Abstract
The overall chemical rate and chemical effect of CF3Br, 2-BTP and 2-BTP/CO2 with hydrocarbon flames are calculated using the perfectly stirred reactor (PSR) model. The chemical effects of CF3Br with CH4/air flames always inhibit combustion. The chemical saturation concentration of CF3Br in stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar is roughly 2.5% and 0.8%, respectively. The overall chemical rate of 2-BTP with moist C3H8/air flames is always less than the uninhibited condition and fluctuates with sub-inerting agent additions. The net chemical effect variation of 2-BTP is more complicated than experimented and calculated flame speeds with 2-BTP added to lean hydrocarbon flames. There are negative chemical effects (chemical combustion effects) with certain sub-inerting 2-BTP concentrations (0.015 ≤ Xa ≤ 0.034), which result in the experimented unwanted combustion enhancement in lean moist C3H8/air flames. CO2 can obviously improve the inhibition effect of 2-BTP in lean moist C3H8/air flames, driving negative chemical effects (enhance combustion) into positive chemical effects (inhibit combustion) with lean moist C3H8/air flames. No enhanced combustion would occur with the blends (2-BTP/CO2) when CO2 addition is larger than 4% in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar.
1. Introduction
Numerous efforts have been made to develop new halon alternatives due to the Montreal Protocol of banning the effective fire suppressant CF3Br. As one of the most promising halon alternatives, CF3CBrCH2 (2-BTP) has a double bond, which can be decomposed into inhibiting species (mostly Br, HBr and CF3) as CF3Br [1]. The double bond lowers both global warming potential (GWP) and ozone depletion potential (ODP) but increases flammability at the same time [2]. The inhibition effectiveness of 2-BTP is comparable with CF3Br, and more efficient than that of hydrofluorocarbon (HFCs). However, many researchers found the unwanted promotion effect caused by 2-BTP added to lean hydrocarbon air flames.
2-BTP failed the US Federal Aviation Administration (FAA) aerosol can test (FAA-ACT) [3] and caused overpressure rise when added in sub-inerting concentrations. In subsequent studies, many numerical and experimental investigations have been performed aiming at further understanding the properties of 2-BTP. Linteris et al. simulated the conditions of the FAA-ACT test and found that the high pressure rise with added 2-BTP only occurred when a large fraction of the available oxidizer in the chamber was consumed, corresponding to the agent added to lean fuel-air [4]. Babushok et al. studied the combustion properties of halogenated fire suppressants [5] and calculated that approximate peak burning velocities of the pure 2-BTP-air flames were 1.15 cm/s, 2.15 cm/s, and 3.5 cm/s for initial mixture temperatures of 300 K, 400 K, and 500 K, respectively [1]. Afterwards, Linteris et al. found when compressively preheated, 2-BTP/air mixture can support low-strain flames which are much more difficult to extinguish [6]. Pagliaro et al. measured the laminar burning velocity of 2-BTP experimentally. They found 2-BTP increases the maximum explosion pressure when added to lean mixtures for constant volume combustion [7]. They further identified that 2-BTP failed to reduce burning velocity effectively at lean conditions due to the fuel-components (CxHy) of the 2-BTP, which considerably shifted overall equivalence ratio φoverall shift toward rich conditions [8]. Xu et al. used the counterflow technique to measure unstretched flame speeds and the extinction stretch rate of premixed and non-premixed hydrocarbon flames. They found 2-BTP to be less effective at reducing the flame speeds of the lean flames as compared to that of the rich flames [9].
Most previous studies have been concentrated on the determination of enhanced combustion effect of 2-BTP when added to lean hydrocarbon flames. Although some important results were obtained, many scientific issues remain unsolved in the understanding of the enhanced effect of 2-BTP on lean hydrocarbon flames, such as the intrinsic chemical and physical effects on flame inhibition. Additionally, the inhibition effectiveness improvement of 2-BTP in lean flames is useful in practice. In this study, perfectly stirred reactor (PSR) simulations are applied. In addition, the aim of the present study is to verify the enhanced combustion chemical effect when sub-inerting 2-BTP loading in lean flames. In addition, the chemical inhibition effect improvement of the binary mixtures of 2-BTP and CO2 is examined.
2. Kinetic Mechanism
The kinetic mechanism of 2-BTP used in this study is assembled from sub-mechanisms available in the literature. The kinetic model consists of three sub-mechanisms. The first block is a high-temperature combustion reaction model of H2/CO/C1–C4 compounds from Wang et al. [10]. The second block is the reactions of the C1–C3 NIST HFC mechanism [11,12], including modifications suggested in more recent work [4,5]. The third block is the chemical kinetic mechanism for 2-Bromo-3,3,3-trifluoropropene (2-BTP) [13]. The entire reaction mechanism for 2-BTP contains 198 species and 1656 elementary reactions.
To validate the assembled kinetic mechanism, calculated laminar burning velocity is compared with experimental ones. The CHEMKIN-PRO [14] premixed code is used to calculate the laminar burning velocity, computations are carried out in a domain of 10 cm, the mesh resolution is progressively refined with final GRAD and CURV equal to 0.05, and grid points are about 500. Figure 1 depicts calculated laminar flame speed of CH4/air mixtures at Φ = 0.6, 0.8, 1.0 and 1.2 with different 2-BTP additions at 298 K and 1 bar. The experimentally measured data from Pagliaro et al [8] are presented for comparison. The correlation between the model and the experiment is excellent for Φ = 1 and 1.2. The model predictions are within 10% of the experimental results. The model slightly over-predicts the flame speed for φ = 0.6 and 0.8. However, the discrepancy gradually diminishes when the agent-loading increases. In general, the predictive ability of the model is good for these conditions.
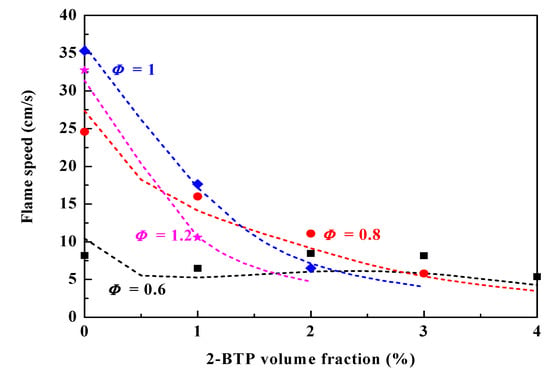
Figure 1.
The measured [8] and calculated laminar flame speed of CH4/air mixtures at Φ = 0.6, 0.8, 1.0 and 1.2 with different 2-BTP additions at 298 K and 1 bar.
3. Numerical Approach Validation
3.1. PSR Model
There are kinetic limitations when using the equilibrium simulations to predict the explosive pressure in FAA-ACT test. However, PSR simulations cannot only understand kinetic limitations [6] but also evaluate inhibition effectiveness of different agents through overall reaction rate during blow-out [15]. The PSR model is a classical idealization of the combustion process in which both physical and chemical mechanisms of fire suppression can be considered. In PSR simulations, chemical equilibrium can be reached for a longer residence time, whereas chemical reactions can be extinguished (blown out) if residence time is below a critical value τchem [15,16]. In addition, the overall chemical rates ωpsr at extinction in the PSR can be calculated (ωpsr = 1/τchem) correspondingly. The overall chemical rates ωpsr have been correlated with inhibition effectiveness with the added suppressant; that is, the stronger agent with slower ωpsr at extinction [4,6].
Premixed laminar burning velocity is the commonly used parameter for ranking various suppressants, as it can be both calculated and measured, for example, through a closed combustion vessel [17]. To verify the PSR model for suppressant ranking, the calculated overall chemical rates are compared with the laminar burning velocity with different agent added into hydrocarbon flames. A steady-state implementation of the PSR model is considered and neglects heat losses from the PSR to the surroundings. Figure 2 shows the overall chemical rate ωpsr at extinction in PSR as a function of CF3Br volume fraction in stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar. The curves perfectly mimic the calculated (Figure 6 in published work [1]) and experimented (Figure 12 in published work [8]) burning velocity of CH4/air flames with added CF3Br. Furthermore, the results can be obtained in a few minutes when using the PSR model, while hours of computation time are required for calculating the laminar burning velocity through premixed code. Thus, it can be inferred that the PSR model is more convenient for suppressant evaluation.
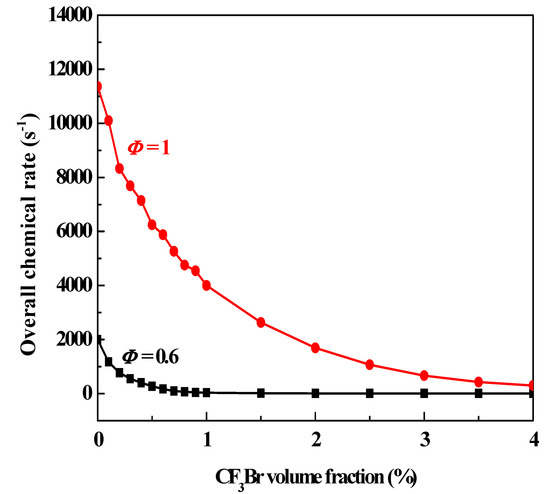
Figure 2.
The overall chemical rate at extinction in PSR as a function of CF3Br volume fraction in stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar.
3.2. Chemical Saturation Effectiveness
In this study, the reduction in overall chemical rate when adding a suppressant into hydrocarbon flames is considered to be its overall inhibition effectiveness. All suppressants have a physical component to suppression. Meanwhile, a chemical suppressant has an additional chemical inhibition component that significantly increases effectiveness. The overall inhibition effectiveness of a suppressant consists of chemical effect and physical effect. The chemical and physical effect of a suppressant can be separated through artificially excluding chemical reactions containing the agent [18,19,20,21]. We introduced a virtual species, identified as “agent*” with the same thermodynamic and transport data as the agent. However, agent* does not participate in any reactions. In this way, the physical effect moiety of the agent can be attained. At the same time, the difference chemical rate between agent and agent* is the chemical effect moiety of the agent under the same conditions. The positive chemical effect value represents inhibit flames chemically, whereas the negative chemical effect value represents enhanced combustion chemically. Table 1 shows the overall effect/chemical effect/physical effect of 3.5% CF3Br added to stoichiometric CH4/air flames at 298 K and 1 bar. The overall chemical rate of stoichiometric CH4/air flames at 298 K and 1 bar is 11,363 s−1, and it decreases to 435 s−1 with 3.5% CF3Br loading. Therefore, the overall inhibition effect of 3.5% CF3Br is the reduction of overall chemical rate, equal to 10,925 s−1. The overall chemical rate with virtual CF3Br* is 7692 s−1, so the physical effect (Yp) of CF3Br is the reduction of the overall chemical rate, equal to 3671 s−1. The chemical effect (Yc) of CF3Br is the difference of overall effect and physical effect, equal to 7258 s−1. The relative physical, and chemical, components of 3.5% CF3Br in the stoichiometric CH4/air flames are 33.6% and 66.4%, respectively. Noto [19] studied the laminar burning velocity reduction of stoichiometric CH4/air flames with 3.5% CF3Br and concluded physical and chemical effectiveness were 35% and 65%. The chemical effectiveness is slightly less than the present study, the discrepancy may attribute to the improved kinetic mechanism of CF3Br.

Table 1.
The overall effect/chemical effect/physical effect of 3.5% CF3Br added to stoichiometric CH4/air flames at 298 K and 1 bar.
Figure 3 shows the chemical effect (left scale)/physical effect (right scale) as a function of CF3Br volume fraction in stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar through PSR model calculations. Both in stoichiometric and lean flames, the physical effects of CF3Br increase monotonously and the chemical effects increase dramatically, followed by decreasing gradually as agent-loading. The chemical effects of CF3Br with CH4/air flames always inhibits the combustion since all the chemical effect values are positive.
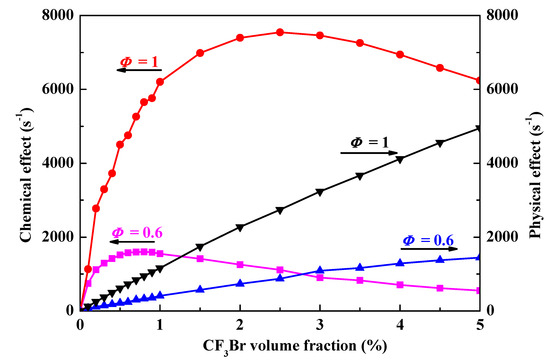
Figure 3.
The chemical effect (left scale)/physical effect (right scale) as a function of CF3Br volume fraction in stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar.
There are two types of chemical suppressants: catalytic ones and non-catalytic ones. Catalytic suppressants reduce concentrations of flame radicals through a regenerative cycle in which one molecule of suppressant recombines several flame radicals, whereas non-catalytic chemical suppressants reduce concentrations of flame radicals by scavenging and are generally less effective [22]. All chemical suppressants exhibit saturation effects [18,19,21] when the reduction of reactive radicals reaches an equilibrium concentration and the additional chemical agents only leads to a thermal effect [23]. CF3Br is a typical chemical suppressant with two chemical inhibiting moieties Br and CF3, of which Br moiety is a catalytic suppressant and CF3 moiety is a non-catalytic suppressant. As seen in Figure 3, chemical effect decreases after its saturation point with CF3Br loading and the additional CF3Br can be considered as an inert gas. The peak chemical effect value in Figure 3 corresponds to the saturation concentration of CF3Br in a stoichiometric and lean (Φ = 0.6) CH4/air flames at 298 K and 1 bar is roughly 2.5% and 0.8%, respectively. The saturation concentrations are consistent with previous research [1,19], and were calculated through reduction of laminar burning velocity.
4. Results and Discussion
4.1. Unwanted Chemical Combustion Enhancement of 2-BTP
To further understand the unwanted combustion enhancement of 2-BTP observed in the FAA-ACT, chemical rates are calculated with various-concentration 2-BTP addition into lean hydrocarbon flames. C3H8/air mixtures at 100% R.H. can best approximate the adiabatic flame temperature and the hydrogen atom content of the total fuel-air mixture in the FAA-ACT [24]. A water vapor volume fraction of 0.025 in the O2/N2/H2O mix corresponds to 100% R.H. at 21 °C. Figure 4 shows the calculated overall chemical rate/chemical effect/physical effect as a function of 2-BTP volume fraction in Φ = 0.6 moist C3H8/air flames at 298 K and 1bar in the PSR model. The overall chemical rate is always less than the uninhibited condition and fluctuates with sub-inerting agent additions, which are controlled by the competition between radical scavenging by the halogenated species (Br and F participate in chain-terminating reactions) and the additional heat release associated with the decomposition of 2-BTP in the lean environment [1]. To provide insight into unwanted experimented combustion enhancement, we have separated the physical and chemical effects of 2-BTP in Figure 4.
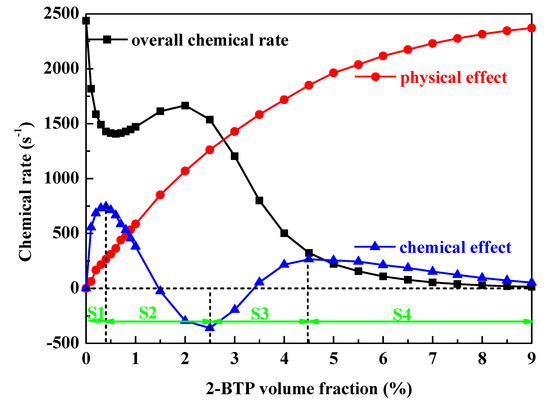
Figure 4.
The overall chemical rate/chemical effect/physical effect as a function of 2-BTP volume fraction in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar.
There are competitive chemical effects when adding 2-BTP to lean flames, including the enhanced effect of fuel-components (CxHy) and suppressive effect of bromine- and fluorine-containing compounds. It is unlikely to make the exact effectiveness clear, but the overall chemical effect value represents the competition result. The positive chemical effect value inhibits flame chemically, whereas the negative chemical effect value enhances combustion chemically. The larger positive chemical effect value accords to more reduction in overall chemical rate, and hence to stronger chemical inhibition effectiveness. The physical effect of 2-BTP increases monotonously but the chemical effect fluctuates obviously as agent-loading. The net chemical effect can be divided into four parts as in Figure 4. With 2-BTP addition, for S1 (0 ≤ Xa ≤ 0.004), the chemical effect increases sharply due to chemical inhibition from the bromine, and then for S2 (0.005 ≤ Xa ≤ 0.025), although fluorine-containing compounds increase in the radical pool but the heat release forms the agent reaction dominants, net chemical effect decrease, and for S3 (0.025 < Xa ≤ 0.045), with more inhibiting species of Br, HBr and CF3, chemical inhibition effect dominants, finally, for S4 (Xa >0.045), chemical effect decreases as the saturation point is achieved. The net chemical effect variation is more complicated than experimented [7] and calculated [1] flame speeds, with 2-BTP adding to lean hydrocarbon flames. It is noted that for S2 and S3, the chemical effect is negative with certain (0.015 ≤ Xa ≤ 0.034) sub-inerting agent concentrations. It is speculated that the negative chemical effect contributes to the experimented unwanted combustion enhancement.
4.2. 2-BTP Inhibition Improvement by CO2
Saso et al. found inert gas (N2 or CO2) enhances the overall inhibition effectiveness of CF3Br but slightly decreases that of CF3H in stoichiometric CH4/air flames [25]. Inert gases are effective at flame suppression through heat extraction and dilution of oxygen. That is, the Br catalytic cycle is enhanced and the inhibition of species CF3 is suppressed as the flame temperature decreases. Since there is Br moiety in 2-BTP, there should be positive synergy between 2-BTP and inert gases. To demonstrate the synergistic effect of binary mixtures containing 2-BTP and CO2, large amounts of computational experiments are performed. As discussed above, the negative chemical effect occurs with sub-inerting agent concentrations, overall chemical rates of the blend (various volume fraction CO2 + a fixed volume fraction 2-BTP (0 ≤ Xa ≤ 0.04)) added into moist C3H8/air flames are calculated. Figure 5 shows the dependence of overall chemical rates on CO2 volume fraction with a fixed 2-BTP adding to moist C3H8/air flames at 298 K and 1bar. As shown in Figure 5, the overall chemical rate decreases as the agent increases for all blend additions, which implies that adding CO2 into 2-BTP improves the inhibition effect significantly. The physical effect always increases as CO2 is added. To inspect chemical effect variation, Figure 6 shows the dependence of chemical effect on 2-BTP volume fraction with varying CO2 addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1bar.
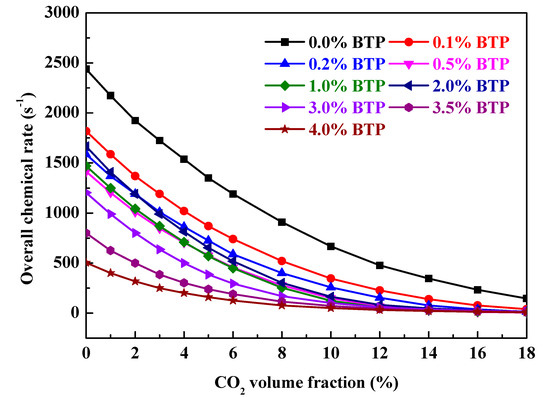
Figure 5.
The dependence of overall chemical rates on CO2 volume fraction with a fixed 2-BTP adding to moist C3H8/air flames at 298 K and 1 bar.
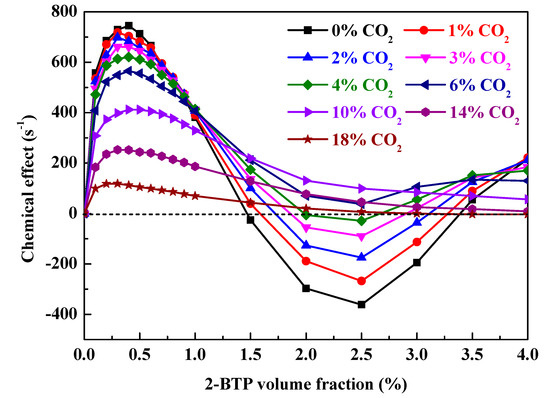
Figure 6.
The dependence of chemical effect on 2-BTP volume fraction with varying CO2 addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar.
As shown in Figure 6, the chemical effect of the blend with various volume fractions of CO2 mimics the chemical effect of 2-BTP in Figure 4. Under the competitive chemical effects of CxHy/Br/CF3, the chemical effect of the blend first increases, then decreases, and finally increases with more agent addition. However, it should be noted that there are no negative chemical effects of 2-BTP when CO2 addition is larger than 4%. That is, no enhanced combustion would occur with sub-concentration of 2-BTP with CO2 addition larger than 4% in Φ = 0.6 moist C3H8/air flames at 298 K and 1bar.
To assess the overall chemical effect variation of CO2 addition, the replot of Figure 6 as the dependence of chemical effect on CO2 volume fraction with varying 2-BTP addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar is shown in Figure 7. The chemical effects decrease monotonously with CO2 addition for 0–0.6% 2-BTP, which may be due to the saturation point having been achieved. The chemical effects first increase then decrease with CO2 addition for 0.7–4% 2-BTP, which implies that CO2 significantly improves the chemical effect of 2-BTP at first and decreases it when saturated. In particular, for the negative chemical effect of sub-inerting 2-BTP concentrations (0.015 ≤ Xa ≤ 0.034), adding CO2 obviously improves chemical inhibition effect, driving combustion enhancement into combustion inhibition.
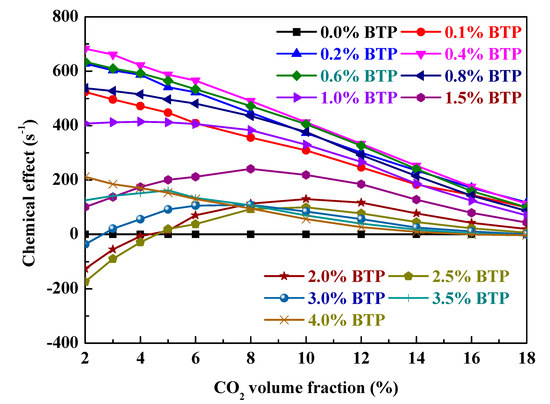
Figure 7.
The dependence of chemical effect on CO2 volume fraction with varying 2-BTP addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar.
The adiabatic temperature is a further parameter for ranking various suppressants [17]. Figure 8 shows the dependence of adiabatic temperature on 2-BTP volume fraction with varying CO2 addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar. The CHEMKIN-PRO [14] equilibrium calculation is used to calculate the adiabatic flame temperature and constant enthalpy; constant pressure solutions are obtained. It is found that the adiabatic flame temperature of C3H8/air is always less with the blend of 2-BTP and CO2 than that with single 2-BTP. That is, the addition of CO2 into 2-BTP could improve inhibition effectiveness of 2-BTP.
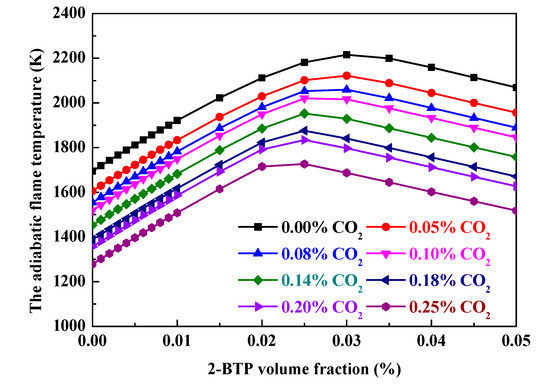
Figure 8.
The dependence of adiabatic temperature on 2-BTP volume fraction with varying CO2 addition in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar.
5. Conclusions
The aim of this study was to understand the enhanced chemical combustion effects of certain 2-BTP loadings on lean flames and the chemical inhibition effect improvement of the binary mixtures of 2-BTP and CO2. The PSR model is used for calculating overall chemical rates. Compared with the premixed code, the PSR model is a time-saving method for evaluating suppressants with detailed physical and chemical mechanisms of fire suppression under consideration. The reduction in overall chemical rate is introduced to assess various suppressants. Chemical and physical effects of the agent are calculated respectively, and it is found that all chemical suppressants show chemical saturation effectiveness. That is, the chemical effect first increases as the agent increases, and when it reaches its saturation point, the chemical effect decreases as the agent further increases.
The calculated overall chemical rate of 2-BTP added into moist C3H8/air flames is always less than the uninhibited condition and fluctuates with sub-inerting agent additions. The physical effect of 2-BTP always inhibits the flame, whereas the chemical effect is complicated. The chemical effect of 2-BTP undergoes four stages under the competitive chemical effects of CxHy/Br/CF3, of which bromine and fluorine-containing compounds participate in chain-terminating reactions to scavenge radicals and the additional heat release associated with the decomposition of 2-BTP in the lean environment. There is a negative chemical effect with certain sub-inerting agent concentrations (0.015 ≤ Xa ≤ 0.034), which result in the experimented unwanted combustion enhancement in moist C3H8/air flames.
All blend addition of CO2 and 2-BTP improves the inhibition effect. CO2 improves the Br catalytic cycle and depresses fluorine-containing and CxHy compounds involved in the reactions. The competition results indicate that CO2 not only enlarges the physical effect of all 2-BTP concentrations, but also improves the chemical inhibition effect of certain 2-BTP concentrations in moist C3H8/air flames. The calculation reveals that no enhanced combustion would occur with sub-concentration of 2-BTP with CO2 addition larger than 4% in Φ = 0.6 moist C3H8/air flames at 298 K and 1 bar, and CO2 obviously improves the chemical inhibition effect of 2-BTP, driving combustion enhancement into combustion inhibition.
The calculation shows that the addition of CO2 into 2-BTP could improve inhibition effectiveness of 2-BTP. The unwanted promotion effect caused by 2-BTP added to lean hydrocarbon air flames could be improved when a certain amount of CO2 is added. In future work, experiments with a closed vessel will be used to further verify inhibition effectiveness of the blend of 2-BTP and CO2.
Author Contributions
P.L. and B.K. conceived and designed the experiments; P.L. performed the experiments; P.L and J.Z. analyzed the data; X.C. contributed analysis tools; P.L. wrote the paper.
Funding
This research was funded by [National Key Technologies Research and Development Program of China] grant number [No. 2018YFC0808500] and The APC was funded by [China Postdoctoral Science Foundation] grant number [No. 2018M632936].
Acknowledgments
The authors gratefully acknowledge the financial support provided by the National Key Technologies Research and Development Program of China (No. 2018YFC0808500) and the China Postdoctoral Science Foundation (No. 2018M632936). The third author would like to thank the Chinese Scholarship Council for financial support to the joint PhD study at University of Adelaide.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Babushok, V.I.; Linteris, G.T.; Burgess, D.R., Jr.; Baker, P.T. Hydrocarbon flame inhibition by c3h2f3br (2-btp). Combust. Flame 2015, 162, 1104–1112. [Google Scholar] [CrossRef]
- Takizawa, K.; Tokuhashi, K.; Kondo, S. Flammability assessment of ch2cfcf3: Comparison with fluoroalkenes and fluoroalkanes. J. Hazard. Mater. 2009, 172, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.W. Behavior of Bromotrifluoropropene and Pentafluoroethane when Subjected to a Simulated Aerosol can Explosion; US Department of Transportation, Federal Aviation Administration: Washington, DC, USA, 2004.
- Linteris, G.T.; Burgess, D.R.; Takahashi, F.; Katta, V.R.; Chelliah, H.K.; Meier, O. Stirred reactor calculations to understand unwanted combustion enhancement by potential halon replacements. Combust. Flame 2012, 159, 1016–1025. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linteris, G.T.; Meier, O.C. Combustion properties of halogenated fire suppressants. Combust. Flame 2012, 159, 3569–3575. [Google Scholar] [CrossRef]
- Linteris, G.T.; Babushok, V.I.; Pagliaro, J.L.; Burgess, D.R., Jr.; Manion, J.A.; Takahashi, F.; Katta, V.R.; Baker, P.T. Understanding overpressure in the faa aerosol can test by c3h2f3br (2-btp). Combust. Flame 2016, 167, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, J.L.; Linteris, G.T.; Sunderland, P.B.; Baker, P.T. Combustion inhibition and enhancement of premixed methane–air flames by halon replacements. Combust. Flame 2015, 162, 41–49. [Google Scholar] [CrossRef]
- Pagliaro, J.L.; Bouvet, N.; Linteris, G.T. Premixed flame inhibition by CF 3 Br and C 3 H 2 F 3 Br (2-BTP). Combust. Flame 2016, 169, 272–286. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, Y.; Qiu, R.; Ren, X. Influence of halon replacements on laminar flame speeds and extinction limits of hydrocarbon flames. Combust. Flame 2017, 182, 1–13. [Google Scholar] [CrossRef]
- Hai, W.; Xiao, Y.; Ameya, V.; Joshi, S.G.; Davis, A.L.; Fokion, E.; Chung, K.L. High-Temperature Combustion Reaction Model of H2/CO/C1-C4 Compounds. 2017. Available online: http://ignis.usc.edu/USC_Mech_II.htm (accessed on 10 May 2007).
- Burgess, D.; Zachariah, M.R.; Tsang, W.; Westmoreland, P.R. Thermochemical and chemical kinetic data for fluorinated hydrocarbons. Prog. Energy Combust. Sci. 1995, 21, 453–529. [Google Scholar] [CrossRef]
- Williams, B.A.; L’esp Érance, D.M.; Fleming, J.W. Intermediate species profiles in low-pressure methane/oxygen flames inhibited by 2-h heptafluoropropane: comparison of experimental data with kinetic modeling. Combust. Flame 2000, 120, 160–172. [Google Scholar] [CrossRef]
- Burgess, D.R.; Babushok, V.I.; Linteris, G.T.; Manion, J.A. A chemical kinetic mechanism for 2-bromo-3,3,3-trifluoropropene (2-btp) flame inhibition. Int. J. Chem. Kinet. 2015, 47, 533–563. [Google Scholar] [CrossRef]
- Kee, R.; Rupley, F.; Miller, J. CHEMKIN-PRO 15112; Reaction Design: San Diego, CA, USA, 2011. [Google Scholar]
- Liu, S.; Soteriou, M.C.; Colket, M.B.; Senecal, J.A. Determination of cup-burner extinguishing concentration using the perfectly stirred reactor model. Fire Saf. J. 2008, 43, 589–597. [Google Scholar] [CrossRef]
- Di Sarli, V. Stability and emissions of a lean pre-mixed combustor with rich catalytic/lean-burn pilot. Int. J. Chem. React. Eng. 2014, 12, 77–89. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Cammarota, F.; Di Sarli, V.; Salzano, E.; Russo, G. Effect of diluents on rapid phase transition of water induced by combustion. AIChE J. 2011, 58, 2810–2819. [Google Scholar] [CrossRef]
- Babushok, V.; Tsang, W.; Linteris, G.T.; Reinelt, D. Chemical limits to flame inhibition. Combust. Flame 1998, 115, 551–560. [Google Scholar] [CrossRef]
- Noto, T.; Babushok, V.; Hamins, A.; Tsang, W. Inhibition effectiveness of halogenated compounds. Combust. Flame 1998, 112, 147–160. [Google Scholar] [CrossRef]
- Liu, F.; Guo, H.; Smallwood, G.J. The chemical effect of co2 replacement of n2 in air on the burning velocity of ch4 and h2 premixed flames. Combust. Flame 2003, 133, 495–497. [Google Scholar] [CrossRef]
- Ren, X.; Jiang, Y.; Xu, W. Numerical investigation of the chemical and physical effects of halogenated fire suppressants addition on methane-air mixtures. J. Fire Sci. 2016, 34, 416–430. [Google Scholar] [CrossRef]
- Williams, B.A.; Fleming, J.W. CF3Br and other suppressants: Differences in effects on flame structure. Proc. Combust. Inst. 2002, 29, 345–351. [Google Scholar] [CrossRef]
- Babkin, V.S.; V’yun, A.V. On the mechanism of laminar flame propagation at high pressures. Combust. Explos. Shock Waves 1971, 7, 203–206. [Google Scholar] [CrossRef]
- Linteris, G.T.; Pagliaro, J.L. Burning Velocity Measurements and Simulations for Understanding the Performance of Fire Suppressants in Aircraft; National Institute of Standards and Technology, U.S. Department of Commerce: Washington, DC, USA, 2016.
- Saso, Y. Binary cf3br- and chf3–inert flame suppressants: Effect of temperature on the flame inhibition effectiveness of cf3br and chf3. Combust. Flame 1999, 118, 489–499. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).