Study of the Hazard of Endogenous Fires in Coal Mines—A Chemometric Approach
Abstract
:1. Introduction
2. Materials and Methods
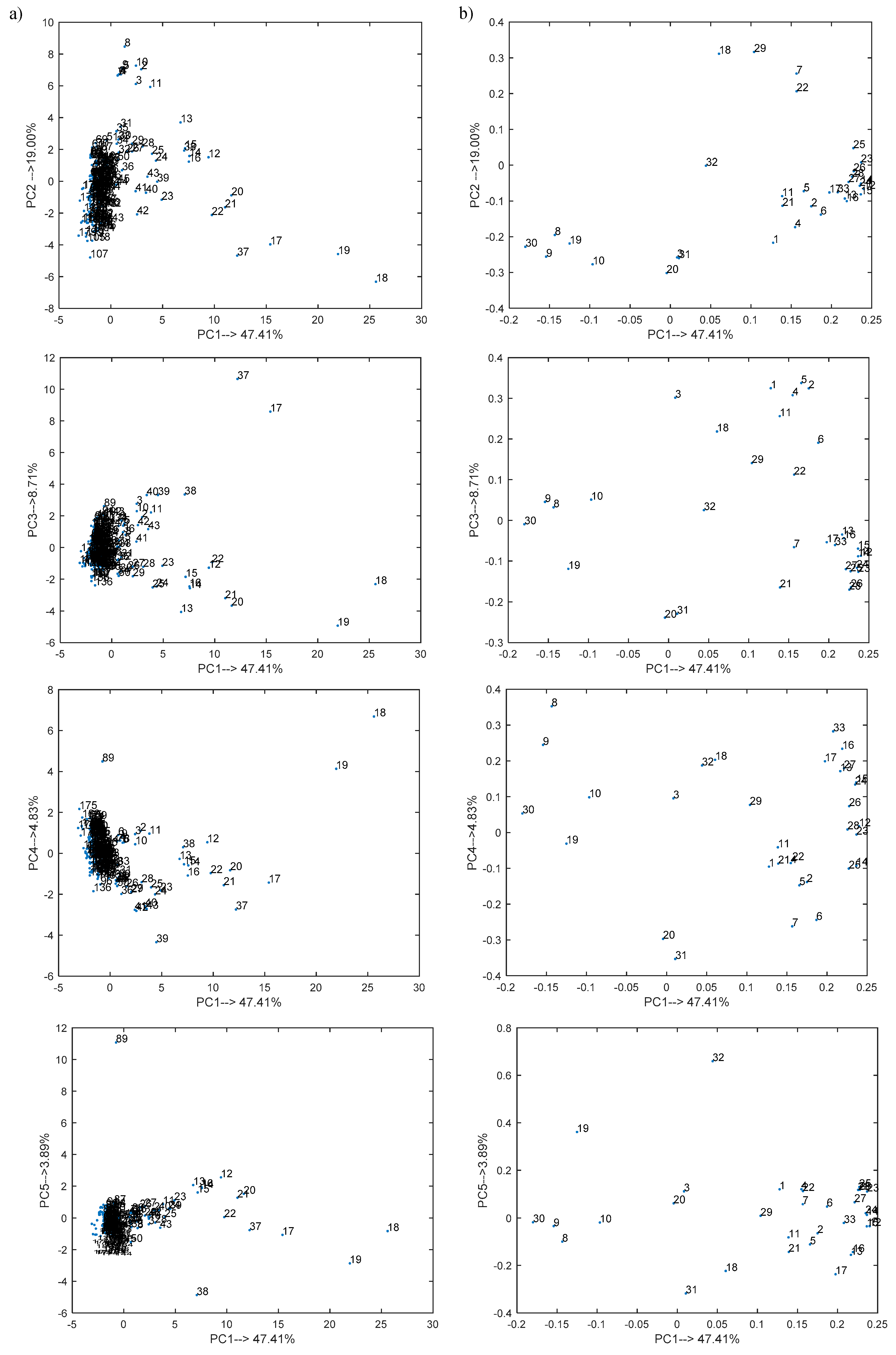
3. Results
- Cluster A collecting objects nos. 1–16, 20–35, and 38–43;
- Cluster B composed of objects nos. 17–19 and 37;
- Cluster C grouping objects nos. 36 and 44–180.
- Class A: Parameters nos. 12–17, 21, 23–28 and 33 (describing concentrations of ethane, ethylene, propane, propylene, acetylene, and carbon monoxide, measured behind the dam at a distance of 20 m; concentration of methane was measured behind the dam at a distance of 20 m, as well as concentrations of ethane, ethylene, propane, propylene, acetylene, carbon monoxide, and hydrogen, measured behind the dam at a distance of 350 m, respectively);
- Class B: Parameters nos. 7, 18, 22 and 29 (describing concentration of oxygen measured in the route, concentrations of oxygen and hydrogen measured behind the dam at a distance of 20 m, and concentration of oxygen measured behind the dam at a distance of 350 m);
- Class C: Parameters nos. 1–6, 11 and 32 (concentrations of ethane, ethylene, propane, propylene, acetylene, carbon monoxide, and hydrogen measured in the route, as well as concentration of methane measured behind the dam at a distance of 350 m); and
- Class D: Parameters nos. 8–10, 19, 20, 30 and 31 (describing concentrations of nitrogen, carbon dioxide, and methane measured in the route, as well as concentrations of nitrogen and carbon dioxide measured behind the dam at a distance of 20 m and 250 m, respectively).
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- BP Statistical Review of World Energy June 2017. Available online: https://www.bp.com (accessed on 10 September 2018).
- International Energy Agency: Coal Information 2018: Overview. Available online: https://www.iea.org (accessed on 10 September 2018).
- Yuan, L.; Smith, A.C. The effect of ventilation on spontaneous heating of coal. J. Loss Prev. Process. Ind. 2012, 25, 131–137. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, T.; Liang, Y.; Wang, Z.A. Review on numerical solutions to self-heating of coal stockpile: Mechanism, theoretical basis, and variable study. Fuel 2016, 182, 80–109. [Google Scholar] [CrossRef]
- Itay, M.; Hill, C.R.; Glasser, D.A. Study of the low temperature oxidation of coal. Fuel Process. Technol. 1989, 21, 81–97. [Google Scholar] [CrossRef]
- Deng, J.; Xiao, Y.; Li, Q.; Lu, J.; Wen, H. Experimental studies of spontaneous combustion and anaerobic cooling of coal. Fuel 2015, 157, 261–269. [Google Scholar] [CrossRef]
- Hertzberg, M. Mine Fire Detection; United States Bureau of Mines Information Circular Number 8768: Pittsburgh, PA, USA, 1978.
- Liu, H.; Wu, C.A. New approach to detect fire source underground mine for preventing spontaneous combustion of sulfide ores. Procedia Eng. 2010, 7, 318–326. [Google Scholar] [CrossRef]
- Baris, K.; Kizgut, S.; Didari, V. Low-temperature oxidation of some Turkish coals. Fuel 2012, 93, 423–432. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Li, J.; Qi, H. Effects of oxygen supply on low-temperature oxidation of coal: A case study of Jurassic coal in Yima, China. Fuel 2017, 202, 446–454. [Google Scholar] [CrossRef]
- Feng, X.; Adamus, A. Overview of research and use of indicator gases of coal spontaneous combustion in China. GeoSci. Eng. 2014, 60, 55–65. [Google Scholar] [CrossRef]
- Xie, J.; Xue, S.; Cheng, W.; Wang, G. Early detection of spontaneous combustion of coal in underground coal mines with development of an ethylene enriching system. Int. J. Coal Geol. 2011, 85, 123–127. [Google Scholar] [CrossRef]
- Zhuang, L.; Wang, J. The Technology of Forecasting and Predicting the Hidden Danger of Underground Coal Spontaneous Combustion. Procedia Eng. 2011, 26, 2301–2305. [Google Scholar] [CrossRef]
- Lu, P.; Liao, G.; Sun, J.H.; Li, P.D. Experimental research on index gas of the coal spontaneous at low-temperature stage. J. Loss Prev. Process. Ind. 2004, 17, 243–247. [Google Scholar] [CrossRef]
- Yuan, L.; Smith, A.C. CO and CO2 emissions from spontaneous heating of coal under different ventilation rates. Int. J. Coal. Geol. 2011, 88, 24–30. [Google Scholar] [CrossRef]
- Tang, Y. Sources of underground CO: Crushing and ambient temperature oxidation of coal. J. Loss Prev. Process. Ind. 2015, 38, 50–57. [Google Scholar] [CrossRef]
- Kim, A.G. Locating fires in abandoned underground coal mines. Int. J. Coal Geol. 2004, 59, 49–62. [Google Scholar] [CrossRef]
- Panigrahi, D.C.; Bhattacharjee, R.M. Development of modified gas indices for early detection of spontaneous heating in coal pillars. J. South Afr. Inst. Min. Metall. 2004, 104, 367–379. Available online: https://www.saimm.co.za/Journal/v104n07p367.pdf (accessed on 15 October 2018).
- Smith, A.C.; Miron, Y.; Lazzara, C.P. Large-Scale Studies of Spontaneous Combustion of Coal; National Institute for Occupational Safety and Health: Washington, DC, USA, 1991. [Google Scholar]
- Singh, A.K.; Singh, R.V.K.; Singh, M.P.; Chandra, H.; Shukla, N.K. Mine fire gas indices and their application to Indian underground coal mine fires. Int. J. Coal Geol. 2007, 69, 192–204. [Google Scholar] [CrossRef]
- Brady, D. The role of gas monitoring in the prevention and treatment of mine fires. In Proceedings of the 2008 Australian Coal Operators’ Conference Coal, Sydney, Australia, 14–15 February 2008; pp. 202–208. [Google Scholar]
- Timko, R.J.; Derick, R.L. Methods to Determine the Status of Mine Atmospheres—An Overview; National Institute for Occupational Safety and Health: Washington, DC, USA, 2006. [Google Scholar]
- Wojtacha-Rychter, K.; Smoliński, A. The interaction between coal and multi-component gas mixtures in the process of coal heating at various temperatures: An experimental study. Fuel 2018, 213, 150–157. [Google Scholar] [CrossRef]
- Joliffe, T. Principal components analysis; Springer: New York, NY, USA, 1986. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Wold, S. Principal components analysis. Chemometr. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Smoliński, A.; Walczak, B. Exploratory analysis of data sets with missing elements and outliers. Chemosphere 2002, 49, 233–245. [Google Scholar] [CrossRef]
- Smoliński, A.; Howaniec, N.; Stańczyk, K. A comparative experimental study of biomass, lignite and hard coal steam gasification. Renew. Energy 2011, 36, 1836–1842. [Google Scholar] [CrossRef]
- Nikolic, K.; Pavlovic, M.; Smolinski, A.; Agbaba, D. The chemometric study and quantitative structure retention relationship modeling of liquid chromatography separation of ziprasidone. Comb. Chem. High Throughput Screen. 2012, 15, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Massart, D.L.; Kaufman, L. The Interpretation of Analytical Data by the Use of Cluster Analysis; JohnWiley & Sons: New York, NY, USA, 1983. [Google Scholar]
- Vogt, W.; Nagel, D.; Sator, H. Cluster Analysis in Clinical Chemistry: A Model; JohnWiley & Sons: New York, NY, USA, 1987. [Google Scholar]
- Vandeginste, B.G.M.; Massart, D.L.; Buydens, L.M.C.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part B; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Howaniec, N.; Smoliński, A.; Cempa-Balewicz, M. Experimental study of nuclear high temperature reactor excess heat use in the coal and energy crops co-gasification process to hydrogen-rich gas. Energy 2015, 84, 455–461. [Google Scholar] [CrossRef]
- Nikolic, K.; Filipic, S.; Smoliński, A.; Kaliszan, R.; Agbaba, D. Partial least square and hierarchical clustering in ADMET modeling: Prediction of blood—brain barrier permeation of α-adrenergic and imidazoline receptor ligands. J. Pharm. Pharm. Sci. 2013, 16, 622–647. [Google Scholar] [CrossRef] [PubMed]
- Smoliński, A.; Stempin, M.; Howaniec, N. Determination of rare earth elements in combustion ashes from selected polish coal mines by wavelength dispersive X-ray fluorescence spectrometry. Spectrochim. Acta Part B Autom. Spectrosc. 2016, 116, 63–74. [Google Scholar] [CrossRef]

| No. | Parameters, ppm | Sample Collection Location |
|---|---|---|
| 1 | concentration of ethane | measured in the route |
| 2 | concentration of ethylene | |
| 3 | concentration of propane | |
| 4 | concentration of propylene | |
| 5 | concentration of acetylene | |
| 6 | concentration of carbon monoxide | |
| 7 | concentration of oxygen | |
| 8 | concentration of nitrogen | |
| 9 | concentration of carbon dioxide | |
| 10 | concentration of methane | |
| 11 | concentration of hydrogen | |
| 12 | concentration of ethane | measured behind the dam at a distance of 20 m |
| 13 | concentration of ethylene | |
| 14 | concentration of propane | |
| 15 | concentration of propylene | |
| 16 | concentration of acetylene | |
| 17 | concentration of carbon monoxide | |
| 18 | concentration of oxygen | |
| 19 | concentration of nitrogen | |
| 20 | concentration of carbon dioxide | |
| 21 | concentration of methane | |
| 22 | concentration of hydrogen | |
| 23 | concentration of ethane | measured behind the dam at a distance of 350 m |
| 24 | concentration of ethylene | |
| 25 | concentration of propane | |
| 26 | concentration of propylene | |
| 27 | concentration of acetylene | |
| 28 | concentration of carbon monoxide | |
| 29 | concentration of oxygen | |
| 30 | concentration of nitrogen | |
| 31 | concentration of carbon dioxide | |
| 32 | concentration of methane | |
| 33 | concentration of hydrogen |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wojtacha-Rychter, K.; Smoliński, A. Study of the Hazard of Endogenous Fires in Coal Mines—A Chemometric Approach. Energies 2018, 11, 3047. https://doi.org/10.3390/en11113047
Wojtacha-Rychter K, Smoliński A. Study of the Hazard of Endogenous Fires in Coal Mines—A Chemometric Approach. Energies. 2018; 11(11):3047. https://doi.org/10.3390/en11113047
Chicago/Turabian StyleWojtacha-Rychter, Karolina, and Adam Smoliński. 2018. "Study of the Hazard of Endogenous Fires in Coal Mines—A Chemometric Approach" Energies 11, no. 11: 3047. https://doi.org/10.3390/en11113047






