Life Cycle Analysis of Endosymbiotic Algae in an Endosymbiotic Situation with Paramecium bursaria Using Capillary Flow Cytometry
Abstract
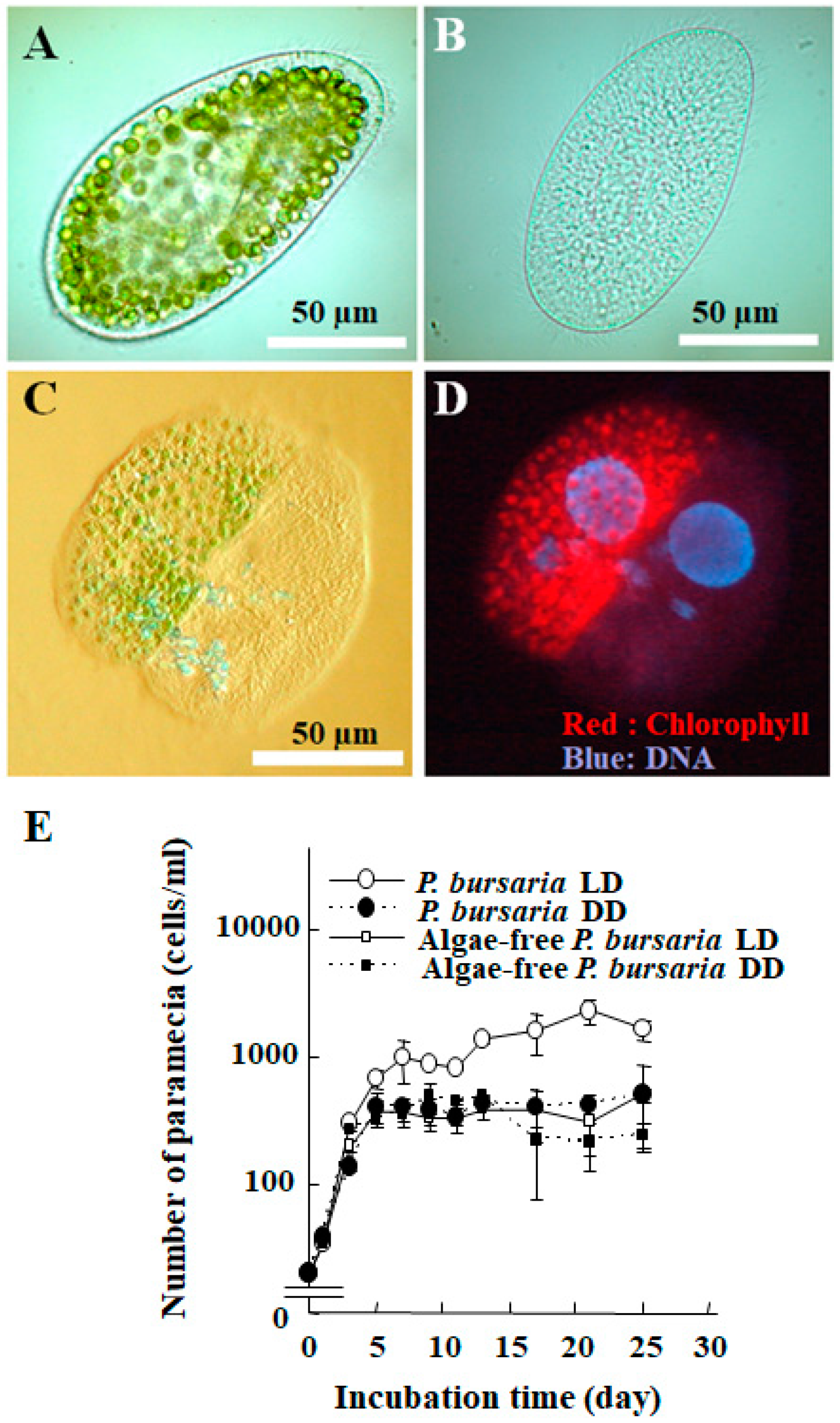
1. Introduction
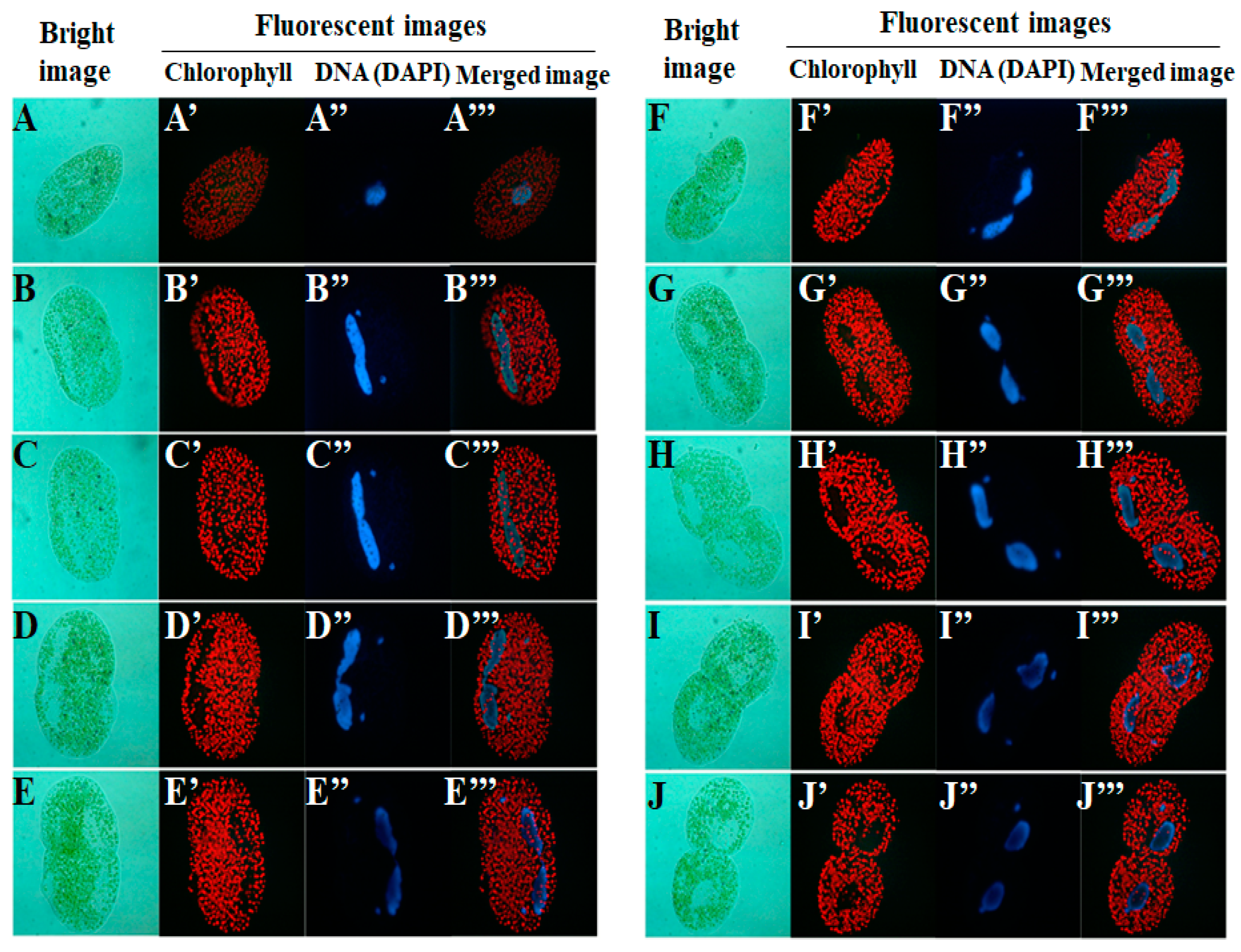
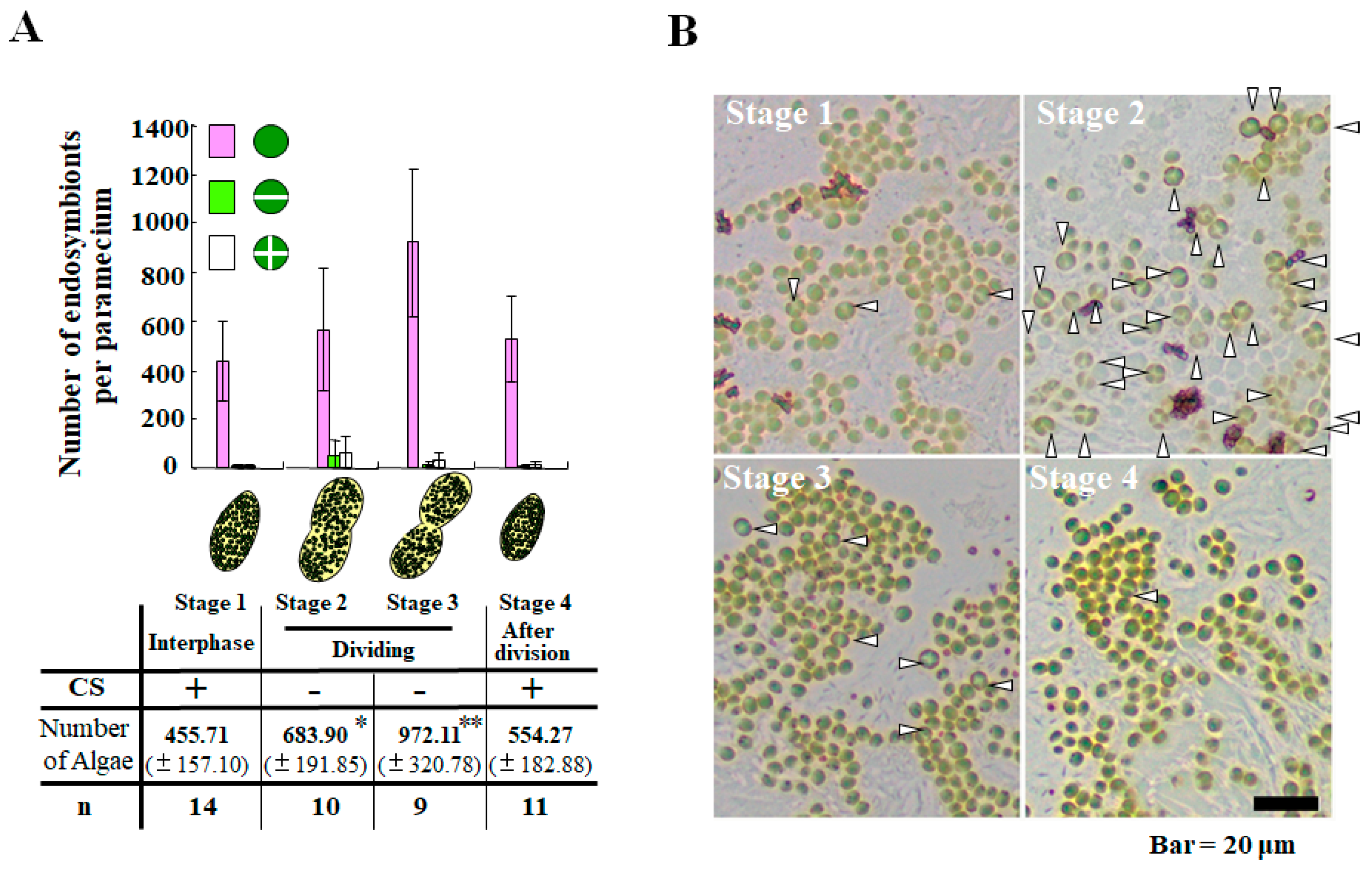
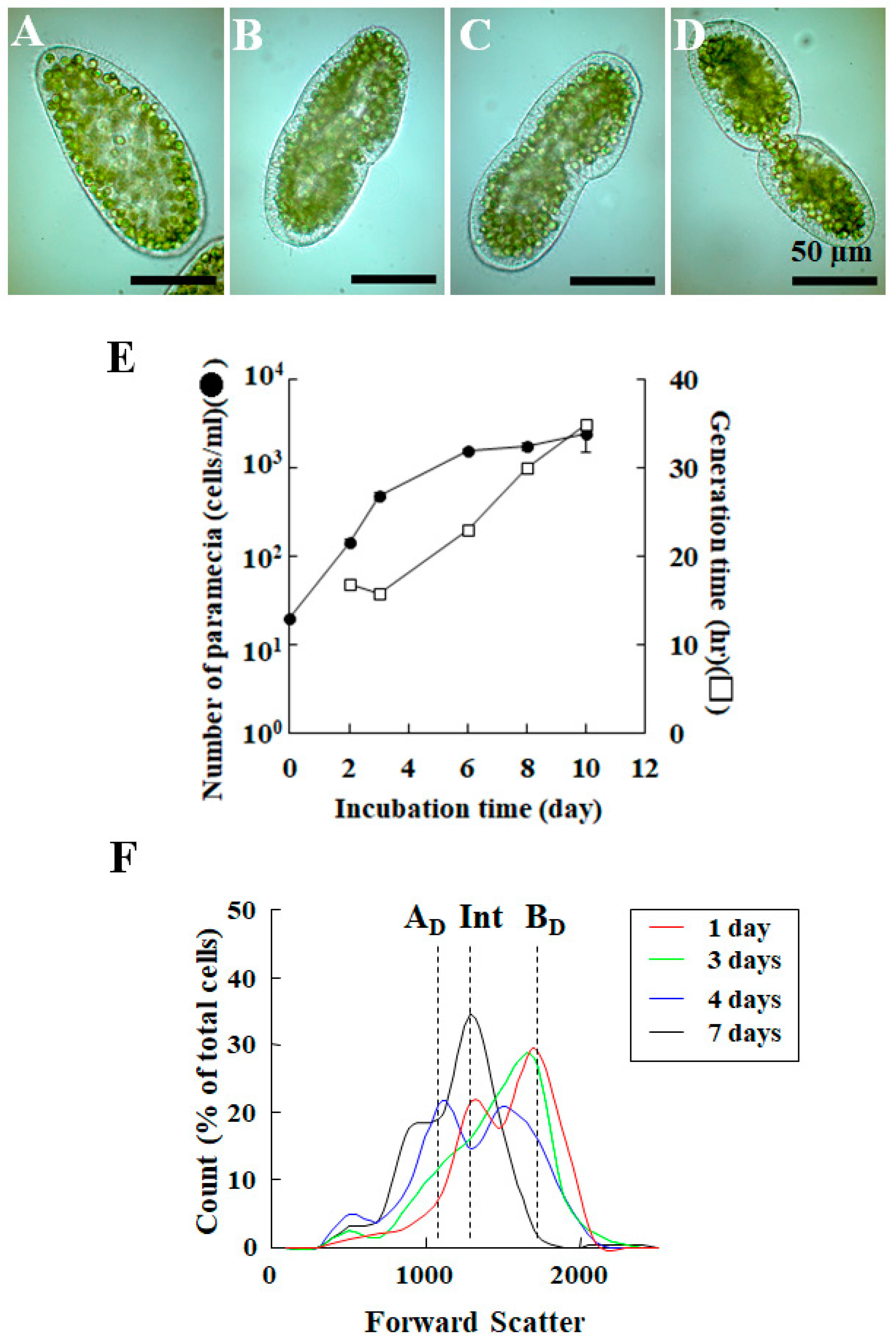
2. Life Cycle of Endosymbionts in P. bursaria Host Cell Analyzed Using Microscopic Analysis
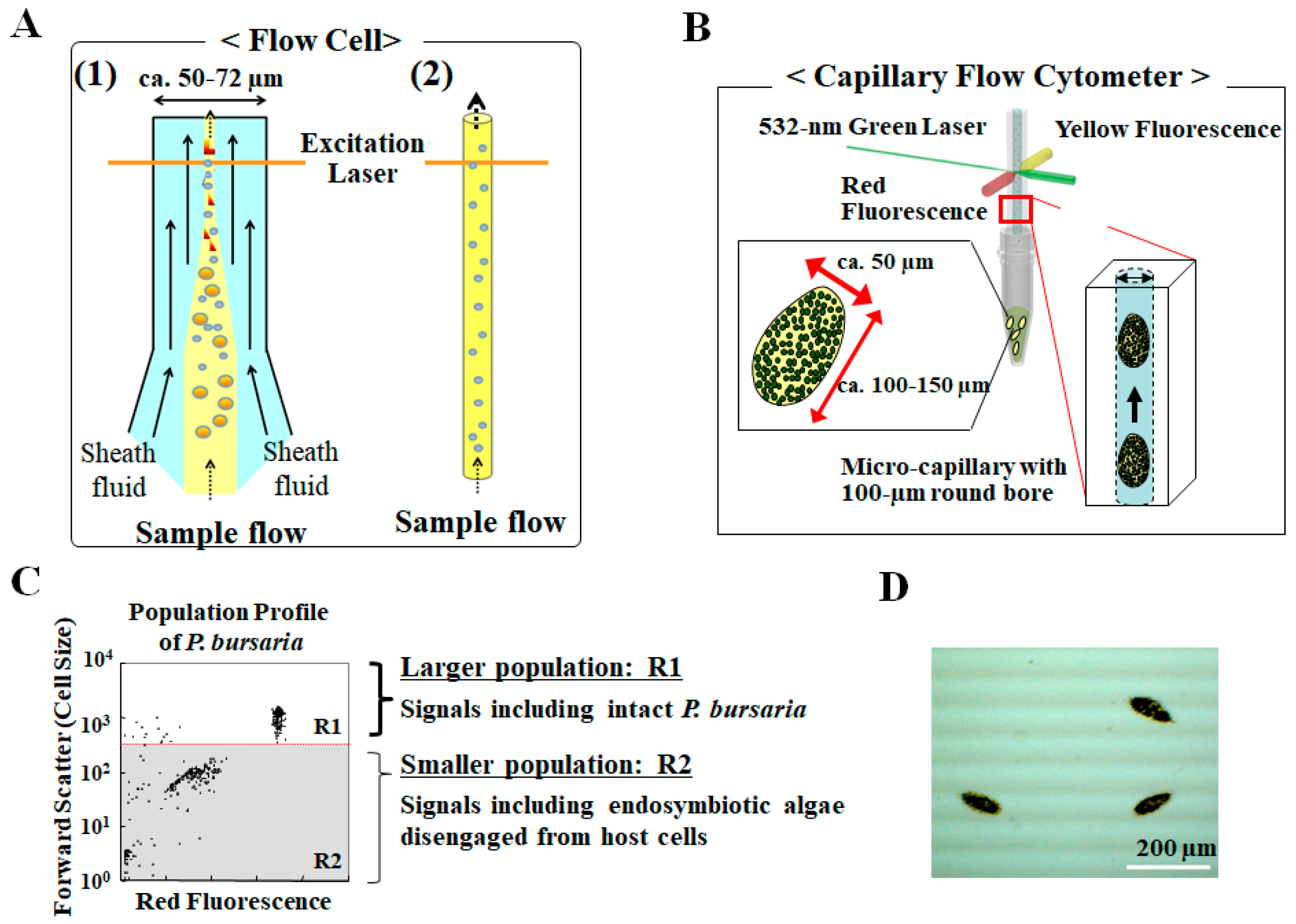
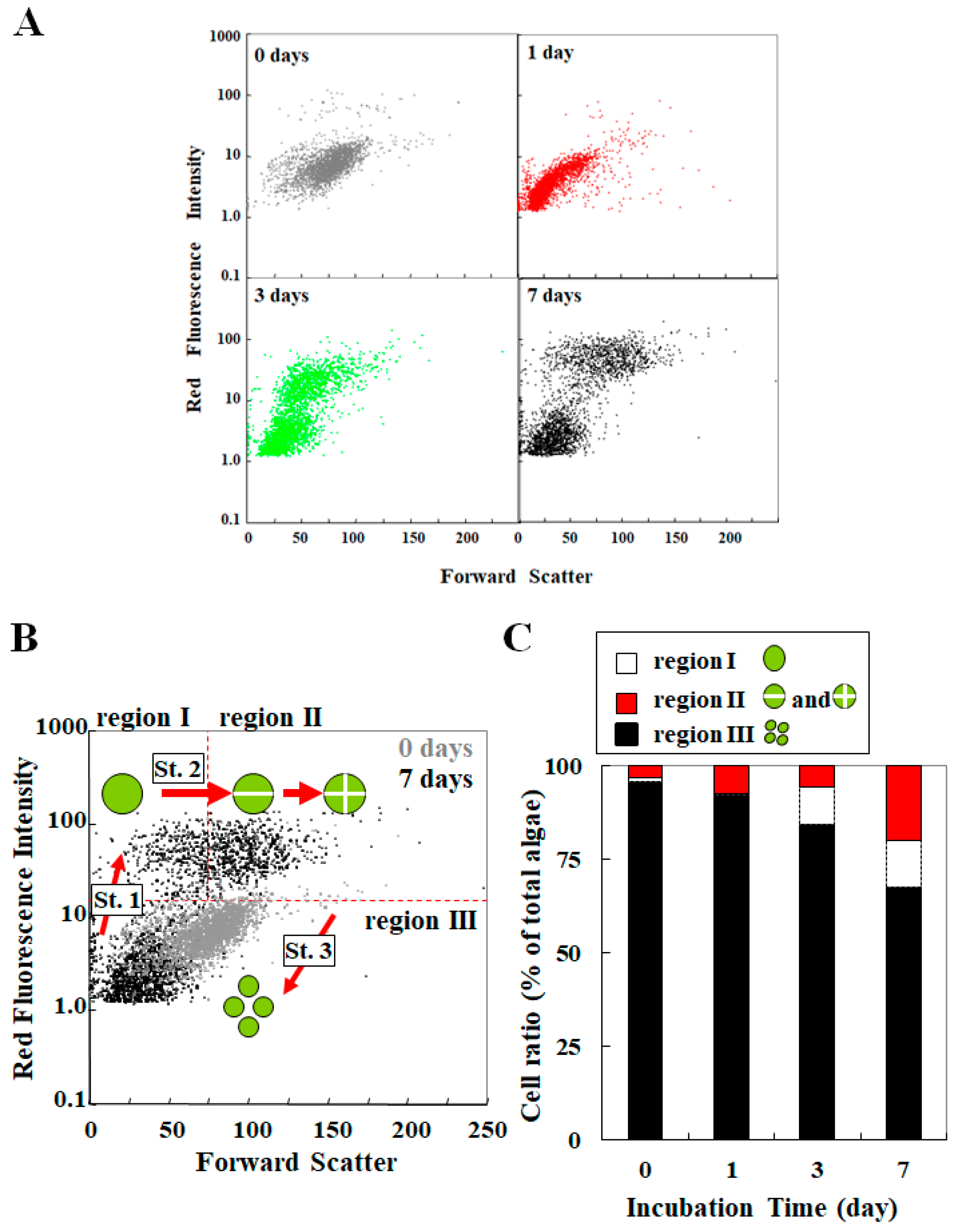
3. Analysis for Studying Endosymbiosis of P. bursaria Using Capillary FCM
3.1. Capillary Type Flow Cytometry, Not Hydrodynamic Focusing with Sheath Fluid, to Detect Intact Paramecium Cells
3.2. Detection of Difference in Cell Cycle of P. bursaria Host Cells Using Capillary FCM
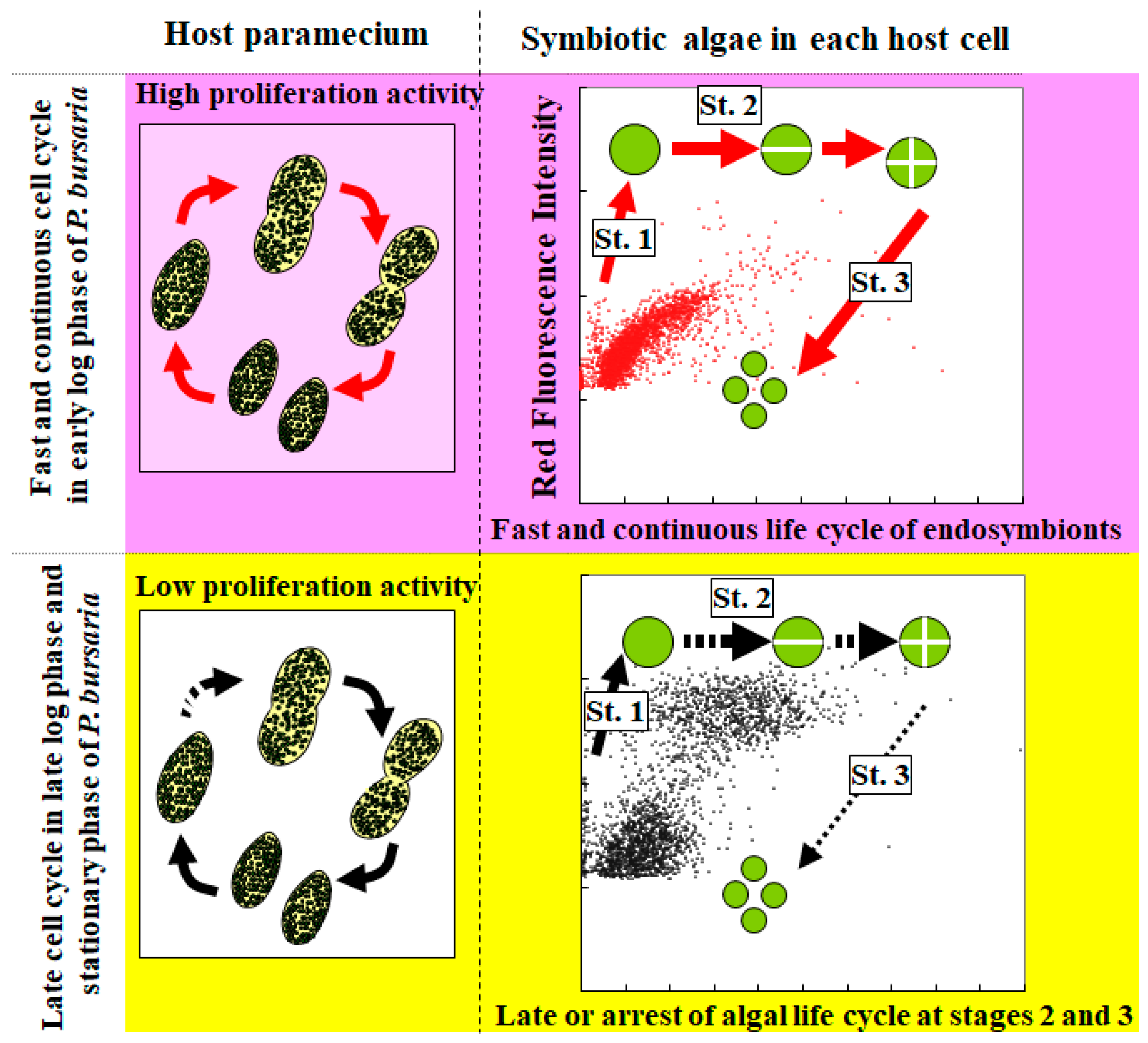
3.3. Cooperative Population Dynamics of Endosymbiotic Algae in P. bursaria Host Cells in Response to the Cell Cycle of Host Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| FCM | flow cytometry |
| LD | light/dark cycle |
| DD | constant dark |
| DAPI | 4′,6-diamidino-2-phenylindole |
| S.D. | standard deviation |
| BP | band pass |
| FSS | forward scatter signals |
| EGT | endosymbiotic gene transfer |
References
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial Applications of Microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Quality Assessment of Microalgae Exposed to Trace Metals Using Flow Cytometry. In Superfood and Functional Food—Development of Superfood and Its Role in Medicine; Shiomi, N., Waisundara, V.Y., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 29–45. [Google Scholar]
- Arashida, R. Characteristics of the microalgae Euglena and its applications in foods and ecological fields. Jpn. Soc. Photosynth. Res. 2012, 22, 33–38. [Google Scholar]
- Chisti, Y. Biodeisel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, B.I.; Kosaka, T.; Hosoya, H. Optical Compartmentation of Vegetating Algae Species as a Basis for Their Growth-Specific Characterization. Cytometry 2002, 48, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, B.I.; Takahashi, T.; Kosaka, T.; Hosoya, H. Life Cycle of Unicellular Algae. Curr. Protoc. Cytom. 2010, 52. [Google Scholar] [CrossRef]
- Takahashi, T. Simultaneous Evaluation of Life Cycle Dynamics between a Host Paramecium and the Endosymbionts of Paramecium bursaria Using Capillary Flow Cytometry. Sci. Rep. 2016, 6, 31638. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Prieto, A.; Weber, A.P.M.; Bhattacharya, D. The Origin and Establishment of the Plastid in Algae and Plants. Annu. Rev. Genet. 2007, 41, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Zimorski, V.; Ku, C.; Martin, W.F.; Gould, S.B. Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol. 2014, 22, 38–48. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Stanton, A.; Archibald, J.M.; Gould, S.B. Streptophyte Terrestrialization in Light of Plastid Evolution. Trends Plant Sci. 2016, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Rumpho, M.E.; Pelletreau, K.N.; Moustafa, A.; Bhattacharya, D. The making of a photosynthetic animal. J. Exp. Biol. 2011, 214, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Muscatine, L.; Karakashian, S.J.; Karakashian, M.W. Soluble extracellular products of algae symbiotic with a ciliate, a sponge and a mutant Hydra. Comp. Biochem. Physiol. 1967, 20, 1–6. [Google Scholar] [CrossRef]
- Ziesenisz, E.; Reisser, W.; Wiessner, W. Evidence of de novo synthesis of maltose excreted by the endosymbiotic Chlorella from Paramecium bursaria. Planta 1981, 153, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Summerer, M.; Sonntag, B.; Sommaruga, R. An experimental test of the symbiosis specificity between the ciliate Paramecium bursaria and strains of the unicellular green alga Chlorella. Environ. Microbiol. 2007, 9, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Shirai, Y.; Kosaka, T.; Hosoya, H. Arrest of cytoplasmic streaming induces algal proliferation in green paramecia. PLoS ONE 2007, 2, e1352. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nagasaki, A. Energy Production Using Microalgae Secreting Carbohydrates. J. Environ. Conserv. Eng. 2011, 40, 662–665. [Google Scholar]
- Fishman, Y.; Zlotkin, E.; Sher, D. Expulsion of Symbiotic Algae during Feeding by the Green Hydra—A Mechanism for Regulating Symbiont Density? PLoS ONE 2008, 3, e2603. [Google Scholar] [CrossRef] [PubMed]
- Matthias, H.; Frederike, A.E.; Kathrin, N.; Thomas, C.B. The Hydra viridis/Chlorella symbiosis. Growth and sexual differentiation in polyps without symbionts. Zoology 2003, 106, 101–108. [Google Scholar]
- Taylor, D.L. The Nutritional Relationship of Anemonia sulcata (PENNANT) and Its Dinoflagellate Symbiont. J. Cell Sci. 1969, 4, 751–762. [Google Scholar] [PubMed]
- Weis, V.M.; Reynolds, W.S.; deBoer, M.D.; Krupp, D.A.A. Host–symbiont specificity during onset of symbiosis between the dinoflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs 2001, 20, 301–308. [Google Scholar] [CrossRef]
- Rowan, R. Thermal adaptation in reef coral symbionts. Nature 2004, 430, 742. [Google Scholar] [CrossRef] [PubMed]
- Richier, S.; Sabourault, C.; Courtiade, J.; Zucchini, N.; Allemand, D.; Furla, P. Oxidative stress and apoptotic events during thermal stress in the symbiotic sea anemone, Anemonia viridis. FEBS J. 2006, 273, 4186–4198. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.; Wolfowicz, I.; Voss, P.A.; Hambleton, E.A.; Guse, A. Development and Symbiosis Establishment in the Cnidarian Endosymbiosis Model Aiptasia sp. Sci. Rep. 2016, 6, 19867. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, I.; Wägele, H. The symbiosis between the ‘solar-powered’ nudibranch Melibe engeli Risbec, 1937 (Dendronotoidea) and Symbiodinium sp. (Dinophyceae). J. Molluscan Stud. 2014, 80, 508–517. [Google Scholar] [CrossRef]
- Nishihara, N.; Horiike, S.; Takahashi, T.; Kosaka, T.; Shigenaka, Y.; Hosoya, H. Cloning and characterization of endosymbiotic algae isolated from Paramecium bursaria. Protoplasma 1998, 203, 91–99. [Google Scholar] [CrossRef]
- Hoshina, R.; Kamako, S.; Imamura, N. Phylogenetic Position of Endosymbiotic Green algae in Paramecium bursaria Ehrenberg from Japan. Plant Boil. (Stuttg.) 2004, 6, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Kimura, K.; Matsuda, S.; Kitaura, M.; Takahashi, T.; Kosaka, T. Symbiotic Algae-free Strains of the Green Paramecium Paramecium bursaria Produced by Herbicide Paraquat. Zool. Sci. 1995, 12, 807–810. [Google Scholar] [CrossRef]
- Takahashi, T.; Yoshii, M.; Kawano, T.; Kosaka, T.; Hosoya, H. New bioassay for the assessment of acrylamide toxicity using a green Paramecium. Toxicol. In Vitro 2005, 19, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Yoshii, M.; Kosaka, T.; Hosoya, H. The Effect of Acrylamide Inducing the Reduction of Nitrobluetetrazolium on the Ciliate and Human Cultured Cells. ITE Lett. Batter. New Technol. Med. 2005, 6, 50–58. [Google Scholar]
- Kodama, Y.; Fujishima, M. Symbiotic Chlorella sp. of the ciliate Paramecium do not prevent acidification and lysosomal fusion of the host digestive vacuoles during infection. Protoplasma 2005, 225, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, N.; Takahashi, T.; Kosaka, T.; Hosoya, H. Characterization of Symbiotic Alagae-free Strains of Paramecium bursaria Produced by the Herbicide Paraquat. J. Protozool. Res. 1996, 6, 60–67. [Google Scholar]
- Miyagishima, S.; Kabeya, Y. Chloroplast division: Squeezing the photosynthetic captive. Curr. Opin. Microbiol. 2010, 13, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, S.; Suzuki, K.; Okazaki, K.; Kabeya, Y. Expression of the Nucleus-Encoded Chloroplast Division Genes and Proteins Regulated by the Algal Cell Cycle. Mol. Biol. Evol. 2012, 29, 2957–2970. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. Direct Evaluation of Endosymbiotic Status in Paramecium bursaria Using a Capillary Flow Cytometry. Cytom. Part A 2014, 85, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, R.G.; Howe, C.J. What makes a chloroplast? Reconstructing the establishment of pohotosynthetic symbioses. J. Cell Sci. 2012, 125, 1865–1875. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.; Woehle, C.; Christa, G.; Wägele, H.; Tielens, A.G.; Jahns, P.; Gould, S.B. Comparison of sister species identifies factors underpinning plastid compatibility in green sea slugs. Proc. R. Soc. B 2015, 282, 20142519. [Google Scholar] [CrossRef] [PubMed]
- Rauch, C.; Vries, J.; Rommel, S.; Rose, L.E.; Woehle, C.; Christa, G.; Laetz, E.M.; Wägele, H.; Tielens, A.G.; Nickelsen, J.; et al. Why it is time to look beyond algal genes in photosynthetic slugs. Genome Biol. Evol. 2015, 7, 2602–2607. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D.E., Jr.; Stoecker, D.K.; Johnson, M.D.; Van Heukelem, W.F.; Sneider, K. Cryptophyte algae are robbed of their organelles by the marine ciliate Mesodinium rubrum. Nature 2000, 405, 1049–1052. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D. Acquired Phototrophy in Ciliates: A Review of Cellular Interactions and Structural Adaptations. J. Eukaryot. Microbiol. 2011, 58, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.D.; Stoecker, D.K. Role of feeding in growth and photophysiology of Myrionecta rubra. Aquat. Microb. Ecol. 2005, 39, 303–312. [Google Scholar] [CrossRef]
- Johnson, M.D.; Oldach, D.; Delwiche, C.F.; Stoecker, D.K. Retention of transcriptionally active cryptophyte nuclei by the ciliate Myrionecta rubra. Nature 2007, 445, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, N.; Horiike, S.; Oka, Y.; Takahashi, T.; Kosaka, T.; Hosoya, H. Microtubule-Dependent Movement of Symbiotic Algae and Granules in Paramecium bursaria. Cell Motil. Cytoskeleton 1999, 43, 85–98. [Google Scholar] [CrossRef]
- Harkins, K.R.; Galbraith, D.W. Factors Governing the Flow Cytometric Analysis and Sorting of Large Biological Particles. Cytometry 1987, 8, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Unruh, T.L.; Deans, J.P. Implementation of a Flow Cytometry Strategy to Isolate and Assess Heterogeneous Membrane Raft Domains. Flow Cytometry—Recent Perspectives; Schmid, I., Ed.; InTechOpen: Rijeka, Croatia, 2012; pp. 169–184. [Google Scholar]
- Gaurav, V.; Kolewe, M.E.; Roberts, S.C. Flow Cytometric Methods to Investigate Culture Heterogeneities for Plant Metabolic Engineering. Methods Mol. Biol. 2010, 643, 243–262. [Google Scholar] [PubMed]
- Hörtnagl, P.H.; Sommaruga, R. Photo-oxidative stress in symbiotic and aposymbiotic strains of the ciliate Paramecium bursaria. Photochem. Photobiol. Sci. 2007, 6, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Gerashchenko, B.I.; Nishihara, N.; Ohara, T.; Tosuji, H.; Kosaka, T.; Hosoya, H. Flow cytometry as a strategy to study the endosymbiosis of algae in Paramecium bursaria. Cytometry 2000, 41, 209–215. [Google Scholar] [CrossRef]
- Martin, W.; Rujan, T.; Richly, E.; Hansen, A.; Cornelsen, S.; Lins, T.; Leister, D.; Stoebe, B.; Hasegawa, M.; Penny, D. Evolutionary analysis of Arabidopsis, cyanobacterial, and chloroplast genomes reveals plastid phylogeny and thousands of cyanobacterial genes in the nucleus. Proc. Natl. Acad. Sci. USA 2002, 99, 12246–12251. [Google Scholar] [CrossRef] [PubMed]
- Timmis, J.N.; Ayliffe, M.A.; Huang, C.Y.; Martin, W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004, 5, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, N.; Inouye, I. Hatena arenicola gen. et sp. nov., a Katablepharid Undergoing Probable Plastid Acquisition. Protist 2006, 157, 401–419. [Google Scholar] [CrossRef] [PubMed]
- Kadano, T.; Kawano, T.; Hosoya, H.; Kosaka, T. Flow cytometric studies of the host-regulated cell cycle in algae symbiotic with green Paramecium. Protoplasma 2004, 223, 133–141. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, T. Life Cycle Analysis of Endosymbiotic Algae in an Endosymbiotic Situation with Paramecium bursaria Using Capillary Flow Cytometry. Energies 2017, 10, 1413. https://doi.org/10.3390/en10091413
Takahashi T. Life Cycle Analysis of Endosymbiotic Algae in an Endosymbiotic Situation with Paramecium bursaria Using Capillary Flow Cytometry. Energies. 2017; 10(9):1413. https://doi.org/10.3390/en10091413
Chicago/Turabian StyleTakahashi, Toshiyuki. 2017. "Life Cycle Analysis of Endosymbiotic Algae in an Endosymbiotic Situation with Paramecium bursaria Using Capillary Flow Cytometry" Energies 10, no. 9: 1413. https://doi.org/10.3390/en10091413
APA StyleTakahashi, T. (2017). Life Cycle Analysis of Endosymbiotic Algae in an Endosymbiotic Situation with Paramecium bursaria Using Capillary Flow Cytometry. Energies, 10(9), 1413. https://doi.org/10.3390/en10091413




