total |
|---|---|---|---|
| 1 | Centre National de la Recherche Scientifique (CNRS) | 30 | 5% |
| 2 | United States Department of Energy | 27 | 5% |
| 3 | Norwegian University of Science & Technology (NTNU) | 24 | 4% |
| 4 | University of Lorraine | 22 | 4% |
| 5 | Chinese Academy of Sciences | 18 | 3% |
| 6 | SINTEF Mat & Chem | 16 | 3% |
| 7 | University System of Georgia | 16 | 3% |
| 8 | Zhejiang University | 16 | 3% |
| 9 | Cooperative Research Centre for Greenhouse Gas Technologies (CO2CRC) | 15 | 3% |
| 10 | University of Melbourne | 12 | 2% |
| ID | Applicant | Patent Families | % Total | Patent Documents | % Total |
|---|---|---|---|---|---|
| 1 | UOP LLC | 56 | 7% | 203 | 7% |
| 2 | General Electric Co. | 29 | 4% | 145 | 5% |
| 3 | Kilimanjaro Energy Inc. | 19 | 2% | 136 | 5% |
| 4 | Fuji Film Corp. | 39 | 5% | 123 | 4% |
| 5 | Shell Internationale Research Maatschappij | 19 | 2% | 120 | 4% |
| 6 | Air Liquide SA | 27 | 3% | 97 | 4% |
| 7 | Membrane Technology & Res. Inc. | 28 | 4% | 89 | 3% |
| 8 | Mitsubishi Hitachi Power Systems Euro GM | 25 | 3% | 81 | 3% |
| 9 | Chevron Corp. | 23 | 3% | 79 | 3% |
| 10 | University of Colorado | 14 | 2% | 74 | 3% |
| 11 | Renaissance Energy Res. Corp. | 12 | 2% | 57 | 2% |
| 12 | Nippon Steel & Sumitomo Metal Corp. | 13 | 2% | 54 | 2% |
| 13 | ExxonMobil Res & Eng Co. | 8 | 1% | 53 | 2% |
| 14 | Nippon Oil Co. Ltd. | 10 | 1% | 47 | 2% |
| 15 | Evonik Degussa GMBH | 3 | <1% | 42 | 2% |
| 16 | Georgia Tech Res. Corp. | 11 | 1% | 42 | 2% |
| 17 | University of Tianjin | 27 | 3% | 41 | 1% |
| 18 | Samsung Electronics Co. Ltd. | 8 | 1% | 40 | 1% |
| 19 | JFE Steel Corp. | 2 | <1% | 38 | 1% |
| 20 | University of Hanyang IUCF-HYU | 8 | 1% | 37 | 1% |
| 21 | Saudi Arabian Oil Co. | 6 | 1% | 32 | 1% |
| 22 | Aramco Services Co. | 4 | 1% | 29 | 1% |
| 23 | Gas Technology Institute | 10 | 1% | 27 | 1% |
| Total | 401 | 51% | 1686 | 61% |
| Company | 1995–2005 | 2007–2017 |
|---|---|---|
| Praxair Technology, Inc., USA | 23 | 6 |
| Membrane Tech. & Research, Inc., USA | 23 | 89 |
| Air Products and Chemicals, Inc., USA | 11 | 24 |
| Air Liquide, France | 11 | 97 |
| Exxon Mobil, USA | 10 | 53 |
| Chevron USA Inc., USA | 9 | 79 |
| UOP LLC, USA | 7 | 203 |
| Engelhard Corporation, USA | 6 | 0 |
| Norsk hydro ASA, Norway | 6 | 0 |
| E.I. Du Pont de Nemours and Company, USA | 5 | 13 |
| ID | Publication Number | Title | Assignee | Year | Cited Documents | Received Citations | Innovation deg. “I” |
|---|---|---|---|---|---|---|---|
| 1 | US20080127632A1 [111] | Carbon dioxide capture systems and methods | General Electric Co. | 2008 | 11 | 107 | 10.7 |
| 2 | US20070004023A1 [115] | Methods, apparatuses, and reactors for gas separations | Trachtenberg, M. | 2007 | 8 | 114 | 10.6 |
| 3 | US20080087165A1 [113] | Method and apparatus for extracting carbon dioxide from air | Kilimanjaro Energy Inc. | 2008 | 71 | 154 | 9.2 |
| 4 | WO2007016271A2 [114] | Removal of carbon dioxide from air | Kilimanjaro Energy Inc. | 2007 | 11 | 87 | 7.6 |
| 5 | US20070022877A1 [116] | Ordered mesopore silica mixed matrix membranes and production methods for making ordered mesopore silica mixed matrix membranes | Kim, S. and Marand, E. | 2007 | 18 | 90 | 7.2 |
| 6 | JP2009195900A [117] | Carbon dioxide separation apparatus | Renaissance Energy Res. Corp. | 2009 | 6 | 60 | 6.8 |
| 7 | US20100251887A1 [118] | Carbon dioxide recovery | University of Kobe Innosepra LLC | 2010 | 7 | 50 | 6.0 |
| 8 | WO2012096055A1 [119] | Composition for formation of carbon dioxide separation membrane, carbon dioxide separation membrane and process for production thereof, and carbon dioxide separation apparatus | Jain Ravi Fuji Film Corp. | 2012 | 8 | 35 | 5.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Míguez, J.L.; Porteiro, J.; Pérez-Orozco, R.; Gómez, M.Á. Technology Evolution in Membrane-Based CCS. Energies 2018, 11, 3153. https://doi.org/10.3390/en11113153
Míguez JL, Porteiro J, Pérez-Orozco R, Gómez MÁ. Technology Evolution in Membrane-Based CCS. Energies. 2018; 11(11):3153. https://doi.org/10.3390/en11113153
Chicago/Turabian StyleMíguez, José Luis, Jacobo Porteiro, Raquel Pérez-Orozco, and Miguel Ángel Gómez. 2018. "Technology Evolution in Membrane-Based CCS" Energies 11, no. 11: 3153. https://doi.org/10.3390/en11113153
APA StyleMíguez, J. L., Porteiro, J., Pérez-Orozco, R., & Gómez, M. Á. (2018). Technology Evolution in Membrane-Based CCS. Energies, 11(11), 3153. https://doi.org/10.3390/en11113153





