Mild Hydrothermal Liquefaction of High Water Content Agricultural Residue for Bio-Crude Oil Production: A Parametric Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
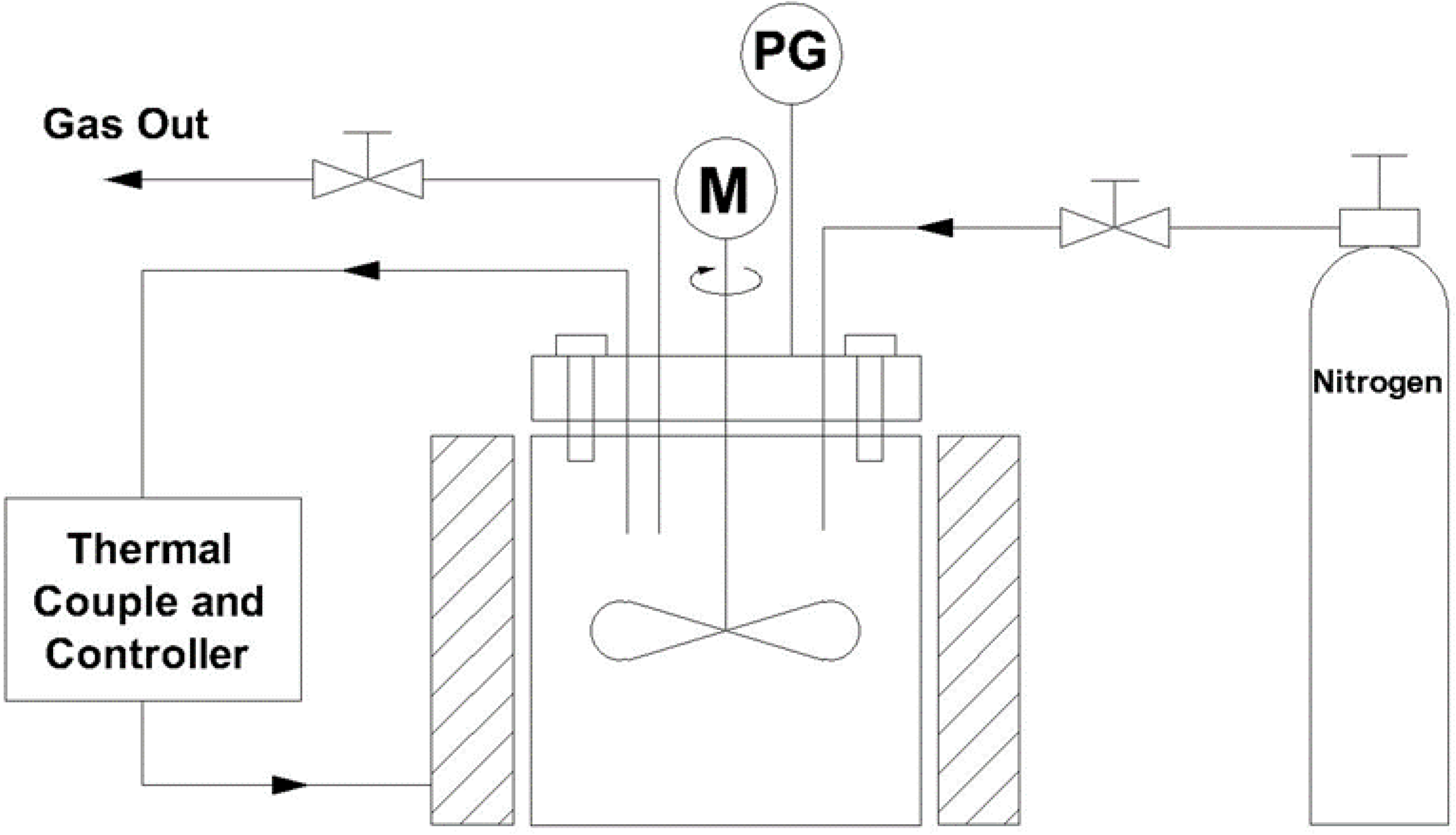
2.2. Hydrothermal Liquefaction of Tomato Plant Wastes
2.3. Characterization
3. Results and Discussion
3.1. Feedstock Characterization and Experimental Design
3.2. Effect of Operating Conditions on HTL and Process Optimization
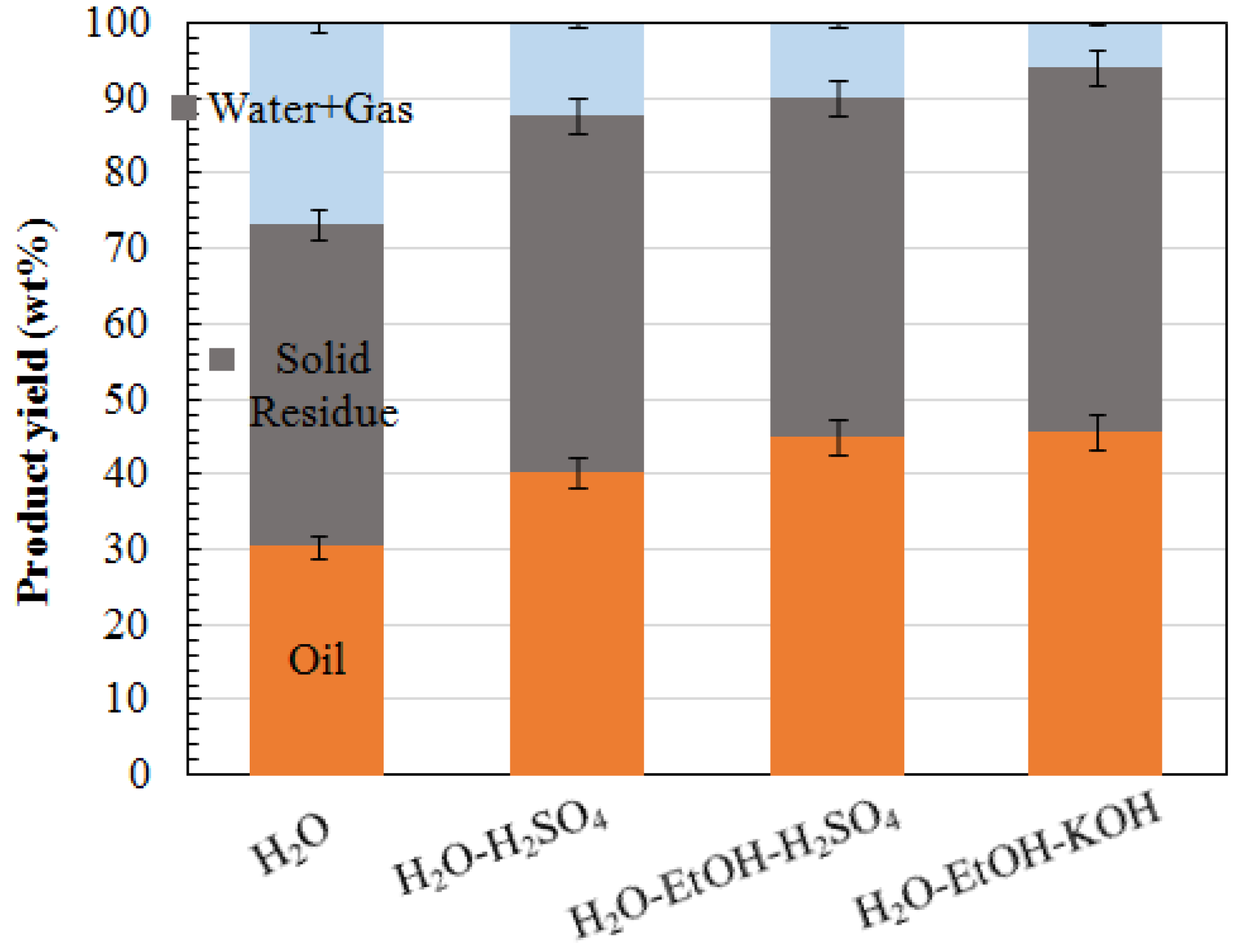
3.2.1. Effect of Reaction Medium and Catalyst
3.2.2. Effects of Reaction Temperature
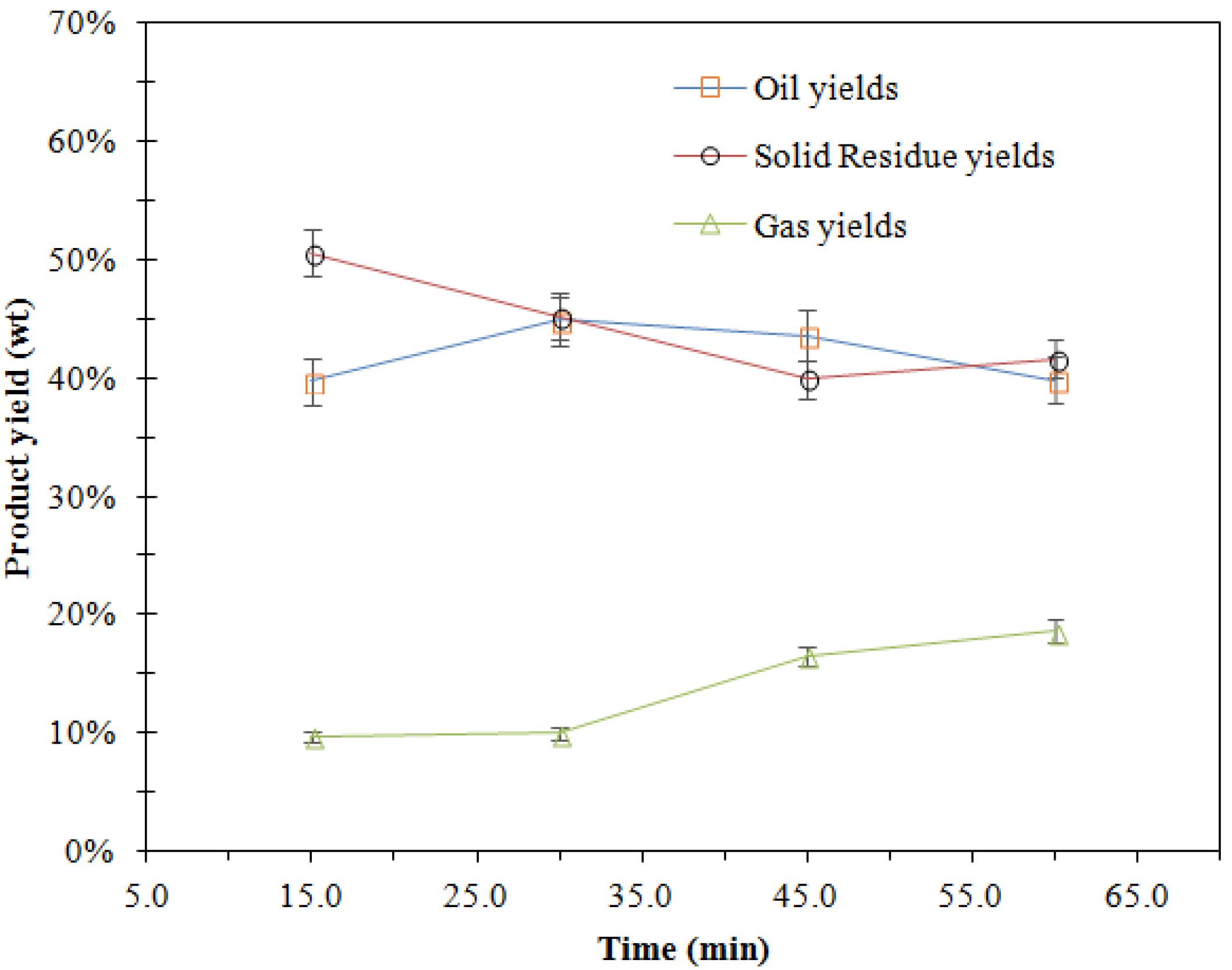
3.2.3. Effects of Holding Time
3.3. Structural Analyses of Bio-Crude Oils
3.4. Elemental Distribution of Oily Products
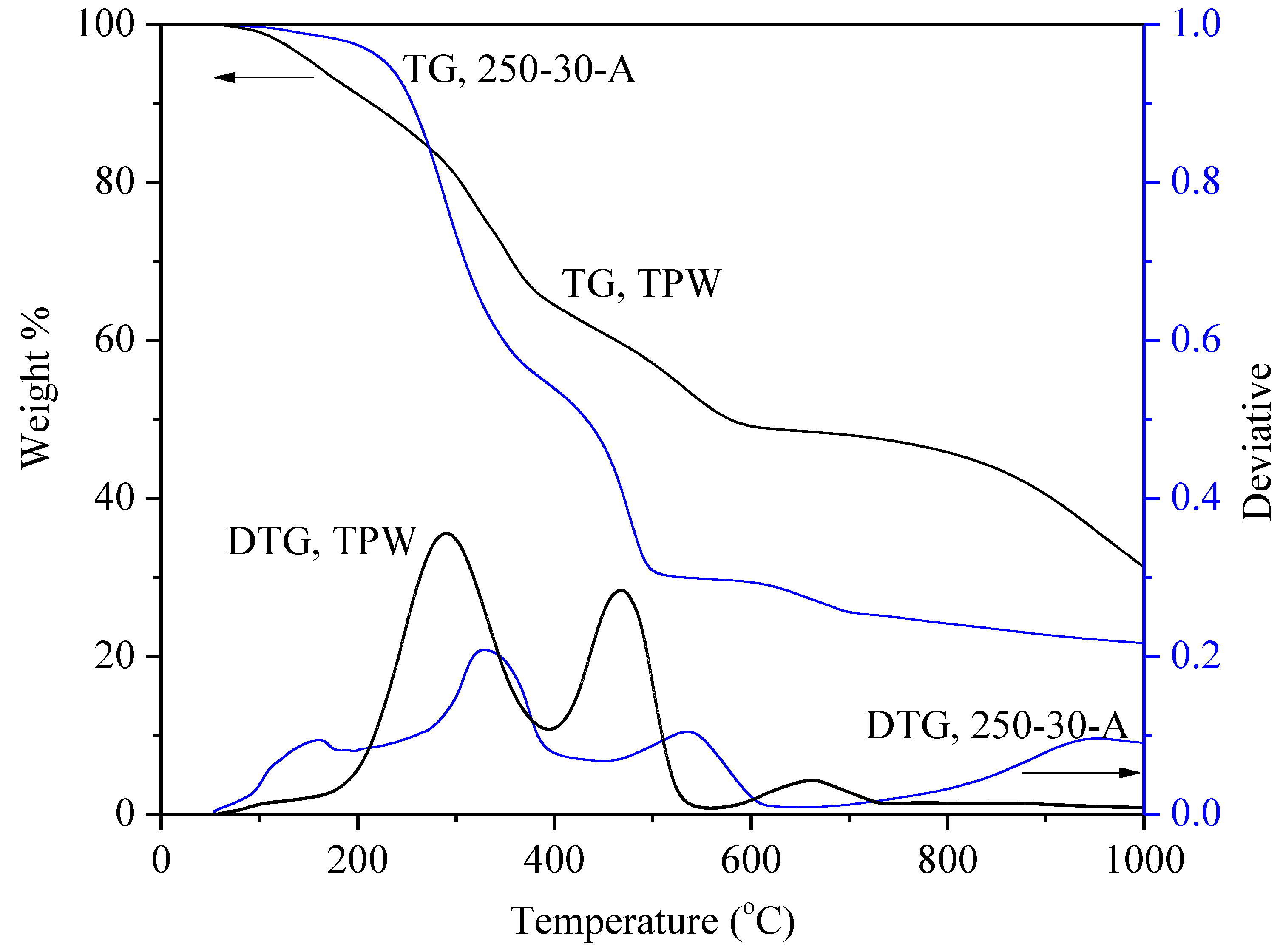
3.5. Thermal Stability, Volatile Matter upon Decomposition and Proximate Analysis of Bio-Crude Oil
3.6. Analysis of Gaseous Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Demirbas, A. Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current status, challenges, and future direction. Energy Fuels 2006, 20, 1727–1737. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of biomass: Deriving more value from waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, M.F. Biorefineries for biofuel upgrading: A critical review. Appl. Energy 2009, 86, S151–S161. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Prins, W.; Ronsse, F.; Brilman, W. Hydrothermal liquefaction (HTL) of microalgae for biofuel production: State of the art review and future prospects. Biomass Bioenergy 2013, 53, 113–127. [Google Scholar] [CrossRef]
- Elliott, D.C.; Biller, P.; Ross, A.B.; Schmidt, A.J.; Jones, S.B. Hydrothermal liquefaction of biomass: Developments from batch to continuous process. Bioresour. Technol. 2015, 178, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.M.; Layzell, D.B. A Canadian biomass inventory: Feedstocks for a bio-based economy. BIOCAP Can. Found. 2003, 18–24. [Google Scholar]
- Reddy, H.K.; Muppaneni, T.; Ponnusamy, S.; Sudasinghe, N.; Pegallapati, A.; Selvaratnam, T.; Seger, M.; Dungan, B.; Nirmalakhandan, N.; Schaub, T.; et al. Temperature effect on hydrothermal liquefaction of Nannochloropsis gaditana and Chlorella sp. Appl. Energy 2016, 165, 943–951. [Google Scholar] [CrossRef]
- Zhu, Y.; Biddy, M.J.; Jones, S.B.; Elliott, D.S.; Schmidt, A.J. Techno-economic analysis of liquid fuel production from woody biomass via hydrothermal liquefaction (HTL) and upgrading. Appl. Energy 2014, 129, 384–394. [Google Scholar] [CrossRef]
- Xu, C.; Etcheverry, T. Hydro-liquefaction of woody biomass in sub-and super-critical ethanol with iron-based catalysts. Fuel 2008, 87, 335–345. [Google Scholar] [CrossRef]
- Taner, F.; Eratik, A.; Ardic, I. Identification of the compounds in the aqueous phases from liquefaction of lignocellulosics. Fuel Process. Technol. 2005, 86, 407–418. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Jin, F.; Tohji, K.; Enomoto, H. Acid catalytic hydrothermal conversion of carbohydrate biomass into useful substances. J. Mater. Sci. 2008, 43, 2472–2475. [Google Scholar] [CrossRef]
- Yin, S.; Pan, Y.; Tan, Z. Hydrothermal conversion of cellulose to 5-hydroxymethyl furfural. Int. J. Green Energy 2011, 8, 234–247. [Google Scholar] [CrossRef]
- Yin, S.; Mehrotra, A.K.; Tan, Z. Alkaline hydrothermal conversion of cellulose to bio-oil: Influence of alkalinity on reaction pathway change. Bioresour. Technol. 2011, 102, 6605–6610. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Toor, S.S.; Rosendahl, L.; Rudolf, A. Hydrothermal liquefaction of biomass: A review of subcritical water technologies. Energy 2011, 36, 2328–2342. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal Liquefaction of Macroalgae Enteromorpha prolifera to Bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Shuping, Z.; Yulong, W.; Mingde, Y.; Kaleem, I.; Chun, L.; Tong, J. Production and characterization of bio-oil from hydrothermal liquefaction of microalgae Dunaliella tertiolecta cake. Energy 2010, 35, 5406–5411. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative Evaluation of Thermochemical Liquefaction and Pyrolysis for Bio-Oil Production from Microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Duan, P.; Savage, P.E. Hydrothermal Liquefaction of a Microalga with Heterogeneous Catalysts. Ind. Eng. Chem. Res. 2011, 50, 52–61. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Scott, J.; Yu, G.; Wang, Z.; Schideman, L.; Zhang, Y.; Strathmann, T.J. Chemical properties of biocrude oil from the hydrothermal liquefaction of Spirulina algae, swine manure, and digested anaerobic sludge. Bioresour. Technol. 2011, 102, 8295–8303. [Google Scholar] [CrossRef] [PubMed]
- Biller, P.; Ross, A.B.; Skill, S.C.; Lea-Langton, A.; Balasundaram, B.; Hall, C.; Riley, R.; Llewellyn, C.A. Nutrient recycling of aqueous phase for microalgae cultivation from the hydrothermal liquefaction process. Algal Res. 2012, 1, 70–76. [Google Scholar] [CrossRef]
- Toor, S.S.; Reddy, H.; Deng, S.; Hoffmann, J.; Spangsmark, D.; Madsen, L.B.; Holm-Nielsen, J.B.; Rosendahl, L.A. Hydrothermal liquefaction of Spirulina and Nannochloropsis salina under subcritical and supercritical water conditions. Bioresour. Technol. 2013, 131, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wei, X.; Zhong, C. Experimental study on the direct liquefaction of Cunninghamia lanceolata in water. Energy 2003, 28, 597–606. [Google Scholar] [CrossRef]
- Rezzoug, S.-A.; Capart, R. Liquefaction of wood in two successive steps: Solvolysis in ethylene-glycol and catalytic hydrotreatment. Appl. Energy 2002, 72, 631–644. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga Laminaria Saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef] [PubMed]



| Moisture Content | 92.2 ± 0.03 |
|---|---|
| Volatile matter | 62 ± 0.52 |
| Ash | 32 ± 0.08 |
| Fixed carbon | 6 ± 0.06 |
| Elemental composition (%) | |
| C | 34.8 ± 0.22 |
| H | 5.0 ± 0.11 |
| O a | 23.4 ± 0.16 |
| N | 4.0 ± 0.08 |
| S | 0.3 ± 0.02 |
| HHV (MJ/kg) | 12.4 b |
| Entry | Name | Temperature (°C) | Holding Time (min) | Solvent | Catalyst | Oil Yield (%) | Solid Residue (%) |
|---|---|---|---|---|---|---|---|
| 1 | 250-30-N-H2O | 250 | 30 | H2O | N/A | 30.46 | 42.79 |
| 2 | 250-30-A-H2O | 250 | 30 | H2O | H2SO4 | 40.42 | 47.37 |
| 3 | 250-30-A | 250 | 30 | H2O and EtOH | H2SO4 | 44.95 | 45.08 |
| 4 | 250-30-B | 250 | 30 | H2O and EtOH | KOH | 45.60 | 48.54 |
| 5 | 220-30-A | 220 | 30 | H2O and EtOH | H2SO4 | 42.35 | 55.16 |
| 6 | 250-30-A | 250 | 30 | H2O and EtOH | H2SO4 | 44.95 | 45.08 |
| 7 | 280-30-A | 280 | 30 | H2O and EtOH | H2SO4 | 40.70 | 41.03 |
| 8 | 250-15-A | 250 | 15 | H2O and EtOH | H2SO4 | 39.71 | 50.58 |
| 9 | 250-30-A | 250 | 30 | H2O and EtOH | H2SO4 | 44.95 | 45.08 |
| 10 | 250-45-A | 250 | 45 | H2O and EtOH | H2SO4 | 41.19 | 41.58 |
| 11 | 250-60-A | 250 | 60 | H2O and EtOH | H2SO4 | 45.88 | 39.86 |
| PK | RT a | Area Pct b | Library/ID | CAS | Qual c |
|---|---|---|---|---|---|
| 1 | 2.68 | 19.35 | Acetic acid | 000064-19-7 | 90 |
| 2 | 3.2 | 2.22 | Thiophene | 000110-02-1 | 38 |
| 3 | 3.67 | 2.77 | 2-Hexanol, 3-methyl- | 002313-65-7 | 59 |
| 4 | 3.89 | 3.75 | 1H-Imidazole, 2-ethenyl- | 043129-93-7 | 35 |
| 5 | 4.27 | 2.51 | 2-Pentanone, 4-hydroxy-4-methyl- | 000123-42-2 | 38 |
| 6 | 4.89 | 1.69 | Pyrimidine, 4,6-dimethyl- | 001558-17-4 | 52 |
| 7 | 5.01 | 36.75 | N,N-Dimethylacetamide | 000127-19-5 | 94 |
| 8 | 8.5 | 9.87 | 4-Pyridinol | 000626-64-2 | 83 |
| 9 | 10.25 | 0.47 | 3-Oxabicyclo[3.3.0]oct-7-en-2-one, 7-methoxy- | 1000155-61-4 | 49 |
| 10 | 12.63 | 0.56 | 6-Isopropenyl-4,8a-dimethyl-4a,5,6,7,8,8a-hexahydro-1H-naphthalen-2-one | 086917-79-5 | 91 |
| 11 | 13.33 | 0.47 | d-Ribitol, 1-deoxy-1-heptylamino- | 1000158-66-6 | 43 |
| 12 | 13.73 | 1.27 | Hexadecanoic acid, ethyl ester | 000628-97-7 | 60 |
| 13 | 13.97 | 3.01 | Benzene, 1-chloro-4-ethoxy- | 000622-61-7 | 38 |
| 14 | 14.34 | 2.05 | Isonipecotic acid, N-(3-cyclopentylpropionyl)-, ethyl ester | 1000361-08-3 | 38 |
| 15 | 14.58 | 2.49 | Benzyl alcohol, 4-methoxy-6-fluoro- | 000405-09-4 | 35 |
| 16 | 14.94 | 4.77 | 2,5-Piperazinedione, 3,6-bis(2-methylpropyl)- | 001436-27-7 | 49 |
| 17 | 15.26 | 1.43 | Benzene, 1-methoxy-3-(methylthio)- | 002388-74-1 | 60 |
| 18 | 15.46 | 2.66 | Phenol, 3,5-dimethoxy- | 000500-99-2 | 46 |
| 19 | 15.73 | 0.63 | Pyrrolidine, 1-(7-nitrodibenzofuran-2-sulfonyl)- | 1000302-59-9 | 46 |
| 20 | 16.69 | 0.77 | Carbamate, N-(2-naphthyl)-, 3-pentynyl ester | 1000197-96-0 | 41 |
| 21 | 18.1 | 0.52 | Benzoic acid, 3-methoxy-, 2-(2-nitrophenyl-2-oxo)ethyl ester | 331277-43-1 | 38 |
| Total | 100 |
| PK | RT | Area Pct | Library/ID | CAS | Qual |
|---|---|---|---|---|---|
| 1 | 3.69 | 19.93 | Acetic acid | 000064-19-7 | 91 |
| 2 | 3.99 | 0.96 | Propanoic acid | 000079-09-4 | 37 |
| 3 | 5.29 | 50.57 | Propanoic acid | 000127-19-5 | 91 |
| 4 | 7.19 | 0.67 | 2-Pyrrolidinone, 1-methyl- | 000872-50-4 | 81 |
| 5 | 7.58 | 2.40 | 2-Propanone, dimethylhydrazone | 013483-31-3 | 40 |
| 6 | 7.9 | 7.20 | Pentanoic acid, 4-oxo- | 000123-76-2 | 80 |
| 7 | 8.18 | 3.64 | 2-Furancarboxylic acid, 4-pentadecyl ester | 1000280-64-0 | 38 |
| 8 | 8.58 | 4.60 | 4-Pyridinol | 000626-64-2 | 91 |
| 9 | 8.75 | 1.61 | 3-Pyridinol | 000109-00-2 | 83 |
| 10 | 15.92 | 0.67 | 2-Methyl aminomethyl-1,3-dioxolane | 057366-77-5 | 30 |
| 11 | 16.29 | 1.31 | Pyrimidine-2,4,6(1H,3H,5H)-trione, 1,3-diethyl-5-[(1,3-diethyl-2,4,6-trioxoperhydro-5-pyrimidinyl)methylene]- | 128644-14-4 | 25 |
| 12 | 16.81 | 2.90 | Thiosemicarbazide, 4-(1-adamantylcarbonyl)- | 300799-69-3 | 35 |
| 13 | 17.03 | 1.09 | Thiosemicarbazide, 4-(1-adamantylcarbonyl)- | 300799-69-3 | 30 |
| 14 | 17.30 | 1.86 | 1H-Pyrrole-2,4-dicarboxylic acid, 5-formyl-3-methyl-, diethyl ester | 002199-60-2 | 38 |
| 15 | 17.61 | 0.59 | N-(5-Chloro-2-methoxy-phenyl)-2-(5-methoxy-1H-benzoimidazol-2-ylsulfanyl)-acetamide | 1000295-83-8 | 15 |
| Total | 100 |
| PK | RT | Area Pct | Library/ID | CAS | Qual |
|---|---|---|---|---|---|
| 1 | 2.8985 | 0.8511 | Cyclopropane, ethenylmethylene- | 019995-92-7 | 37 |
| 2 | 3.1593 | 34.6287 | Acetic acid | 000064-19-7 | 90 |
| 3 | 3.4265 | 6.442 | Acetic acid, hydrazide | 001068-57-1 | 9 |
| 4 | 3.9546 | 9.6236 | Propylene Glycol | 000057-55-6 | 72 |
| 5 | 4.1009 | 3.359 | 2-hydroxy-2-methyl-4-heptanone | 054862-91-8 | 43 |
| 6 | 4.3427 | 5.6599 | Acetamide, N-(aminoiminomethyl)- | 005699-40-1 | 50 |
| 7 | 4.4699 | 4.6055 | 2-Pentanone, 4-hydroxy- | 004161-60-8 | 64 |
| 8 | 4.6289 | 1.6247 | n-Capric acid isopropyl ester | 002311-59-3 | 27 |
| 9 | 4.9025 | 2.1625 | Pyrazine, 2,5-dimethyl- | 000123-32-0 | 53 |
| 10 | 5.0743 | 1.5347 | 2-Cyclopenten-1-one, 2-methyl- | 001120-73-6 | 30 |
| 11 | 5.1443 | 17.7941 | N,N-Dimethylacetamide | 000127-19-5 | 91 |
| 12 | 6.6712 | 0.9744 | Pyrazine, 2-ethyl-3,5-dimethyl- | 013925-07-0 | 38 |
| 13 | 7.2438 | 0.5655 | Ethanone, 1-(1-cyclohexen-1-yl)- | 000932-66-1 | 43 |
| 14 | 7.6191 | 0.4386 | 6-Methyl-2,3-dihydropyran-2,4-dione | 000541-98-0 | 49 |
| 15 | 14.0193 | 2.155 | 3-(Ethyl-hydrazono)-butan-2-one | 1000194-94-0 | 35 |
| 16 | 14.3947 | 0.9634 | 2-Methylcyclohexylamine | 007003-32-9 | 27 |
| 17 | 14.9355 | 1.9235 | Cyclohexane, 1,2,3-trimethyl- | 001678-97-3 | 25 |
| 18 | 15.2981 | 1.7533 | Cyclohexa-2,4-dienecarboxylic acid, 4-hydroxy-1-methyl-6-oxo-, ethyl ester | 1000304-34-0 | 45 |
| 19 | 15.4763 | 2.9403 | l-Proline, N-valeryl-, hexyl ester | 1000345-48-3 | 53 |
| Total | 100 |
| Sample | N/% | C/% | H/% | S/% | O/% | Ash/% | HHV/MJ/kg |
|---|---|---|---|---|---|---|---|
| TPW | 4.0 | 34.8 | 5.0 | 0.3 | 23.4 | 32.5 | 12.4 |
| 250-30-A | 5.5 | 31.8 | 4.1 | 1.9 | 30.9 | 25.8 | 12.3 |
| Residue | 1.3 | 30.9 | 4.1 | 3.7 | 0.1 | 59.9 | 16.3 |
| 250-30-A-OF | 4.6 | 48.6 | 5.4 | 0.8 | 31.5 | 9.1 | 18.5 |
| 250-30-A-AF | 7.7 | 20.2 | 3.4 | 0.0 | 39.8 | 28.9 | 4.6 |
| Entry | Name | CH4 | CO | CO2 | N2 |
|---|---|---|---|---|---|
| 1 | 250-30-A | 0 | 1.17 | 12.93 | 81.80 |
| 2 | 250-30-B | 0.01 | 0.37 | 6.86 | 88.99 |
| 3 | 220-30-A | 0.01 | 0.73 | 10.88 | 86.43 |
| 4 | 250-30-A | 0 | 1.17 | 12.93 | 81.80 |
| 5 | 280-30-A | 0.02 | 2.45 | 8.20 | 79.96 |
| 6 | 250-15-A | 0.00 | 1.19 | 6.68 | 83.02 |
| 7 | 250-30-A | 0 | 1.17 | 12.93 | 81.80 |
| 8 | 250-45-A | 0.01 | 2.11 | 13.96 | 82.66 |
| 9 | 250-60-A | 0.01 | 1.27 | 10.40 | 85.72 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Minaret, J.; Yuan, Z.; Dutta, A.; Xu, C. Mild Hydrothermal Liquefaction of High Water Content Agricultural Residue for Bio-Crude Oil Production: A Parametric Study. Energies 2018, 11, 3129. https://doi.org/10.3390/en11113129
Zhang Y, Minaret J, Yuan Z, Dutta A, Xu C. Mild Hydrothermal Liquefaction of High Water Content Agricultural Residue for Bio-Crude Oil Production: A Parametric Study. Energies. 2018; 11(11):3129. https://doi.org/10.3390/en11113129
Chicago/Turabian StyleZhang, Yongsheng, Jamie Minaret, Zhongshun Yuan, Animesh Dutta, and Chunbao (Charles) Xu. 2018. "Mild Hydrothermal Liquefaction of High Water Content Agricultural Residue for Bio-Crude Oil Production: A Parametric Study" Energies 11, no. 11: 3129. https://doi.org/10.3390/en11113129
APA StyleZhang, Y., Minaret, J., Yuan, Z., Dutta, A., & Xu, C. (2018). Mild Hydrothermal Liquefaction of High Water Content Agricultural Residue for Bio-Crude Oil Production: A Parametric Study. Energies, 11(11), 3129. https://doi.org/10.3390/en11113129




_Xu.png)

