Effects of Organosolv Pretreatment Using Temperature-Controlled Bench-Scale Ball Milling on Enzymatic Saccharification of Miscanthus × giganteus
Abstract
:1. Introduction
2. Results and Discussion
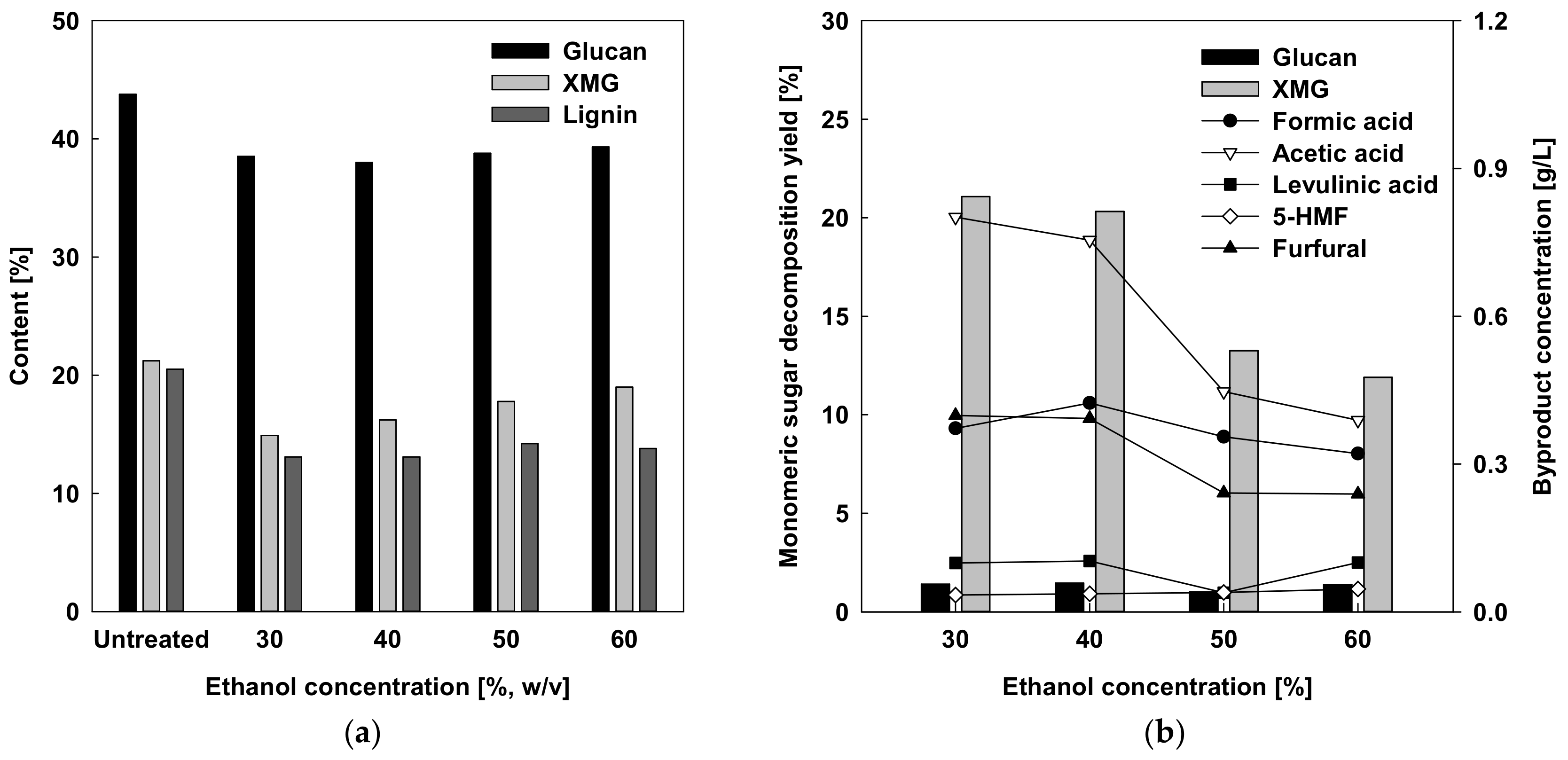
2.1. Effects of Ethanol Concentration on Compositions of Pretreated Solids and Liquids Using Small Scale Batch Reactor
2.2. Organosolv Pretreatment Using Bench-Scale Ball-Milling Reactor
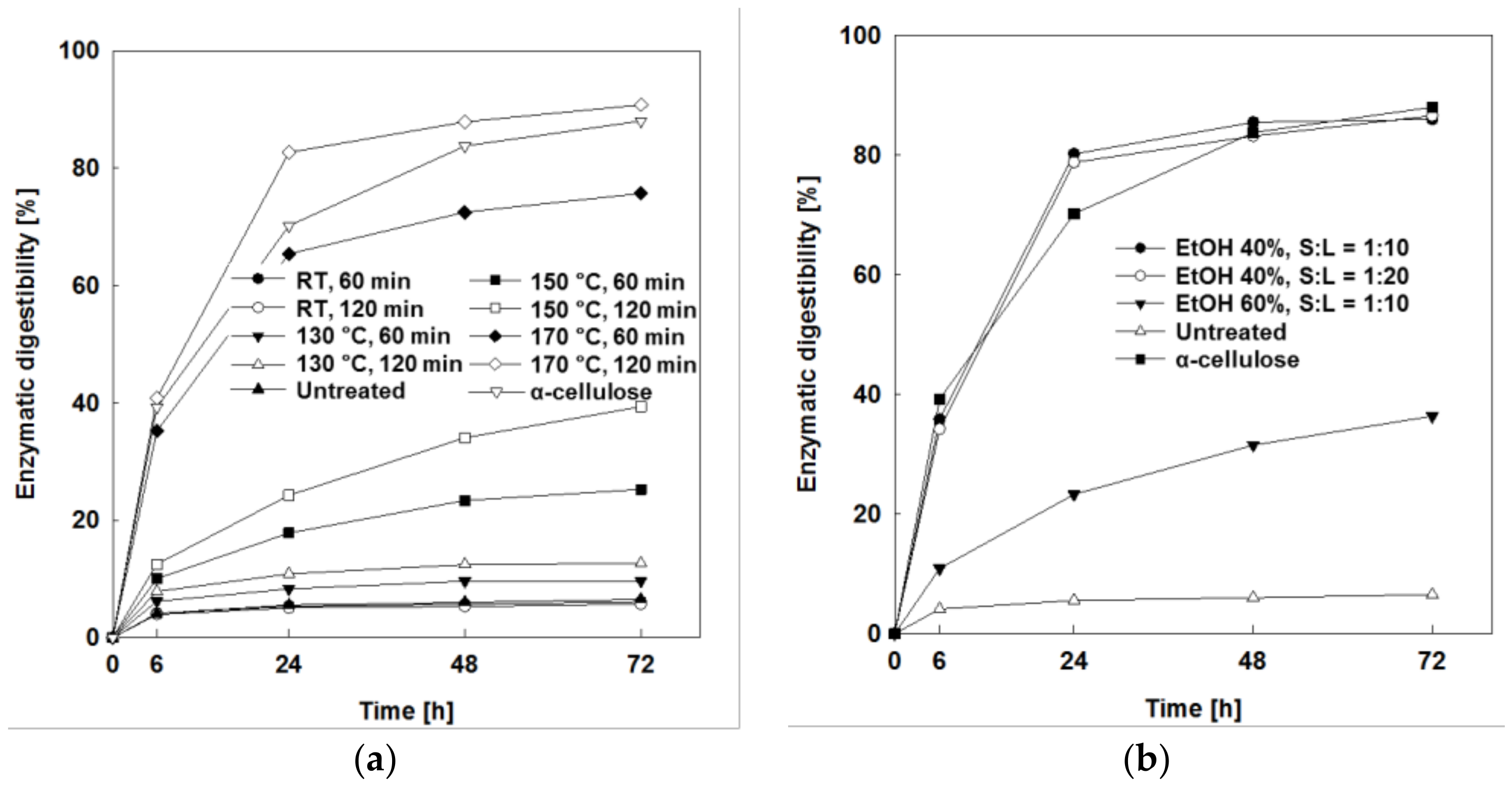
2.2.1. Effects of Reaction Temperature and Time
2.2.2. Effects of Liquid/Solid (L/S) Ratio
2.2.3. Enzymatic Digestibility Testing of Pretreated Solids
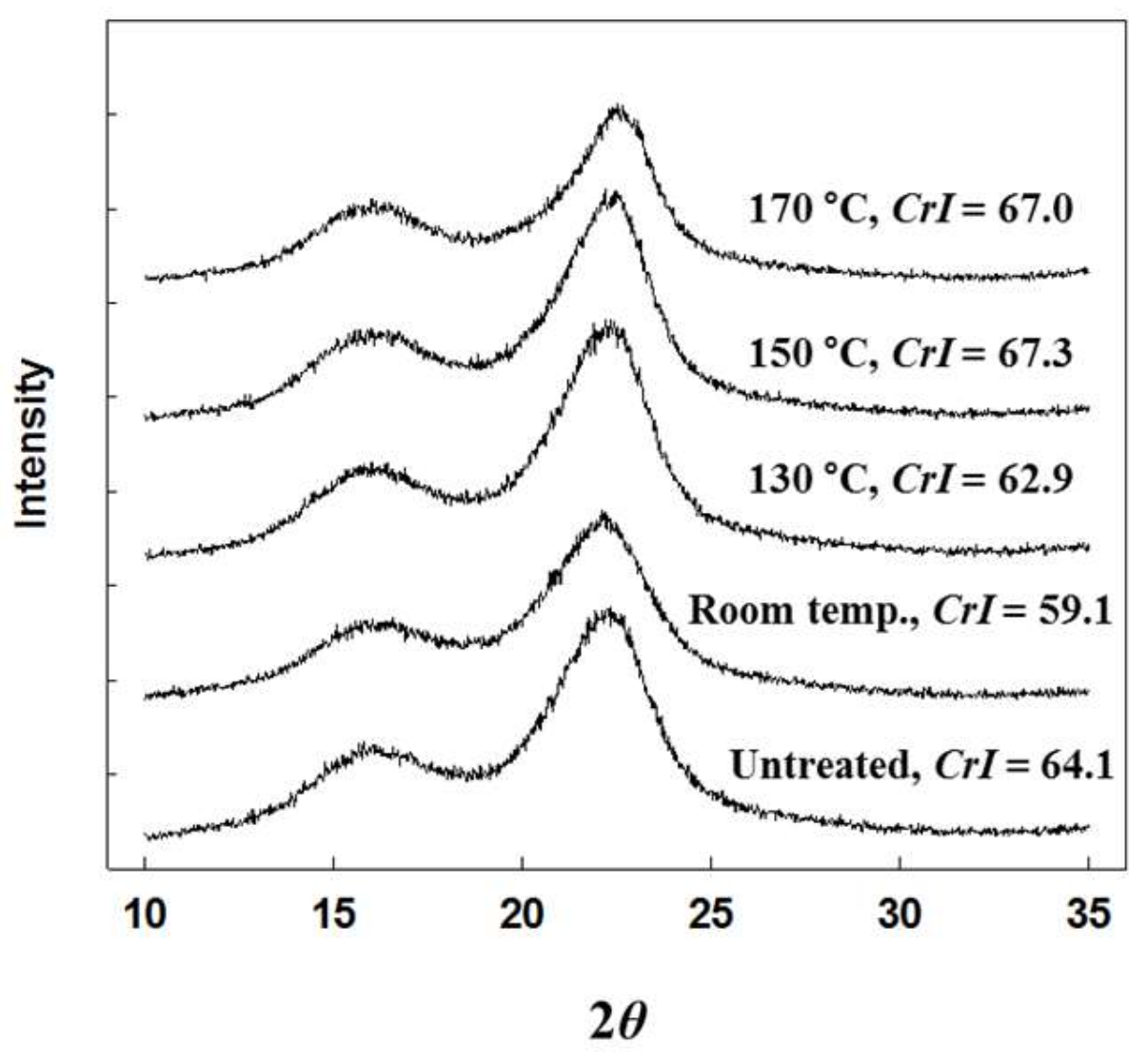
2.3. Crystallinity Index (CrI) of Untreated and Treated MG
3. Materials and Methods
3.1. Materials
3.2. Experimental Setup and Operation
3.2.1. Small Batch Organosolv Pretreatment Reactor
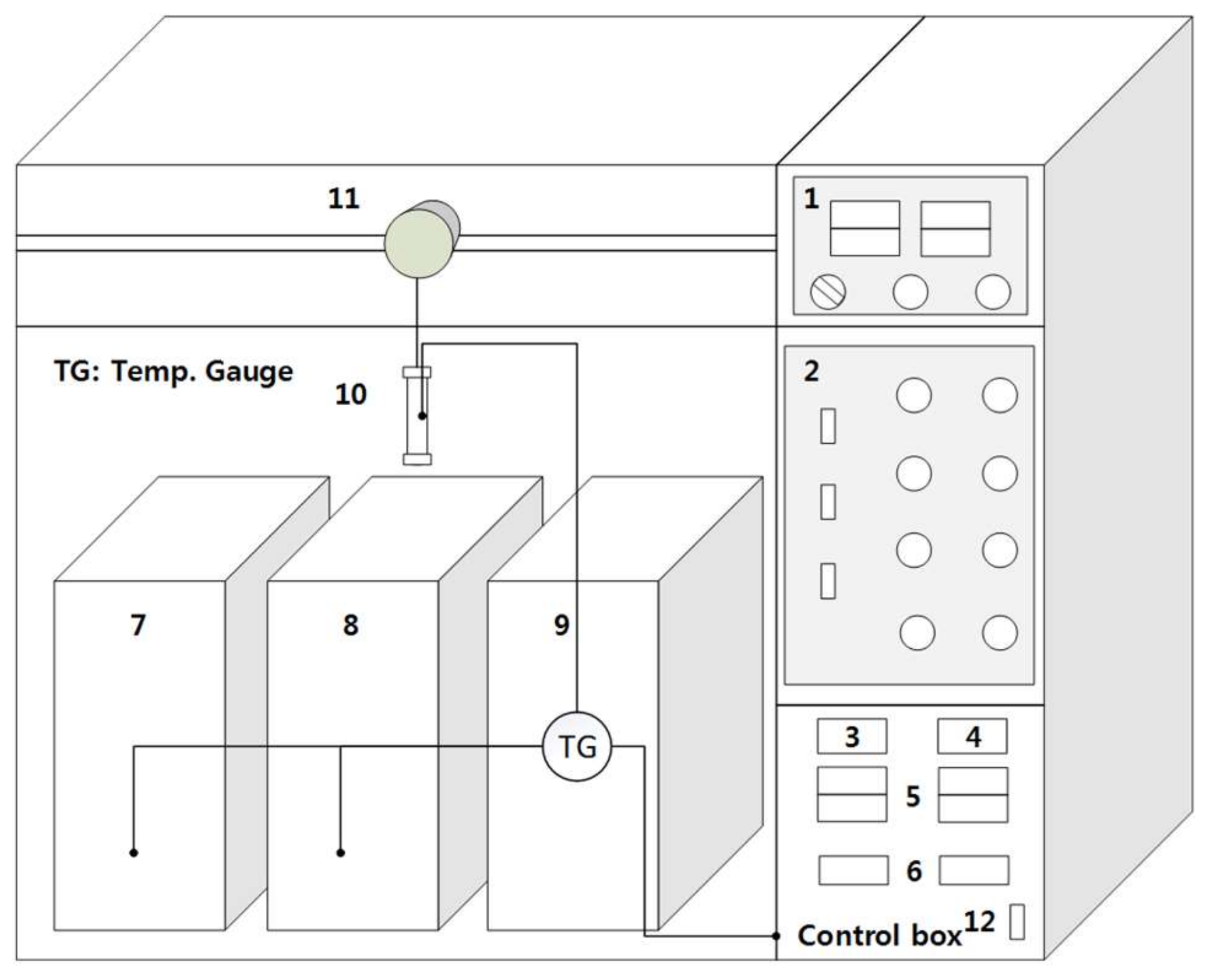
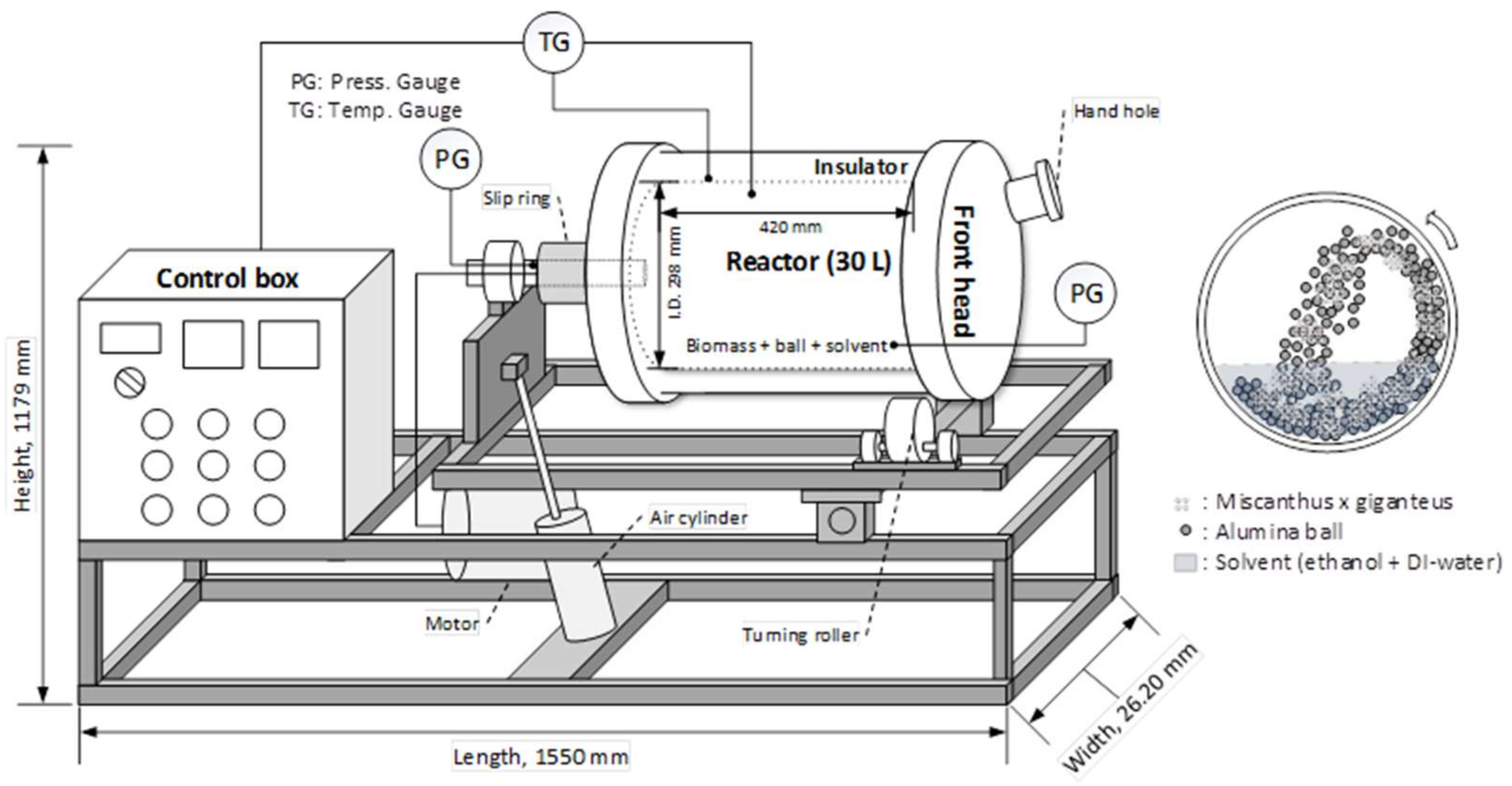
3.2.2. Bench Scale Ball-Milling Pretreatment Reactor
3.3. Enzymatic Digestibility Test
3.4. Analytical Methods
3.5. Crystallinity Measurement
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix


References
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, T.H. Overview of technical barriers and implementation of cellulosic ethanol in the US. Energy 2014, 66, 13–19. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Szczodrak, J.; Fiedurek, J. Technology for conversion of lignocellulosic biomass to ethanol. Biomass Bioenergy 1996, 10, 367–375. [Google Scholar] [CrossRef]
- Rao, L.V.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Kadla, J.F.; Ehara, K.; Gilkes, N.; Saddler, J.N. Organosolv ethanol lignin from hybrid poplar as a radical scavenger: Relationship between lignin structure, extraction conditions, and antioxidant activity. J. Agric. Food Chem. 2006, 54, 5806–5813. [Google Scholar] [CrossRef] [PubMed]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: Optimization of process yields. Biotechnol. Bioeng. 2006, 94, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Geng, A.; Xin, F.; Ip, J.Y. Ethanol production from horticultural waste treated by a modified organosolv method. Bioresour. Technol. 2012, 104, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.C.; Wen, J.L.; Xu, F.; Sun, R.C. Structural and thermal characterization of hemicelluloses isolated by organic solvents and alkaline solutions from Tamarix austromongolica. Bioresour. Technol. 2011, 102, 5947–5951. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kwon, J.H.; Kim, T.H.; Choi, W.I. Impact of planetary ball mills on corn stover characteristics and enzymatic digestibility depending on grinding ball properties. Bioresour. Technol. 2017, 241, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273. [Google Scholar] [CrossRef]
- Zhao, H.; Kwak, J.H.; Wang, Y.; Franz, J.A.; White, J.M.; Holladay, J.E. Effects of crystallinity on dilute acid hydrolysis of cellulose by cellulose ball-milling study. Energy Fuels 2006, 20, 807–811. [Google Scholar] [CrossRef]
- Khullar, E.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Effect of particle size on enzymatic hydrolysis of pretreated Miscanthus. Ind. Crops Prod. 2013, 135, 11–17. [Google Scholar] [CrossRef]
- Liu, H.; Pang, B.; Zhao, Y.; Lu, J.; Han, Y.; Wang, H. Comparative study of two different alkali-mechanical pretreatments of corn stover for bioethanol production. Fuel 2018, 221, 21–27. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, Y.; Xu, H.; Yang, Q.; Xiong, C.; Kuga, S.; Matsumoto, Y. Facile dissolution of wood pulp in aqueous NaOH/urea solution by ball milling pretreatment. Ind. Crops Prod. 2018, 118, 48–52. [Google Scholar] [CrossRef]
- Dai, L.; Li, C.; Zhang, J.; Cheng, F. Preparation and characterization of starch nanocrystals combining ball milling with acid hydrolysis. Carbohydr. Polym. 2018, 180, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.; Rova, U.; Christakopoulos, P. Organosolv fractionation of softwood biomass for biofuel and biorefinery applications. Energies 2017, 11, 50. [Google Scholar] [CrossRef]
- Teramoto, Y.; Tanaka, N.; Lee, S.H.; Endo, T. Pretreatment of eucalyptus wood chips for enzymatic saccharification using combined sulfuric acid-free ethanol cooking and ball milling. Biotechnol. Bioeng. 2008, 99, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Joshee, N.; Liu, S. Utilization of hardwood in biorefinery: A kinetic interpretation of pilot-scale hot-water pretreatment of Paulownia elongata woodchips. J. Biobased Mater. Bioenergy 2016, 10, 339–348. [Google Scholar] [CrossRef]
- Liu, S. Woody biomass: Niche position as a source of sustainable renewable chemicals and energy and kinetics of hot-water extraction/hydrolysis. Biotechnol. Adv. 2010, 28, 563–582. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.Q.; You, T.T.; Wang, W.; Xu, F.; Sun, R.C. Synergistic benefits of ionic liquid and alkaline pretreatments of poplar wood. Part 2: Characterization of lignin and hemicelluloses. Bioresour. Technol. 2013, 136, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Weinwurm, F.; Turk, T.; Denner, J.; Whitmore, K.; Friedl, A. Combined liquid hot water and ethanol organosolv treatment of wheat straw for extraction and reaction modeling. J. Clean. Prod. 2017, 165, 1473–1484. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeon, Y.J.; Oh, K.K.; Kim, T.H. Production of furfural and cellulose from barley straw using acidified zinc chloride. Korean J. Chem. Eng. 2013, 30, 1339–1346. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Selig, M.; Weiss, N.; Ji, Y. Enzymatic Saccharification of Lignocellulosic Biomass; NREL/TP-510–42629; National Renewable Energy Laboratory: Golden, CO, USA, 2018. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Tmpleton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510–42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Cao, Y.; Tan, H. Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb. Technol. 2005, 36, 314–317. [Google Scholar] [CrossRef]

| Conditions | Treated Solids | Liquid Hydrolyzate | Total Cons. 1 | |||||
|---|---|---|---|---|---|---|---|---|
| Temp. | Time | Glucan | XMG 2 | Lignin 3 | Glucan | XMG | Glucan | XMG |
| [°C] | [min] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] | [wt.%] |
| Untreated | 43.8 | 21.2 | 20.5 | - | - | - | - | |
| RT 4 | 30 | 43.5 | 20.9 | 20.2 | 0.4 | 0.5 | 99.7 | 99.1 |
| 60 | 43.3 | 21.0 | 20.0 | 0.4 | 0.7 | 99.5 | 99.8 | |
| 120 | 43.0 | 20.7 | 20.3 | 0.6 | 0.8 | 98.9 | 98.5 | |
| 130 | 30 | 41.7 | 20.1 | 18.3 | 0.7 | 2.1 | 96.0 | 97.0 |
| 60 | 42.3 | 20.2 | 18.5 | 0.9 | 2.6 | 97.5 | 97.7 | |
| 120 | 42.7 | 20.0 | 16.7 | 0.8 | 3.4 | 98.5 | 97.7 | |
| 150 | 30 | 42.1 | 19.0 | 15.4 | 1.1 | 8.4 | 97.3 | 98.0 |
| 60 | 41.5 | 17.6 | 14.1 | 1.4 | 15.4 | 96.3 | 98.4 | |
| 120 | 40.7 | 14.4 | 12.3 | 2.0 | 30.0 | 95.1 | 97.8 | |
| 170 | 30 | 41.2 | 10.1 | 10.6 | 1.9 | 40.9 | 96.0 | 88.3 |
| 60 | 40.8 | 4.2 | 10.0 | 1.9 | 65.3 | 95.0 | 85.3 | |
| 120 | 35.4 | 2.0 | 7.8 | 4.1 | 74.1 | 85.0 | 83.6 | |
| No. | Conditions | Treated Solid | Liquid Hydrolyzate | Total Conservation | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EtOH | L/S | Time | Glucan | XMG | Lignin | Glucan | XMG | Glucan | XMG | |
| [%] | [-] | [min] | [%] | [%] | [%] | [%] | [%] | [%] | [%] | |
| Untreated | 43.8 | 21.2 | 20.5 | - | - | - | - | |||
| 1 | 40 | 10 | 30 | 41.2 | 10.1 | 10.6 | 1.9 | 40.9 | 96 | 88.3 |
| 60 | 40.8 | 4.2 | 10 | 1.9 | 65.3 | 95 | 85.3 | |||
| 90 | 37.5 | 3.3 | 9.6 | 1.8 | 68.6 | 87.6 | 84 | |||
| 120 | 35.4 | 2 | 7.8 | 4.1 | 74.1 | 85 | 83.6 | |||
| 2 | 40 | 20 | 30 | 39.6 | 12.8 | 11.8 | 1.7 | 35.5 | 92.3 | 95.8 |
| 60 | 38.8 | 8.8 | 9.1 | 2.3 | 56.5 | 91 | 98 | |||
| 90 | 37.8 | 6.3 | 8.5 | 2.2 | 57.4 | 88.5 | 87.1 | |||
| 120 | 36.6 | 5.3 | 8 | 2.4 | 59.2 | 86.1 | 84.1 | |||
| 3 | 60 | 10 | 30 | 42.3 | 18.3 | 12.9 | 1.8 | 9.8 | 98.5 | 95.8 |
| 60 | 39.5 | 15.7 | 11.5 | 1.9 | 16.8 | 92.2 | 91 | |||
| 90 | 38.8 | 13.6 | 9.4 | 2 | 24.5 | 90.6 | 88.4 | |||
| 120 | 37.5 | 8.7 | 8.6 | 2.1 | 45.3 | 87.8 | 86.3 | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.H.; Im, D.; Oh, K.K.; Kim, T.H. Effects of Organosolv Pretreatment Using Temperature-Controlled Bench-Scale Ball Milling on Enzymatic Saccharification of Miscanthus × giganteus. Energies 2018, 11, 2657. https://doi.org/10.3390/en11102657
Kim TH, Im D, Oh KK, Kim TH. Effects of Organosolv Pretreatment Using Temperature-Controlled Bench-Scale Ball Milling on Enzymatic Saccharification of Miscanthus × giganteus. Energies. 2018; 11(10):2657. https://doi.org/10.3390/en11102657
Chicago/Turabian StyleKim, Tae Hoon, Dongjoong Im, Kyeong Keun Oh, and Tae Hyun Kim. 2018. "Effects of Organosolv Pretreatment Using Temperature-Controlled Bench-Scale Ball Milling on Enzymatic Saccharification of Miscanthus × giganteus" Energies 11, no. 10: 2657. https://doi.org/10.3390/en11102657
APA StyleKim, T. H., Im, D., Oh, K. K., & Kim, T. H. (2018). Effects of Organosolv Pretreatment Using Temperature-Controlled Bench-Scale Ball Milling on Enzymatic Saccharification of Miscanthus × giganteus. Energies, 11(10), 2657. https://doi.org/10.3390/en11102657






