Quantifying Cathode Water Transport via Anode Relative Humidity Measurements in a Polymer Electrolyte Membrane Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polarization Curve
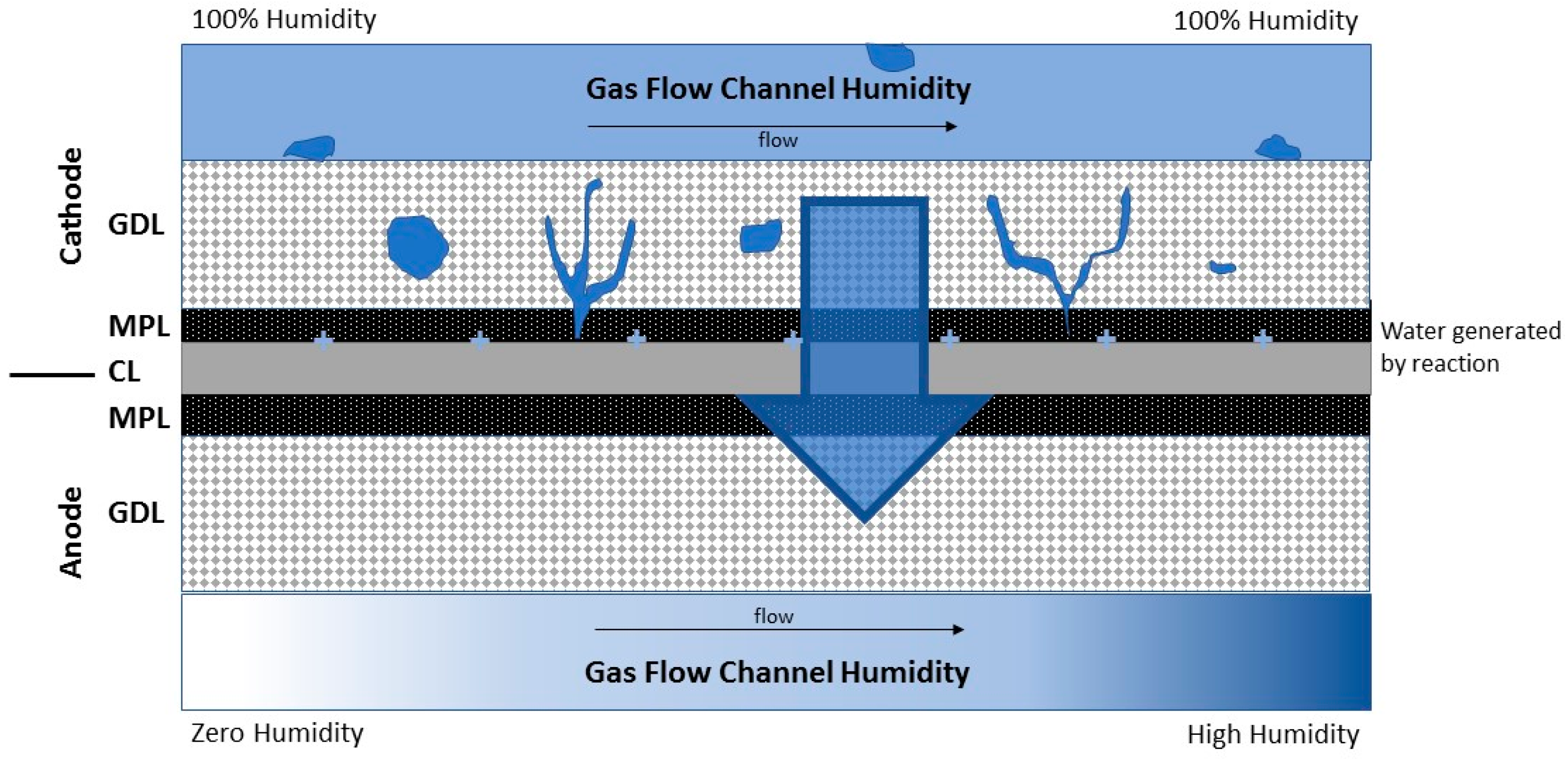
2.2. Anode Water Removal
2.3. Anode Pressure Drop Calibration Curve
3. Quantification of In-Situ Data to Determine Average Anode Relative Humidity and Net Cell Water Flux
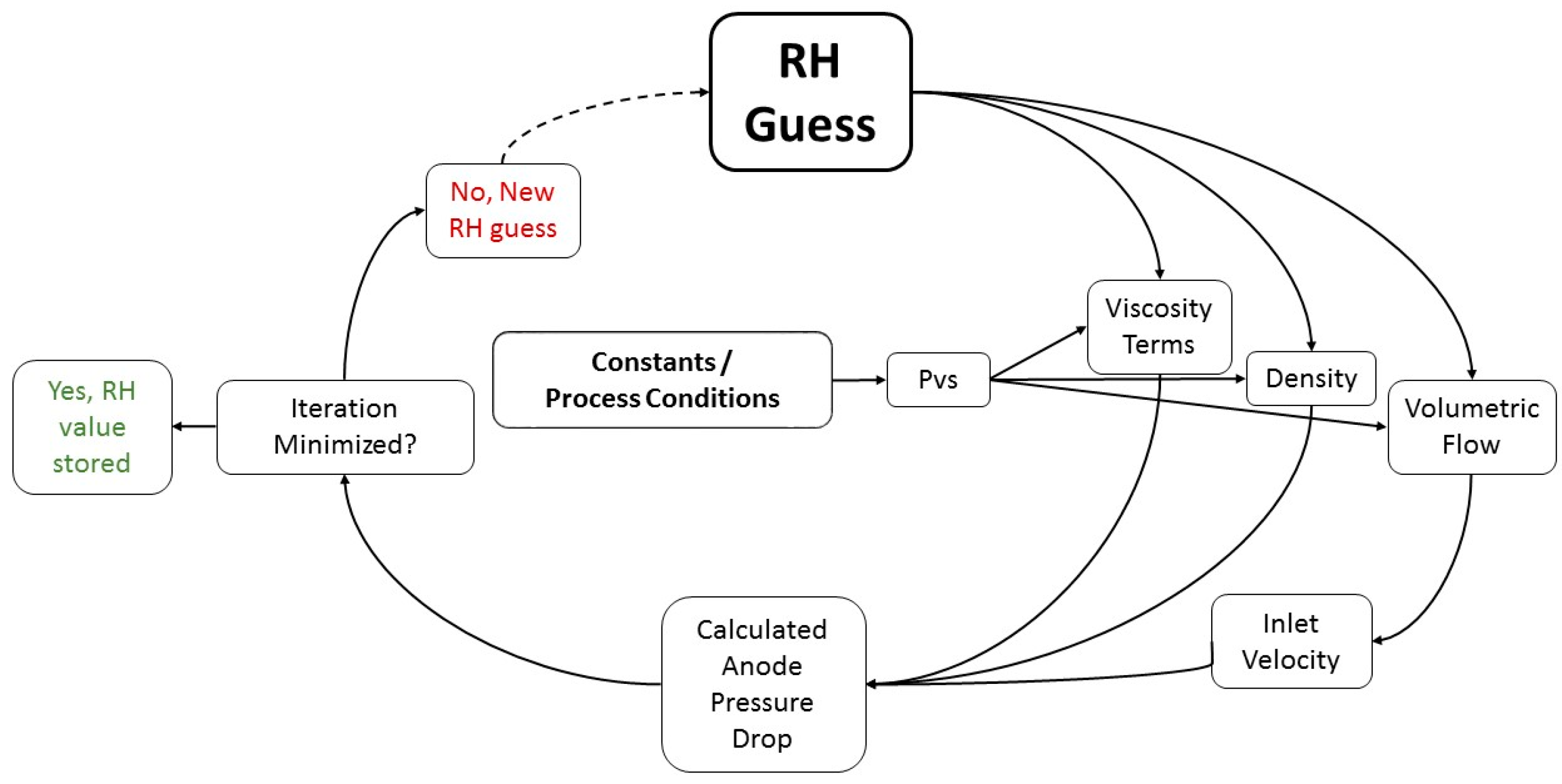
3.1. Ex-Situ Determination of Set-up Specific Parameters Related to Pressure Drop
3.2. Quantification of In-Situ Data to Determine Anode Relative Humidity
3.3. Quantification of Net Cell Water Flux
4. Experimental Results and Discussion
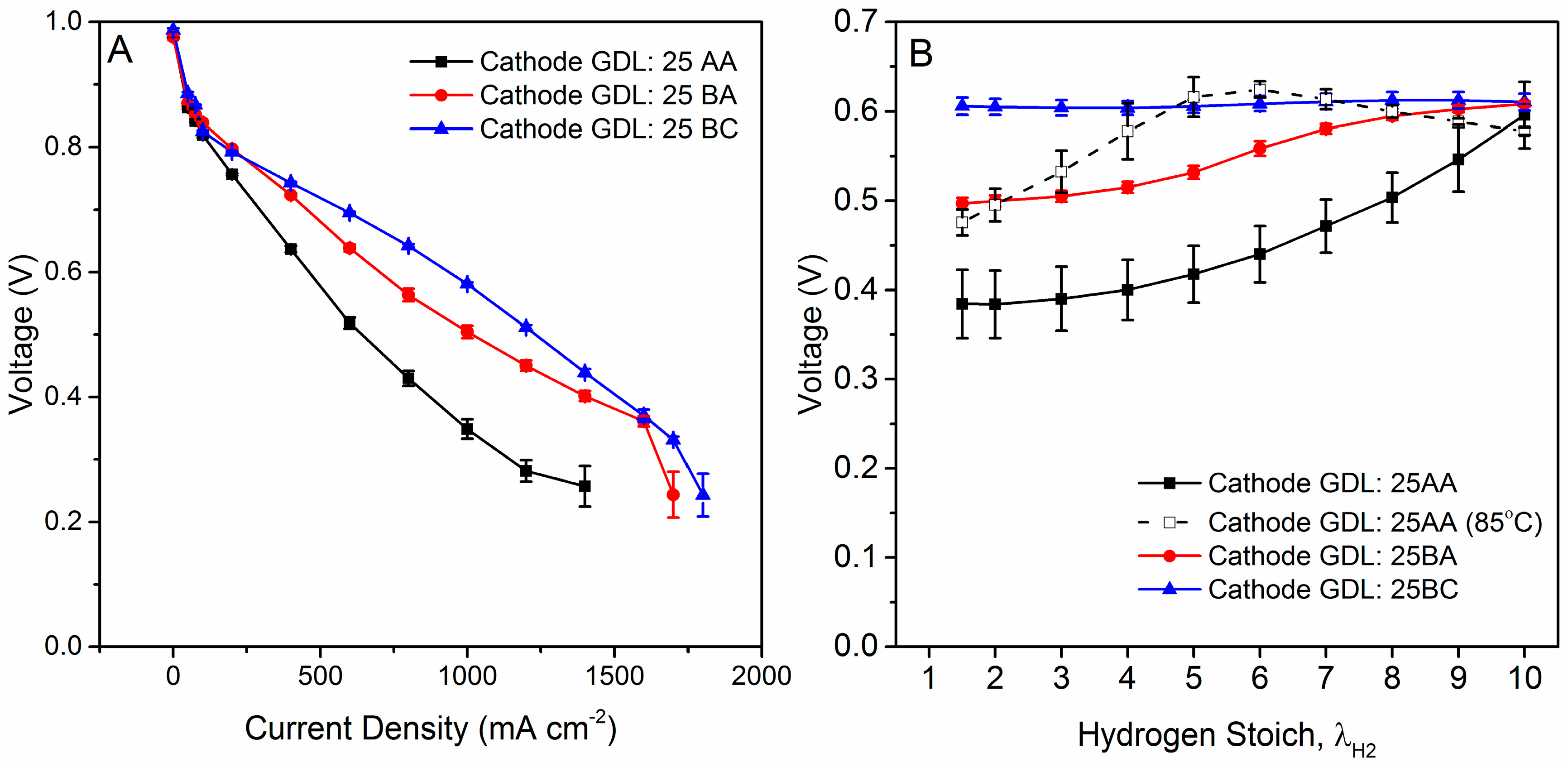
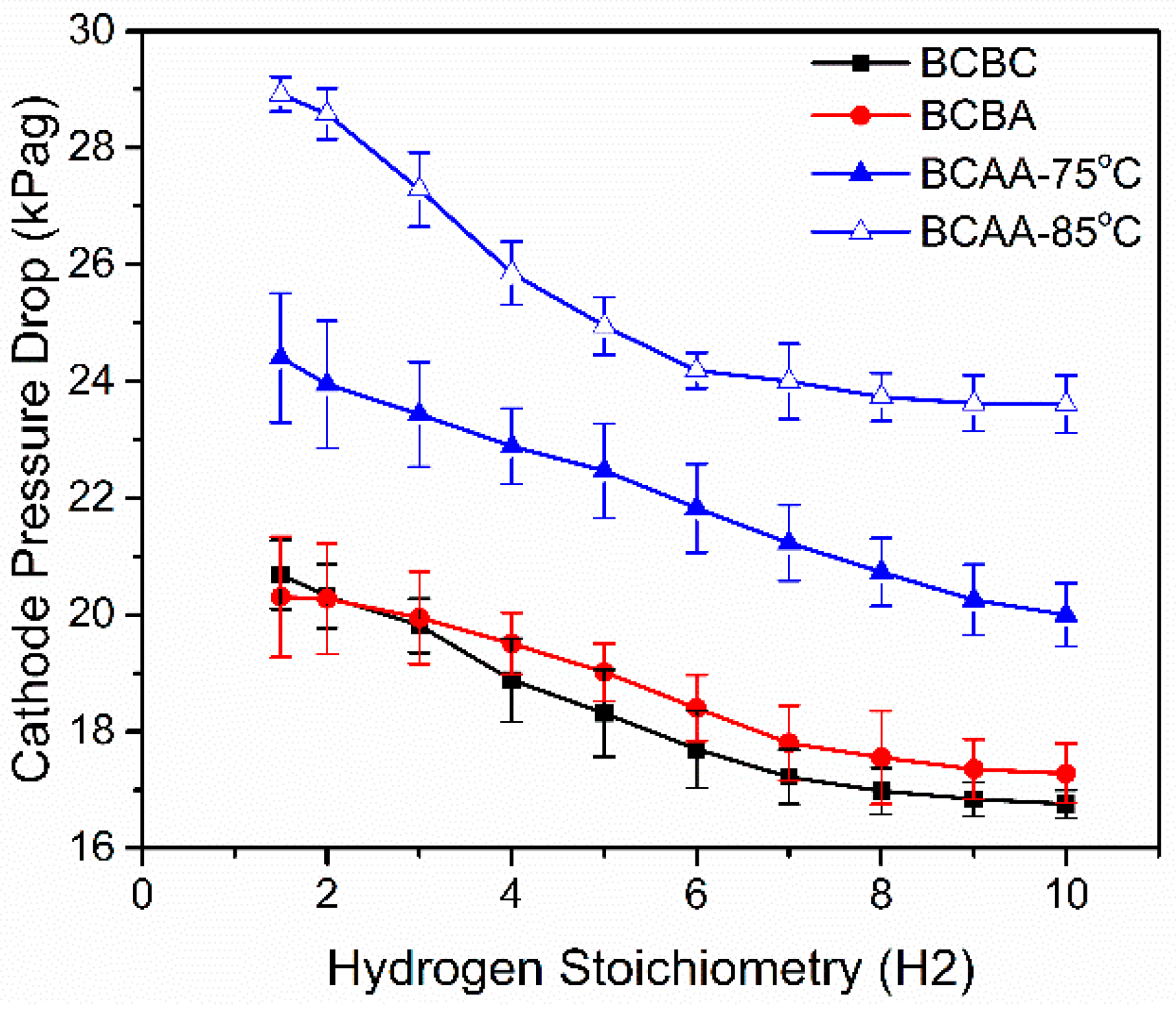
4.1. Polarization Curves and Qualitative AWR Results
4.2. Ex-Situ System Parameters in Pressure Drop Analysis
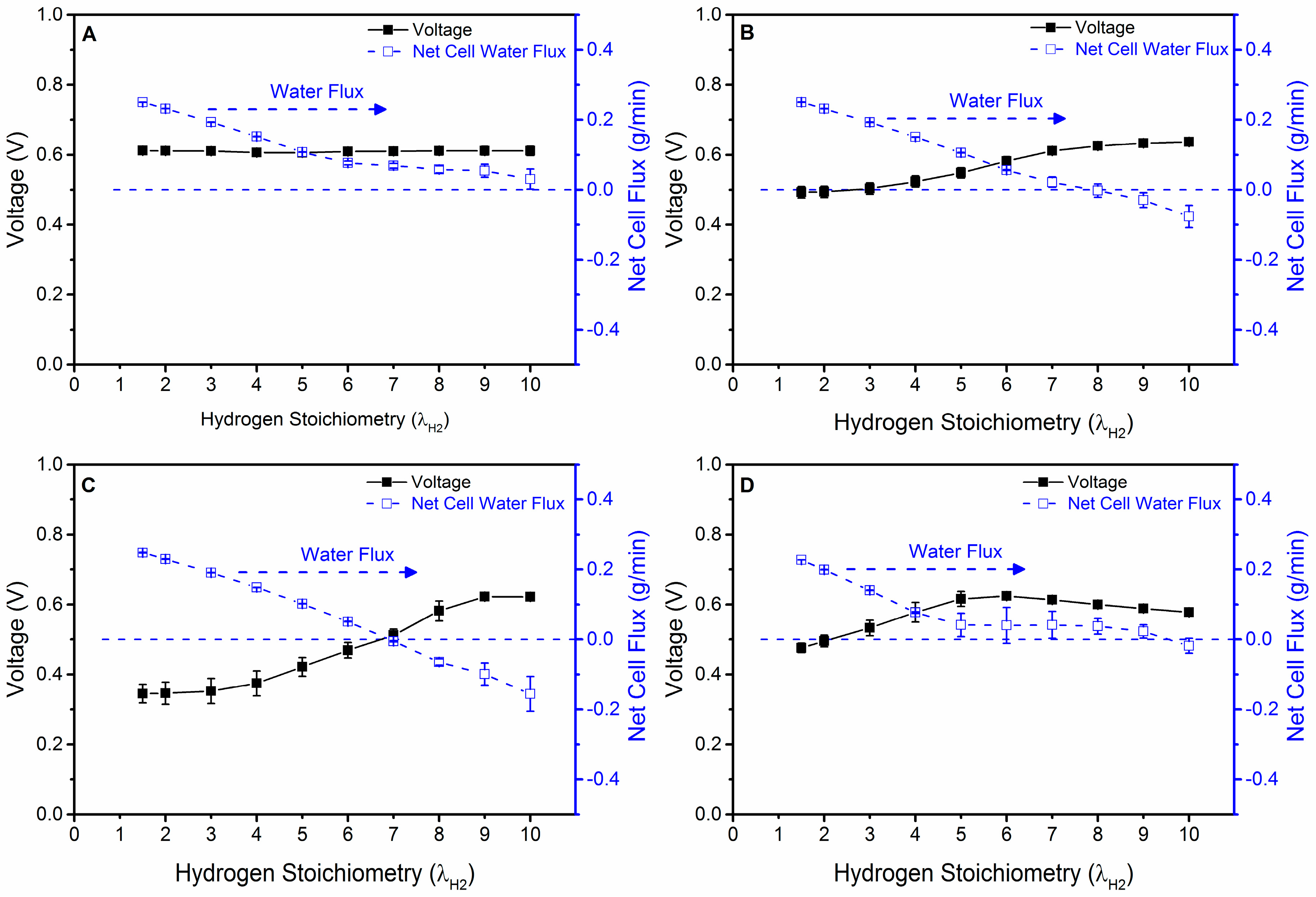
4.3. Quantification of Water Removed
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Constants Used in Equations
| Fluid | µ0 | C |
|---|---|---|
| Air | 1.81 × 10−5 | 120 |
| Hydrogen | 0.92 × 10−5 | 72 |
| Water Vapor | 1.02 × 10−5 | 660 |
| Coefficients for Vapor Pressure Equation | |
|---|---|
| a = | −5800.2206 |
| b = | 1.3914493 |
| c = | −0.0486402 |
| d = | 0.41764768 × 10−4 |
| e = | −0.14452093 × 10−7 |
| f = | 6.5459673 |
References
- United States Department of Energy. Full Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan; United States Department of Energy: Washington, DC, USA, 2012; pp. 1–58.
- Wang, Y.; Chen, K.S.; Mishler, J.; Cho, S.C.; Adroher, X.C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 2011, 88, 981–1007. [Google Scholar] [CrossRef]
- Yousfi-Steiner, N.; Moçotéguy, P.; Candusso, D.; Hissel, D.; Hernandez, A.; Aslanides, A. A review on pem voltage degradation associated with water management: Impacts, influent factors and characterization. J. Power Sources 2008, 183, 260–274. [Google Scholar] [CrossRef]
- Ous, T.; Arcoumanis, C. Degradation aspects of water formation and transport in proton exchange membrane fuel cell: A review. J. Power Sources 2013, 240, 558–582. [Google Scholar] [CrossRef]
- Pei, P.; Li, Y.; Xu, H.; Wu, Z. A review on water fault diagnosis of pemfc associated with the pressure drop. Appl. Energy 2016, 173, 366–385. [Google Scholar] [CrossRef]
- Hussaini, I.S.; Wang, C.Y. Dynamic water management of polymer electrolyte membrane fuel cells using intermittent RH control. J. Power Sources 2010, 195, 3822–3829. [Google Scholar] [CrossRef]
- Song, M.; Pei, P.; Zha, H.; Xu, H. Water management of proton exchange membrane fuel cell based on control of hydrogen pressure drop. J. Power Sources 2014, 267, 655–663. [Google Scholar] [CrossRef]
- Damour, C.; Benne, M.; Grondin-Perez, B.; Chabriat, J.-P.; Pollet, B.G. A novel non-linear model-based control strategy to improve pemfc water management—The flatness-based approach. Int. J. Hydrogen Energy 2015, 40, 2371–2376. [Google Scholar] [CrossRef]
- Pahon, E.; Yousfi Steiner, N.; Jemei, S.; Hissel, D.; Moçoteguy, P. A signal-based method for fast pemfc diagnosis. Appl. Energy 2016, 165, 748–758. [Google Scholar] [CrossRef]
- Alink, R.; Haußmann, J.; Markötter, H.; Schwager, M.; Manke, I.; Gerteisen, D. The influence of porous transport layer modifications on the water management in polymer electrolyte membrane fuel cells. J. Power Sources 2013, 233, 358–368. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chang, M.-H. Effect of cathode microporous layer composition on proton exchange membrane fuel cell performance under different air inlet relative humidity. J. Power Sources 2013, 232, 306–309. [Google Scholar] [CrossRef]
- Li, A.; Chan, S.H. Understanding the role of cathode structure and property on water management and electrochemical performance of a pem fuel cell. Int. J. Hydrogen Energy 2013, 38, 11988–11995. [Google Scholar] [CrossRef]
- Lemoine-Nava, R.; Hanke-Rauschenbach, R.; Mangold, M.; Sundmacher, K. The gas diffusion layer in polymer electrolyte membrane fuel cells: A process model of the two-phase flow. Int. J. Hydrogen Energy 2011, 36, 1637–1653. [Google Scholar] [CrossRef]
- Qin, C.; Rensink, D.; Fell, S.; Majid Hassanizadeh, S. Two-phase flow modeling for the cathode side of a polymer electrolyte fuel cell. J. Power Sources 2012, 197, 136–144. [Google Scholar] [CrossRef]
- Fazeli, M.; Hinebaugh, J.; Fishman, Z.; Tötzke, C.; Lehnert, W.; Manke, I.; Bazylak, A. Pore network modeling to explore the effects of compression on multiphase transport in polymer electrolyte membrane fuel cell gas diffusion layers. J. Power Sources 2016, 335, 162–171. [Google Scholar] [CrossRef]
- Kalidindi, A.R.; Taspinar, R.; Litster, S.; Kumbur, E.C. A two-phase model for studying the role of microporous layer and catalyst layer interface on polymer electrolyte fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 9297–9309. [Google Scholar] [CrossRef]
- Meng, H.; Han, B.; Ruan, B. Numerical modeling of liquid water transport inside and across membrane in pem fuel cells. Asia-Pac. J. Chem. Eng. 2013, 8, 104–114. [Google Scholar] [CrossRef]
- Qin, Y.; Du, Q.; Yin, Y.; Jiao, K.; Li, X. Numerical investigation of water dynamics in a novel proton exchange membrane fuel cell flow channel. J. Power Sources 2013, 222, 150–160. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.S. Advanced control of liquid water region in diffusion media of polymer electrolyte fuel cells through a dimensionless number. J. Power Sources 2016, 315, 224–235. [Google Scholar] [CrossRef]
- Grötsch, M.; Mangold, M. A two-phase pemfc model for process control purposes. Chem. Eng. Sci. 2008, 63, 434–447. [Google Scholar] [CrossRef]
- Berning, T.; Odgaard, M.; Kær, S.K. A study of multi-phase flow through the cathode side of an interdigitated flow field using a multi-fluid model. J. Power Sources 2010, 195, 4842–4852. [Google Scholar] [CrossRef]
- Ding, Y.; Bi, X.; Wilkinson, D.P. 3D simulations of the impact of two-phase flow on pem fuel cell performance. Chem. Eng. Sci. 2013, 100, 445–455. [Google Scholar] [CrossRef]
- Yau, T.C.; Cimenti, M.; Bi, X.T.; Stumper, J. Water transport and schröder’s paradox in fuel cell membrane electrode assemblies. J. Power Sources 2013, 224, 285–289. [Google Scholar] [CrossRef]
- Morgan, J.M.; Datta, R. Understanding the gas diffusion layer in proton exchange membrane fuel cells. I. How its structural characteristics affect diffusion and performance. J. Power Sources 2014, 251, 269–278. [Google Scholar] [CrossRef]
- Chevalier, S.; Lee, J.; Ge, N.; Yip, R.; Antonacci, P.; Tabuchi, Y.; Kotaka, T.; Bazylak, A. In operando measurements of liquid water saturation distributions and effective diffusivities of polymer electrolyte membrane fuel cell gas diffusion layers. Electrochim. Acta 2016, 210, 792–803. [Google Scholar] [CrossRef]
- Bazylak, A. Liquid water visualization in pem fuel cells: A review. Int. J. Hydrogen Energy 2009, 34, 3845–3857. [Google Scholar] [CrossRef]
- Krüger, P.; Markötter, H.; Haußmann, J.; Klages, M.; Arlt, T.; Banhart, J.; Hartnig, C.; Manke, I.; Scholta, J. Synchrotron X-ray tomography for investigations of water distribution in polymer electrolyte membrane fuel cells. J. Power Sources 2011, 196, 5250–5255. [Google Scholar] [CrossRef]
- Deevanhxay, P.; Sasabe, T.; Tsushima, S.; Hirai, S. Effect of liquid water distribution in gas diffusion media with and without microporous layer on pem fuel cell performance. Electrochem. Commun. 2013, 34, 239–241. [Google Scholar] [CrossRef]
- Banerjee, R.; Ge, N.; Lee, J.; George, M.G.; Chevalier, S.; Liu, H.; Shrestha, P.; Muirhead, D.; Bazylak, A. Transient liquid water distributions in polymer electrolyte membrane fuel cell gas diffusion layers observed through in-operando synchrotron X-ray radiography. J. Electrochem. Soc. 2017, 164, F154–F162. [Google Scholar] [CrossRef]
- Owejan, J.P.; Trabold, T.A.; Jacobson, D.L.; Baker, D.R.; Hussey, D.S.; Arif, M. In situ investigation of water transport in an operating pem fuel cell using neutron radiography: Part 2—Transient water accumulation in an interdigitated cathode flow field. Int. J. Heat Mass Transf. 2006, 49, 4721–4731. [Google Scholar] [CrossRef]
- Hussaini, I.S.; Wang, C.Y. Visualization and quantification of cathode channel flooding in pem fuel cells. J. Power Sources 2009, 187, 444–451. [Google Scholar] [CrossRef]
- Owejan, J.P.; Owejan, J.E.; Gu, W.; Trabold, T.A.; Tighe, T.W.; Mathias, M.F. Water transport mechanisms in pemfc gas diffusion layers. J. Electrochem. Soc. 2010, 157, B1456–B1464. [Google Scholar] [CrossRef]
- Anderson, R.; Blanco, M.; Bi, X.; Wilkinson, D.P. Anode water removal and cathode gas diffusion layer flooding in a proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2012, 37, 16093–16103. [Google Scholar] [CrossRef]
- Battrell, L.; Trunkle, A.; Eggleton, E.; Zhang, L.; Anderson, R. Investigation of water transport within a proton exchange membrane fuel cell by diffusion layer saturation analysis. In Proceedings of the 2016 14th International Conference on Fuel Cell Science, Engineering and Technology, Charlotte, NC, USA, 26–30 June 2016; p. V001T005A002. [Google Scholar]
- Cindrella, L.; Kannan, A.M.; Lin, J.F.; Saminathan, K.; Ho, Y.; Lin, C.W.; Wertz, J. Gas diffusion layer for proton exchange membrane fuel cells—A review. J. Power Sources 2009, 194, 146–160. [Google Scholar] [CrossRef]
- Li, H.; Tang, Y.; Wang, Z.; Shi, Z.; Wu, S.; Song, D.; Zhang, J.; Fatih, K.; Zhang, J.; Wang, H.; et al. A review of water flooding issues in the proton exchange membrane fuel cell. J. Power Sources 2008, 178, 103–117. [Google Scholar] [CrossRef]
- Kim, M.; Jung, N.; Eom, K.; Yoo, S.J.; Kim, J.Y.; Jang, J.H.; Kim, H.-J.; Hong, B.K.; Cho, E. Effects of anode flooding on the performance degradation of polymer electrolyte membrane fuel cells. J. Power Sources 2014, 266, 332–340. [Google Scholar] [CrossRef]
- Voss, H.H.; Wilkinson, D.P.; Pickup, P.G.; Johnson, M.C.; Basura, V. Anode water removal: A water management and diagnostic technique for solid polymer fuel cells. Electrochim. Acta 1995, 40, 321–328. [Google Scholar] [CrossRef]
- Barbir, F. Pem Fuel Cells: Theory and Practice; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]


| Criteria | Description |
|---|---|
| Active Area | 50 cm2 |
| Cathode Channel Dimensions | 1.57 mm (w) × 0.99 mm (d) |
| Anode Channel Dimensions | 1.27 mm (w) × 0.51 mm (d) |
| Compression Pressure | 100 kPaG |
| Flow Field Design | Single serpentine channel |
| Flow Field Pattern | Semi Co-Flow |
| Sigracet Gas Diffusion Layer | % Polytetrafluoroethylene | Microporous Layer | Thickness (µm) | Porosity |
|---|---|---|---|---|
| 25 BC | 5% | Yes | 235 | 0.80 |
| 25 BA | 5% | No | 190 | 0.88 |
| 25 AA | 0% | No | 190 | 0.88 |
| Stoich | Trial 1 | Trial 2 | Trial 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Voltage | Anode RH | Net Cell Water Flux (g/min) | Voltage | Anode RH | Net Cell Water Flux (g/min) | Voltage | Anode RH | Net Cell Water Flux (g/min) | |
| 1.5 | 0.37 | 100.00% | 0.25 | 0.31 | 100.00% | 0.25 | 0.36 | 100.00% | 0.25 |
| 2 | 0.37 | 100.00% | 0.23 | 0.30 | 100.00% | 0.23 | 0.37 | 100.00% | 0.23 |
| 3 | 0.38 | 100.00% | 0.19 | 0.30 | 100.00% | 0.19 | 0.38 | 100.00% | 0.19 |
| 4 | 0.40 | 100.00% | 0.15 | 0.33 | 100.00% | 0.15 | 0.39 | 100.00% | 0.15 |
| 5 | 0.44 | 100.00% | 0.10 | 0.39 | 100.00% | 0.10 | 0.44 | 100.00% | 0.10 |
| 6 | 0.48 | 100.00% | 0.05 | 0.44 | 100.00% | 0.05 | 0.49 | 100.00% | 0.05 |
| 7 | 0.52 | 100.00% | −0.01 | 0.50 | 100.00% | 0.00 | 0.52 | 100.00% | 0.00 |
| 8 | 0.59 | 100.00% | −0.07 | 0.57 | 98.90% | −0.07 | 0.58 | 95.86% | −0.06 |
| 9 | 0.63 | 95.70% | −0.13 | 0.62 | 89.19% | −0.10 | 0.62 | 83.01% | −0.07 |
| 10 | 0.62 | 91.82% | −0.20 | 0.62 | 85.59% | −0.16 | 0.62 | 75.72% | −0.10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Battrell, L.; Trunkle, A.; Eggleton, E.; Zhang, L.; Anderson, R. Quantifying Cathode Water Transport via Anode Relative Humidity Measurements in a Polymer Electrolyte Membrane Fuel Cell. Energies 2017, 10, 1222. https://doi.org/10.3390/en10081222
Battrell L, Trunkle A, Eggleton E, Zhang L, Anderson R. Quantifying Cathode Water Transport via Anode Relative Humidity Measurements in a Polymer Electrolyte Membrane Fuel Cell. Energies. 2017; 10(8):1222. https://doi.org/10.3390/en10081222
Chicago/Turabian StyleBattrell, Logan, Aubree Trunkle, Erica Eggleton, Lifeng Zhang, and Ryan Anderson. 2017. "Quantifying Cathode Water Transport via Anode Relative Humidity Measurements in a Polymer Electrolyte Membrane Fuel Cell" Energies 10, no. 8: 1222. https://doi.org/10.3390/en10081222
APA StyleBattrell, L., Trunkle, A., Eggleton, E., Zhang, L., & Anderson, R. (2017). Quantifying Cathode Water Transport via Anode Relative Humidity Measurements in a Polymer Electrolyte Membrane Fuel Cell. Energies, 10(8), 1222. https://doi.org/10.3390/en10081222





