A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry
Abstract
:1. Introduction
2. Organics and Chemicals Introduced to the Effluent
2.1. Wastewater Organic Loading
2.2. Cleaning with Chemicals
2.3. Disinfecting Chemicals
3. Abattoir Wastewater Treatment Methods
3.1. Physical Treatments
3.2. Chemical Treatments
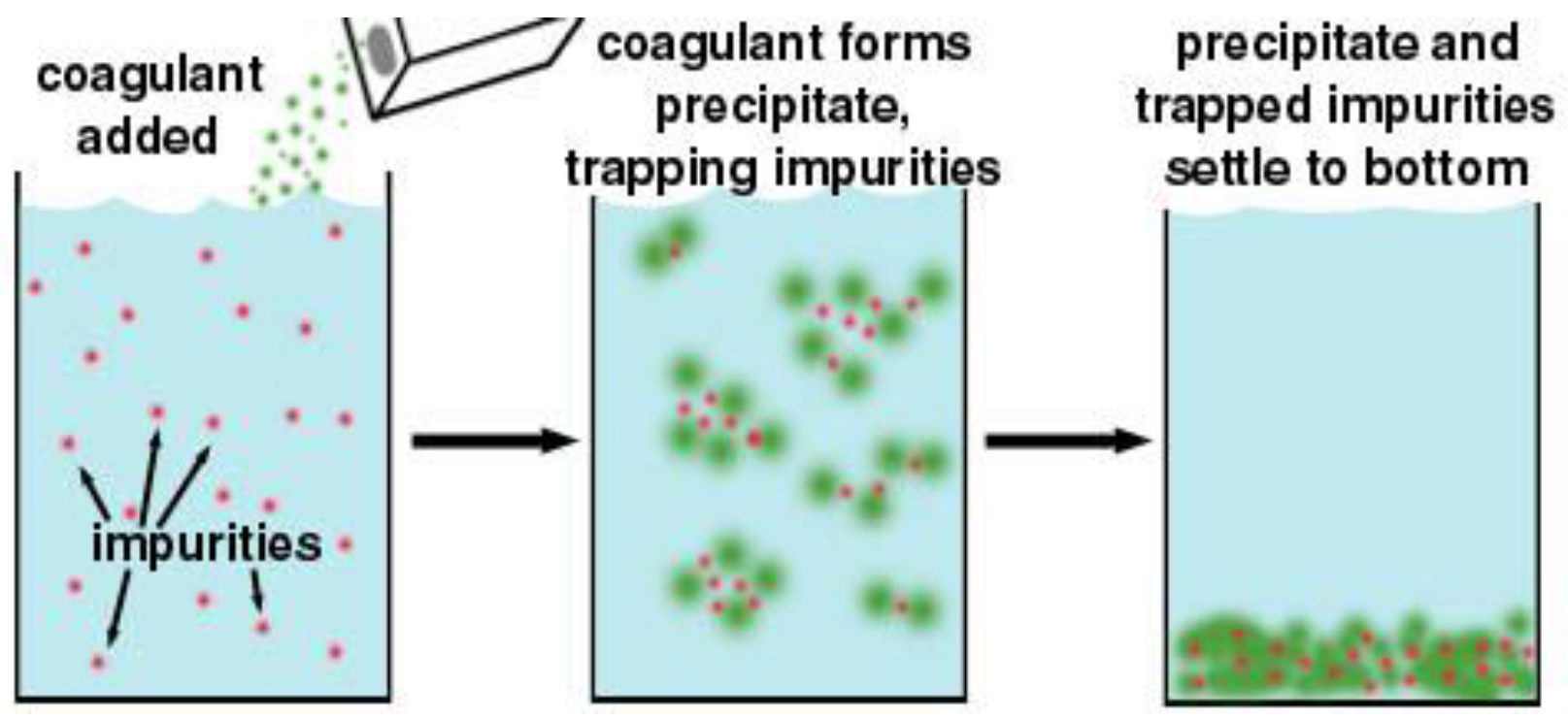
3.2.1. Coagulation-Flocculation-Sedimentation
3.2.2. Electrochemical Methods
3.2.3. Chemical DAF Unit
3.2.4. By-Products
3.3. Biological Treatment
3.3.1. Anaerobic Digestion Process
3.3.2. Aerobic Treatment
3.4. Combined Processes for Producing High Quality Effluent
3.5. Energy-Generating Treatment Systems
4. Industrial Practice and Examples
5. Wastewater Treatment and Energy
6. Innovative Coagulants
7. Optimal and Practical Solutions
- Optimize fresh water usage;
- Improve separation of blood from the wastewater system;
- Removal of solid waste from production area floors before wet cleaning;
- Installation of sludge trap and fat separator.
8. Economical Analysis
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dąbrowski, W.; Żyłka, R.; Rynkiewicz, M. Evaluation of energy consumption in agro-industrial wastewater treatment plant. J. Ecol. Eng. 2016, 17, 73–78. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.F.; Mehrvar, M. Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: A review on trends and advances. J. Environ. Manag. 2015, 161, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Dillon, P. Water reuse in the meat industry—Opportunities and issues. Available online: www.meatupdate.csiro.au (accessed on 10 September 2014).
- Food and Agriculture Organization of the United Nations (FAO). Cleaning and Sanitation in Meat Plants. Available online: http://www.fao.org/docrep/010/ai407e/AI407E26.htm (accessed on 10 September 2014).
- Al-Shaikhli, S. Optimisation of Coagulation/Flocculation Process Using Natural Coagulants in Raw Water Treatment. Master’s Thesis, The University of Southern Queensland, Toowoomba, Australia, 2013. [Google Scholar]
- De Haas, D.; Dancey, M. Wastewater Treatment Energy Efficiency, a Review with Current Australian Prespectives. Available online: http://www.watercentre.org/awa-journal-november-2015-article/ (accessed on 10 April 2017).
- Moore, L. Energy Use at Water and Wastewater Treatment Plants. Available online: http://www.tnenvironment.com/Pres12/MooreL.pdf (accessed on 10 September 2014).
- Tee, P.F.; Abdullah, M.O.; Tan, I.A.W.; Rashid, N.K.A.; Amin, M.A.M.; Nolasco-Hipolito, C.; Bujang, K. Review on hybrid energy systems for wastewater treatment and bio-energy production. Renew. Sustain. Energy Rev. 2016, 54, 235–246. [Google Scholar] [CrossRef]
- Hamawand, I. Anaerobic digestion process and bio-energy in meat industry: A review and a potential. Renew. Sustain. Energy Rev. 2015, 44, 37–51. [Google Scholar] [CrossRef]
- Kroyer, G. Impact of food processing on the environment—An overview. LWT Food Sci. Technol. 1995, 28, 547–552. [Google Scholar] [CrossRef]
- Luoa, J.; Lindsey, S.; Xueb, J. Irrigation of meat processing wastewater onto land. Agric. Ecosyst. Environ. 2004, 103, 123–148. [Google Scholar] [CrossRef]
- Sampson, B.; Hilless, K.; Laganistra, M. Wastewater, Report by GHD Pty Ltd, Brisbane, Australia. Available online: http://www.ampc.com.au/site/assets/media/reports/Resources/Wastewater-enviromental-best-practice-manual.pdf (accessed on 12 August 2014).
- Aboulhassan, M.A.; Souabi, S.; Yaacoubi, A.; Baudu, M. Removal of surfactant from industrial wastewaters by coagulation flocculation process. Int. J. Environ. Sci. Technol. 2006, 3, 327–332. [Google Scholar] [CrossRef]
- Johns, M.R. Developments in wastewater treatment in the meat processing industry: A review. Bioresour. Technol. 1995, 54, 203–216. [Google Scholar] [CrossRef]
- Husband, P. Physical-Chemical Treatment, CSIRO Meat Research Laboratory. Available online: http://www.meatupdate.csiro.au/data/Waste_water_and_odour_07.pdf (accessed on 17 July 2014).
- Long, J.H.; Aziz, T.N.; Francis, L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Pittaway, P. Using covered anaerobic ponds to treat abattoir wastewater, reduce greenhouse gases and generate bio energy. In MLA Publication A.ENV.0107; AMPC: Sydney, Australia, 2011. [Google Scholar]
- Australian Meat Processor Corporation. Waste Solids. Available online: http://www.ampc.com.au/site/assets/media/reports/Resources/Wastesolids-enviromental-best-practice-manual.pdf (accessed on 10 September 2014).
- Lawrence, M. Treatment Slaughterhouse Wastewater, North Dakota State University, Power Point Slides. Available online: www.ndsu.edu/.../Treatment%20of%20Slaughterhouse%20Wastewater_ (accessed on 19 July 2014).
- Waeger, F.; Delhaye, T.; Fuchs, W. The use of ceramic microfiltration and ultrafiltration membranes for particle removal from anaerobic digester effluents. Sep. Purif. Technol. 2010, 73, 271–278. [Google Scholar] [CrossRef]
- Avula, R.Y.; Nelson, H.M.; Singh, R.K. Recycling of poultry process wastewater by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2009, 10, 1–8. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Saez, J.; Lorens, M.; Soler, A.; Ortuno, J.F. Nutrient removal and sludge production in the coagulation–flocculation process. Water Res. 2001, 36, 2910–2919. [Google Scholar] [CrossRef]
- Satyanarayan, S.; Ramakant; Vanerkar, A.P. Conventional approach for abattoir wastewater treatment. Environ. Technol. 2005, 26, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Mittal, G. Treatment of wastewater from abattoirs before land application—A review. Bioresour. Technol. 2006, 97, 1119–1135. [Google Scholar] [CrossRef] [PubMed]
- Bazrafshan, E.; Biglari, H.; Mostafapour, F.K. Determination of hydrophobic and hydrophilic fractions of natural organic matter in raw water of Zahedan water treatment plant. J. Health Scope 2012, 1, 25–28. [Google Scholar] [CrossRef]
- Al-Mutairi, N.Z. Coagulant toxicity and effectiveness in a slaughterhouse wastewater treatment plant. Ecotoxicol. Environ. Saf. 2006, 65, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Arvanitoyannis, I.S.; Ladas, D. Meat waste treatment methods and potential uses. Int. J. Food Sci. Technol. 2008, 43, 543–559. [Google Scholar] [CrossRef]
- Amuda, O.S.; Alade, A. Coagulation/flocculation process in the treatment of abattoir wastewater. Desalination 2006, 196, 22–31. [Google Scholar] [CrossRef]
- Aguilar, M.I.; Saez, J.; Llorens, M.; Soler, A.; Ortuno, J.F. Microscopic observation of particle reduction in slaughterhouse wastewater by coagulation–flocculation using ferric sulfate as coagulant and different coagulant aids. Water Res. 2003, 37, 2233–2241. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Residual oil and suspended solid removal using natural adsorbents chitosan, bentonite and activated carbon: A comparative study. Chem. Eng. J. 2005, 108, 179–185. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Sumathi, S.; Hameed, B.H. Coagulation of residue oil and suspended solid in palm oil mill effluent by chitosan, alum and PAC. Chem. Eng. J. 2006, 118, 99–105. [Google Scholar] [CrossRef]
- Al-Juboori, R.A.; Aravinthan, V.; Yusaf, T.; Bowtell, L. Assessing the application and downstream effects of pulsed mode ultrasound as a pre-treatment for alum coagulation. Ultrason. Sonochem. 2016, 31, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Asselin, M.; Drogui, P.; Benmoussa, H.; Blais, J.F. Effectiveness of electrocoagulation process in removing organic compounds from slaughterhouse wastewater using monopolar and bipolar electrolytic cells. Chemosphere 2008, 72, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M.; Senturk, E.; Bayramoglu, M. Treatment of poultry slaughterhouse wastewaters by electrocoagulation. J. Hazard. Mater. 2006, 133, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, M.; Kobya, M.; Eyvaz, M.; Senturk, E. Technical and economic analysis of electrocoagulation for the treatment of poultry slaughterhouse wastewater. Sep. Purif. Technol. 2006, 51, 404–408. [Google Scholar] [CrossRef]
- Masse, D.I.; Masse, L. Characterization of wastewater from hog slaughterhouses in Eastern Canada and evaluation of their in-plant wastewater treatment systems. Can. Agric. Eng. 2000, 42, 139–146. [Google Scholar]
- Karpati, A.; Szabo, L. Suitable pretreatment of sewage resulting in pollution drop in meat processing. In Food Industries and the Environment International Syrup; Hollo, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1984; pp. 367–376. [Google Scholar]
- Travers, S.; Lovett, D. Pressure flotation of slaughterhouse wastewaters using carbon dioxide. Water Resour. Res. 1985, 19, 1479–1482. [Google Scholar]
- Edgerton, B. Bioenergy Commercialisation for Australia’s Dairy Industry; Australian Government, RIRDC: Wagga Wagga, Australian, 2009.
- Petrury, R.; Lettinga, G. Digestion of a milk-fat emulsion. Bioresour. Technol. 1997, 61, 141–149. [Google Scholar] [CrossRef]
- Cuetos, M.J.; Gomez, X.; Otero, M.; Moran, A. Anaerobic digestion and co-digestion of slaughterhouse waste (SHW): Influence of heat and pressure pre-treatment in biogas yield. Waste Manag. 2010, 30, 1780–1789. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Oliveira, R.; Alves, M.M. Co-digestion of cow manure, food waste and intermittent input of fat. Bioresour. Technol. 2009, 100, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Q.; Fu, G.; Li, Y. Semi-continuous anaerobic co-digestion of thickened waste activated sludge and fat, oil and grease. Waste Manag. 2011, 31, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Nakhla, G.; Al-Sabawi, M.; Bassi, A.; Liu, V. Anaerobic treatability of high oil and grease rendering wastewater. J. Hazard. Mater. 2003, B102, 243–255. [Google Scholar] [CrossRef]
- Gavala, H.N.; Ahring, B.K. Inhibition of the anaerobic digestion process by linear alkylbenzene sulfonates. Biodegradation 2002, 13, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.T.; Campos, E.; Sánchez-Leal, J.; Ribosa, I. Effect of linear alkylbenzene sulphonates (LAS) on the anaerobic digestion of sewage sludge. Water Res. 2006, 40, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Armendáriz, B.; Martínez-Carrera, D.; Calixto-Mosqueda, M.; Alba, J.; Rodríguez-Vázquez, R. Filamentous fungi remove weathered hydrocarbons from polluted soil of tropical México. Rev. Int. Contam. Ambient. 2010, 26, 193–199. [Google Scholar]
- Muller, M.T. Anaerobic Biodegradation and Toxicity of Alcohol Ethoxylates. Ph.D. Thesis, ETH Zurich, Switzerland, 2000. [Google Scholar]
- Labatut, R.A.; Gooch, C.A. Monitoring of Anaerobic Digestion Process to Optimize Performance and Prevent System Failure. Available online: https://dspace.library.cornell.edu/bitstream/1813/36531/1/21.Rodrigo.Labatut.pdf (accessed on 01 October 2014).
- Novak, J.T.; Park, C.-M. Effect of Aluminium and Iron on Odors, Digestion Efficiency, and Dewatering Properties Phase Iv’; IWA Publishing: London, UK, 2010. [Google Scholar]
- Warman, R.W. Effects on Anaerobic Digestion of Employing Polyelectrolytes and Ferric Chloride as Aids to Clarification of Domestic Sewage’. Master’s Thesis, The University of British Colombia, West Mall, Vancouver, BC, Canada, 1975. [Google Scholar]
- Marianna, M.; Attila, T.; Viktória, O. Digestate: A new nutrient source—Review. In Biogas; Kumar, S., Ed.; InTech: Rijeka, Croatia, 2012; ISBN 978-953-51-0204-5. [Google Scholar]
- Edalatmanesh, M.; Dhib, R.; Mehrvar, M. Kinetic modeling of aqueous phenol degradation by UV/H2O2 process. Inc. Int. J. Chem. Kinet. 2008, 40, 34–43. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Zhang, Y.; Yu, T.; Kamagata, Y. Nitrification performance and microbial community dynamics in a submerged membrane bioreactor with complete sludge retention. J. Biotechnol. 2006, 123, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Henry, L.G.; Liu, R.; Huang, X.; Zhan, X. Nitrogen removal from slaughterhouse wastewater through partial nitrification followed by denitrification in intermittently aerated sequencing batch reactors at 11 °C. Environ. Technol. 2014, 35, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Hartnett, J.P.; Fridman, A.; Cho, Y.I.; Greene, G.A.; Bar-Cohen, A. (Eds.) Transport Phenomena in Plasma; Elsevier Science: London, UK, 2007. [Google Scholar]
- Chu, H.-Q.; Cao, D.-W.; Jin, W.; Dong, B.-Z. Characteristics of bio-diatomite dynamic membrane process for municipal wastewater treatment. J. Membr. Sci. 2008, 325, 271–276. [Google Scholar] [CrossRef]
- Pollice, A.; Laera, G.; Saturno, D.; Giordano, C. Effects of sludge retention time on the performance of a membrane bioreactor treating municipal sewage. J. Membr. Sci. 2008, 317, 65–70. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Sroka, E. Integrated system of activated sludge–reverse osmosis in the treatment of the wastewater from the meat industry. Process Biochem. 2005, 40, 1517–1523. [Google Scholar] [CrossRef]
- López-López, A.; Vallejo-Rodríguez, R.; Méndez-Romero, D.C. Evaluation of a combined anaerobic and aerobic system for the treatment of slaughterhouse wastewater. Environ. Technol. 2010, 31, 319–326. [Google Scholar] [CrossRef] [PubMed]
- McPhail, N. Review of Removal of Fats, Oil and Greases from Effluents from Meat Processing Plants; Australian Meat Processor Corporation (AMPC) Report; AMPC: Sydney, Australia, 2015. [Google Scholar]
- Gao, J.L.; Oloibiri, V.; Chys, M.; Wandel, S.D.; Decostere, B.; Audenaert, W.; He, Y.L.; Van Hulle, S.W.H. Integration of autotrophic nitrogen removal, ozonation and activated carbon filtration for treatment of landfill leachate. Chem. Eng. J. 2015, 275, 281–287. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, C.; Rocha, K.C.; El-Din, M.G.; Liu, Y. Treatment of oil sands process-affected water using moving bed biofilm reactors: With and without ozone pretreatment. Bioresour. Technol. 2015, 192, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Diya’uddeen, B.H.; Rahim Pouran, S.; Abdul Aziz, A.R.; Nashwan, S.M.; Wan Daud, W.M.A.; Shaaban, M.G. Hybrid of Fenton and sequencing batch reactor for petroleum refinery wastewater treatment. J. Ind. Eng. Chem. 2015, 25, 186–191. [Google Scholar] [CrossRef]
- Bustillo-Lecompte, C.F.; Mehrvar, M.; Quiñones-Bolaños, E. Combined anaerobic-aerobic and UV/H2O2 processes for the treatment of synthetic slaughterhouse wastewater. J. Environ. Sci. Health Part A 2013, 48, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Lecompte, C.F.; Mehrvar, M.; Quiñones-Bolaños, E. Cost-effectiveness analysis of TOC removal from slaughterhouse wastewater using combined anaerobic–aerobic and UV/H2O2 processes. J. Environ. Manag. 2014, 134, 145–152. [Google Scholar] [CrossRef] [PubMed]
- De Nardi, I.R.; Del Nery, V.; Amorim, A.K.B.; dos Santos, N.G.; Chimenes, F. Performances of SBR, chemical–DAF and UV disinfection for poultry slaughterhouse wastewater reclamation. Desalination 2011, 269, 184–189. [Google Scholar] [CrossRef]
- Kim, B.; Gautier, M.; Prost-Boucle, S.; Molle, P.; Michel, P.; Gourdon, R. Performance evaluation of partially saturated vertical-flow constructed wetland with trickling filter and chemical precipitation for domestic and winery wastewaters treatment. Ecol. Eng. 2014, 71, 41–47. [Google Scholar] [CrossRef]
- Li, W.; Krantz, W.B.; Cornelissen, E.R.; Post, J.W.; Verliefde, A.R.D.; Tang, C.Y. A Novel Hybrid Process of Reverse Electrodialysis and Reverse Osmosis for Low Energy Seawater Desalination and Brine Management. Appl. Energy 2013, 104, 592–602. [Google Scholar] [CrossRef]
- Kong, W.; Guo, Q.; Wang, X.; Yue, X. Electricity generation from wastewater using an anaerobic fluidized bed microbial fuel cell. Ind. Eng. Chem. Res. 2011, 50, 12225–12232. [Google Scholar] [CrossRef]
- Liu, X.-W.; Wang, Y.-P.; Huang, Y.-X.; Sun, X.-F.; Sheng, G.-P.; Raymond, J.; Zeng, F.-L.; Dong, F.; Wang, S.-G.; Tong, Z.-H.; et al. Integration of a microbial fuel cell with activated sludge process for energy-saving wastewater treatment: Taking a sequencing batch reactor as an example. Biotechnol. Bioeng. 2011, 108, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Australian Meat Accreditation Listing. Available online: https://www.ausmeat.com.au/docs/AUS-MEAT%20Accreditation%20Listing.pdf (accessed on 12 November 2014).
- Kaygusuz, K. Energy for sustainable development: A case of developing countries. Renew. Sustain. Energy Rev. 2012, 16, 1116–1126. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, H.; Chen, S.; Dichtl, N.; Dai, X.; Li, N. Effects of thermal hydrolysis on organic matter solubilization and anaerobic digestion of high solid sludge. Chem. Eng. J. 2015, 264, 174–180. [Google Scholar] [CrossRef]
- Delforno, P.T.; Júnior, L.V.G.; Noronha, F.M.; Sakamoto, K.I.; Varesche, A.B.A.; Oliveira, M.V. Microbial diversity of a full-scale UASB reactor applied to poultry slaughterhouse wastewater treatment: Integration of 16S rRNA gene amplicon and shotgun metagenomic sequencing. MicrobiologyOpen 2017. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Lecompte, C.F.; Mehrvar, M. Treatment of actual slaughterhouse wastewater by combined anaerobic–aerobic processes for biogas generation and removal of organics and nutrients: An optimization study towards a cleaner production in the meat processing industry. J. Clean. Prod. 2017, 141, 278–289. [Google Scholar] [CrossRef]
- Jenicek, P.; Kutil, J.; Benes, O.; Todt, V.; Zabranska, J.; Dohanyos, M. Energy self sufficient sewage wastewater treatment plants: Is optimized anaerobic sludge digestion the key? Water Sci. Technol. 2013, 68, 1739–1744. [Google Scholar] [CrossRef] [PubMed]
- Davarnejad, R.; Nasiri, S. Slaughterhouse wastewater treatment using an advanced oxidation process: Optimization study. Environ. Pollut. 2017, 223, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sheboygan-WWTP. Available online: http://www.sheboyganwwtp.com/index.php (accessed on 1 April 2017).
- Wang, H.; Yang, Y.; Keller, A.A.; Li, X.; Feng, S.; Dong, Y.; Li, F. Comparative analysis of energy intensity and carbon emissions in wastewater treatment in USA, Germany, China and South Africa. Appl. Energy 2016, 184, 873–881. [Google Scholar] [CrossRef]
- BMZ. Climate Change Mitigation in the Water Sector. 2012. Available online: http://agriwaterpedia.info/images/9/9e/00_GIZ_Climate_Change_Mitigation_in_the_Water_Sector.pdf (accessed on 28 January 2015).
- De Haas, D.W.; Foley, J.; Marshall, B.; Dancey, M.; Vierboom, S.; Bartle-Smith, J. Benchmarking wastewater treatment plant energy use in Australia. In Proceedings of the Ozwater’15 Conference, Adelaide, Australia, 12–14 May 2015. [Google Scholar]
- Ge, H.; Batstone, D.J.; Keller, J. Operating aerobic wastewater treatment at very short sludge ages enables treatment and energy recovery through anaerobic sludge digestion. Water Res. 2013, 47, 6546–6557. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Li, Y.; Li, X.; Luo, P.; Wang, H.; Robinson, P.Z.; Wang, X.; Wu, J.; Li, F. The feasibility and challenges of energy self-sufficient wastewater treatment plants. Appl. Energy 2017. [Google Scholar] [CrossRef]
- Bodik, I.; Kubaská, M. Energy and sustainability of operation of a wastewater treatment plant. Environ. Prot. Eng. 2013, 39, 15–24. [Google Scholar]
- Kenway, S.J.; Priestley, A.; Cook, S.; Seo, S.; Inman, M.; Gregory, A.; Hall, M. Energy Use in the Provision and Consumption of Urban Water in Australia and New Zealand. Available online: http://www.clw.csiro.au/publications/waterforahealthycountry/2008/wfhc-urban-water-energy.pdf (accessed on 1 April 2017).
- Mizuta, K.; Shimada, M. Benchmarking energy consumption in municipal wastewater treatment plants in Japan. Water Sci. Technol. 2010, 62, 2256–2262. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zeng, S.; Chen, J.; He, M.; Yang, W. Operational energy performance assessment system of municipal wastewater treatment plants. Water Sci. Technol. 2010, 62, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Kim, B.H.; Yaakob, Z.; Daud, W.R.W. Activated carbon nanofibers as an alternative cathode catalyst to platinum in a two-chamber microbial fuel cell. Int. J. Hydrogen Energy 2011, 36, 746–752. [Google Scholar] [CrossRef]
- Ghasemi, M.; Daud, W.R.W.; Hassan, H.S.A.; Oh, S.-E.; Ismail, M.; Rahimnejad, M.; Jahim, J.M. Nano-structured carbon as electrode material in microbial fuel cells: A comprehensive review. J. Alloy. Compd. 2013, 580, 245–255. [Google Scholar] [CrossRef]
- Lim, S.S.; Daud, W.R.W.; Md Jahim, J.; Ghasemi, M.; Chong, P.S.; Ismail, M. Sulfonated poly(ether ketone)/poly(ether sulfone) composite membranes as an alternative proton exchange membrane in microbial fuel cells. Int. J. Hydrogen Energy 2012, 37, 11409–11424. [Google Scholar] [CrossRef]
- McCarty, P.L.; Bae, J.; Kim, J. Domestic wastewater treatment as a net energy producer-can this be achieved? Environ. Sci. Technol. 2011, 45, 7100–7106. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Carliell-Marquet, C.; Kansal, A. Energy pattern analysis of a wastewater treatment plant. Appl. Water Sci. 2012, 2, 221–226. [Google Scholar] [CrossRef]
- Chuentongaram, Y. Chemical Treation of the Slaughterhouse Wastewater with Chitosan and/or Ferric Chloride as a Preliminary Treatment. Master’s Thesis, Mahidol University, Salaya, Thailand, 2004. [Google Scholar]
- Morales, A.F.; Mensez, N.R.; Tamayo, D.M. Treatment of Slaughterhouse Wastewater Using Moringa Oleifera Lam Seeds as Coagulant. Tropical and Subtropical Agroecosystems, North America. 2009. Available online: http://www.veterinaria.uady.mx/ojs/index.php/TSA/article/view/282 (accessed on 26 August 2014).
- Lagasi, J.E.; Agunwamba, J.C.; Aho, M. Comparative studies on the use of ordinary and de-oiled moringa oleifera in the treatment of abattoir waste water. Int. J. Eng. Sci. (IJES) 2014, 3, 1–7. [Google Scholar]
- Khwaja, A.R.; Vasconcellos, S.R. Methods for Recovering Tallow from Wastewater. U.S. Patent 7,943,048 B2, 17 May 2011. [Google Scholar]
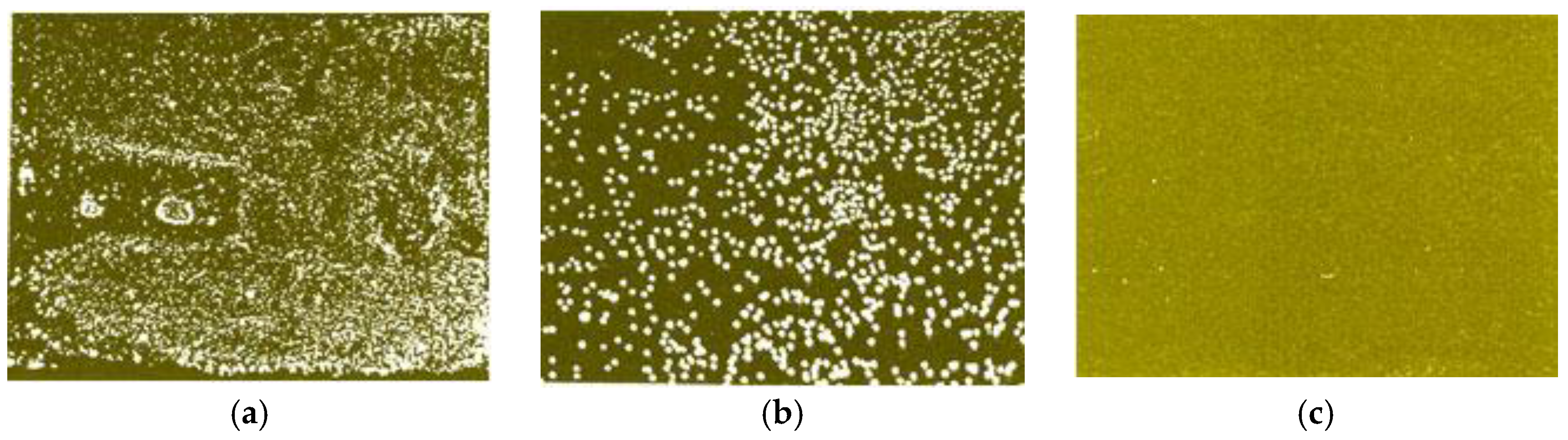



| Symbols, Units | Parameter | Range | Mean |
|---|---|---|---|
| TOC (mg/L) | Total Organic Carbon | 70–1200 | 546 |
| BOD5 (mg/L) | Biological Oxygen Demand | 150–4635 | 1209 |
| COD (mg/L) | Chemical Oxygen Demand | 500–15,900 | 4221 |
| TN (mg/L) | Total Nitrogen | 50–841 | 427 |
| TSS (mg/L) | Total Suspended Solid | 270–6400 | 1164 |
| pH | pH | 4.90–8.10 | 6.95 |
| TP (mg/L) | Total Phosphorus | 25–200 | 50 |
| Ortho-PO4 (mg/L) | Orthophosphate | 20–100 | 25 |
| Orhto-P2O5 (mg/L) | - | 10–80 | 20 |
| K (mg/L) | Potassium | 0.01–100 | 90 |
| Colour (mg/L Pt scale) | - | 175–400 | 290 |
| Turbidity (FAU) | - | 200–300 | 275 |
| Type | SS | BOD5 | FOG |
|---|---|---|---|
| Sewer disposal pollutant limits | <1000–<1500 mg/L | <300–<3000 mg/L | <50–<200 mg/L |
| Surface water disposal pollutant limits | <10–<15 mg/L | <5–<10 mg/L | −15 mg/L |
| Cleaning Agent | Purpose | Chemicals |
|---|---|---|
| Alkaline | Generally suitable for removing organic dirt, protein residues and fat. | Sodium hydroxide (caustic soda), sodium carbonate (soda ash), and sodium metasilicate. |
| Acid | Used particularly for removal of encrusted residues of dirt or protein or inorganic deposits (“scaling”). | Inorganic acids: phosphoric acid, nitric acid, sulphuric acid and hydrochloric acid. Organic acids: gluconic acid, tartaric acid, citric acid, acetic acid and sulphamic acid. |
| Neutral | Less effective than alkaline or acid cleaning agents, but have mild impact on skin and materials and are useful for manual cleaning of smooth surfaces without encrusted dirt. | Silicates may be used as anti-corrosive agents in alkaline detergents but will deposit on stainless steel and it is therefore important to know on which materials to use. |
| Foam cleaning | A relatively new cleaning method, in particular for larger-scale plants. | n/a |
| Detergents | Used to improve dirt loosening properties. | Anionic, nonionic and cationic surface active agents. |
| Name | Chemicals | Use | Substances | Hazardous and Dangerous Status |
|---|---|---|---|---|
| Alkaline, TOPAX 625 | Sodium hydroxide solution | Cleaning product | Up to 3% in water. Sodium hydroxide <10%, sodium hypochlorite <10%, sodium metasilicate <10% | Classified as hazardous substance and dangerous goods |
| Acid, TOPAX 56 | Phosphoric acid solution | Cleaning product | Up to 3% in water. (2-(2-butoxyethoxy) ethanol <10%, phosphoric acid 10–30%, isotridecyl ester <10% | Classified as hazardous substance and dangerous goods |
| Disinfectant | Composition | Purpose |
|---|---|---|
| Chlorine containing compounds | Na- or Ca-hypochlorite (Na/Ca O Cl), gaseous chlorine (Cl2) (Hypochlorous acid) | Effective against a wide range of bacteria, penetrates cell walls, but has a corroding effect on equipment |
| Aldehydes | Formaldedyde, phenoles/kresols, alcohols, alkalines (pH 10 or higher) (e.g., NaOH), acids (some organic acids) | Destruction of microorganisms, may be corrosive |
| Quaternary ammonium compounds (QUATS) | Amphotensids | Effect on cell walls, not corrosive, odourless, additional cleaning properties (surfactant) |
| Oxygen releasing substances | Peroxide compounds (H2O2), per-acetic acid | Penetrate into cells, good effect on all microorganisms including spores and virus, odourless, may be corrosive at concentrations greater than 1% |
| Name | Sanitizing Agents | Use | Substances | Hazardous and Dangerous Status |
|---|---|---|---|---|
| SANIMAXX | n/a | Sanitizer | Up to 3% in water. Quaternary ammonium compound, di-c8-10-alkyldimethyl chlorides <10%, quaternary ammonium compounds, benzyl-c12-c16-alkyldimethyl, chloride <10%. | Classified as hazardous substance and not dangerous goods |
| XY-12 | Hypochlor-ite solution | Sanitizer | Up to 3% in water. Sodium hypochlorite 10–30% | Classified as non hazardous substance and non dangerous goods |
| Treatment Method | Performance | Energy Consumption |
|---|---|---|
| Coarse and fine screening | The first step involves coarse screening so that large particles (above 1 cm) are removed. This is important to prevent accumulation of these particles which may disrupt mechanical equipment. Primary screening can remove 5–20% BOD and 5–30% TSS. | Low, no pumping is required |
| Primary sedimentation | Skimming and sedimentation processes are able to remove floating and sediment objects, e.g., 20% to 30% BOD, 40% to 50% TSS, and 50% to 60% grease. This process is more efficient than the screening unit but this comes with high capital, operation and maintenance costs. | Low, no pumping is required |
| Dissolved air flotation (DAF) | Usually before anaerobic treatment, the wastewater stream is diverted to the DAF unit so that blood, fat, oil and grease constituents are reduced. A dissolved air flotation (DAF) system can be used to continually or periodically recover fats and protein by scraping. If the dissolved air flotation process is controlled well, at least 30% to 35% removal of BOD, 60% removal of TSS and 80% of FOGs removal is achievable. | High, air pumping is required |
| Parameter | World Bank Standards | EU Standards | US Standards | Canadian Standards | Australian Standards |
|---|---|---|---|---|---|
| BOD (mg/L) | 30 | 25 | 26 | 5–30 | 6–10 |
| COD (mg/L) | 125 | 125 | n/a | n/a | 3 × BOD |
| TSS (mg/L) | 50 | 35 | 30 | 5–30 | 10–15 |
| TN (mg/L) | 10 | 10 | 8 | 1 | 0.1–15 |
| Coagulant | COD Removal Efficiency (%) | BOD Removal Efficiency (%) | TSS Removal Efficiency (%) |
|---|---|---|---|
| Al2(SO4)3 (Alum) | 33.1–87 | 30–88 | 31–97 |
| Fe2(SO4)3 (ferric sulphite) | 64–78 | 81–91 | 43–98 |
| PAX-18 | 69–80 | 45–79 | 57–97 |
| Al2(SO4)3 + AP | 46–87 | 62–90 | 86–97 |
| Fe2(SO4)3 + AP | 59–90 | 62–93 | 81–98 |
| PAX-18 + AP | 69–80 | 79–90 | 88–98 |
| Al2(SO4)3 + AP polyelectrolyte | 79.1 | 86.3 | 85.4 |
| Treatment | COD Removal Efficiency (%) | TSS Removal Efficiency (%) | Reference |
|---|---|---|---|
| DAF and chemicals | 32–92 | 70–97 | [37] |
| DAF at pH 4–4.5 | 71 | 78 | [38] |
| DAF and chemicals | 38–71 | 37–63 | [24] |
| DAF with air | 40 | 60 | [38] |
| WWTP | TS Removal (%) | VS Removal (%) | COD Removal (%) | Organic N Removal (%) | Iron Content (mg/g TS) |
|---|---|---|---|---|---|
| A | 30.4 | 39.4 | 52.0 | 26.1 | 39.6 |
| B | 29.7 | 36.7 | 45.4 | 12.6 | 37.4 |
| C | 27.1 | 42.5 | 62.7 | 44.3 | 41.2 |
| D | 19.9 | 26.6 | 68.0 | 32.1 | 1.87 |
| E | 32.5 | 43.9 | 65.3 | 41.2 | 8.7 |
| F | 39.4 | 47.2 | 35.9 | 42.4 | 15.42 |
| G | 31.2 | 37.8 | 49.8 | 50.4 | 38.2 |
| Abattoir | Cleaning Chemicals | WWT Chemicals | Comments |
|---|---|---|---|
| A | yes | No | Only one physical treatment unit (screening), then series of five anaerobic ponds and then a facultative pond |
| B | yes | No | Screening, DAF, anaerobic pond and a very long serpentine pond |
| C | yes | Yes, polymer for dewatering sludge | Tertiary screening, and anaerobic pond |
| D | yes | n/a | No treatment, just evaporation pond |
| E | yes | n/a | No treatment, just evaporation pond |
| F | yes | n/a | No treatment, just evaporation pond |
| G | yes | n/a | No treatment, just evaporation pond |
| H | yes | Yes, polymer (zeta) in the flocculating system | Screening, flocculating tank, DAF, anaerobic pond, storage tank which then used for irrigation |
| I | yes | No | Screening, DAF, two anaerobic pond parallel, aerobic pond, settling pond, water recycled for washing (cattle and yards) and watering grass and gardens, trucks washing and feedlots, the extra water go to pond five where the water used for irrigation of crops (crops for cattle feeds only). No water leaves the plants. |
| J | yes | Yes, aluminium sulphate and lime in the primary DAF and sodium hypochlorite in the tertiary DAF (pH control). | Screening, scrubbing, decanter, primary DAF, anaerobic and aerobic ponds, settling, tertiary DAF, chlorine |
| K | yes | Yes, 1. Coagulant Catfloc 2. Anionic flocculant 3. pH control sulphuric acid 98% sodium hydroxide 46% | In the DAF mixing tanks. |
| L | yes | Only chlorine | Screening (two), anaerobic and aerobic ponds, chlorine, and then for irrigation |
| M | yes | Yes, chemical DAF, ferric sulphate and anionic polymer | Shaker screening, balance tank, chemical DAF, equalizer tank, DAF aeration and then to sewage |
| Purpose | Lower Energy Intensity | Upper Energy Intensity | Energy Intensity Used for Projections |
|---|---|---|---|
| - | GJ/ML | GJ/ML | GJ/ML |
| Water Treatment and Pumping | - | - | - |
| Conventional water treatment plant | 0.36 | 1.8 | 1.08 |
| Conventional water pumping | 0.25 | 6.26 | 1.96 |
| Reverse osmosis on treated wastewater for reuse | 3.6 | 5.4 | 4.5 |
| Reverse osmosis on sea water | 12.6 | 14.4 | 13.5 |
| Pumping energy for reuse | 3.6 | 7.2 | 5.4 |
| Pumping energy for desalination | 3.6 | 7.2 | 5.4 |
| Waste water Treatment and Pumping | - | - | - |
| Primary wastewater treatment plant | 0.5 | 1.0 | 0.8 |
| Secondary wastewater treatment plant | 1.0 | 2.0 | 1.65 |
| Tertiary wastewater treatment plant | 2.0 | 5.0 | 3.25 |
| Conventional wastewater pumping | 0.25 | 1.55 | 0.74 |
| Unit | Electrical Energy (kWh/m3) | Manual Energy (kWh/m3) | Chemical Energy (kWh/m3) | Total Energy (kWh/m3) |
|---|---|---|---|---|
| Sump | 0.2 | 0.003 | - | 0.203 |
| Primary settling tank (PST) | 0.09 | 0.019 | 0.096 | 0.205 |
| Dosing Tank | 0.04 | 0.046 | - | 0.086 |
| Rotatory biological contractor (RBC) | 0.09 | 0.002 | - | 0.092 |
| Secondary settling tank (SST) | 0.17 | 0.008 | - | 0.178 |
| Disinfection tank | 0.03 | 0.006 | - | 0.036 |
| Sand filter | - | 0.01 | - | 0.01 |
| Carbon filter | - | 0.01 | - | 0.01 |
| Treated water tank | 0.18 | - | 0.003 | 0.183 |
| Sludge storage tank | - | 0.027 | - | 0.027 |
| Total | 0.8 | 0.131 | 0.099 | 1.03 |
| Some Natural Coagulants | Chemicals | Advantages | Disadvantages |
|---|---|---|---|
| Natural polymers | Sodium alginate | Can be effective when used with alum | Less efficient than synthetic polymers |
| Chitosan | Inexpensive additives for increasing settling velocity, and reducing coagulant dosage. | ||
| Starch | |||
| Moringa oleifera | |||
| Psyllium |
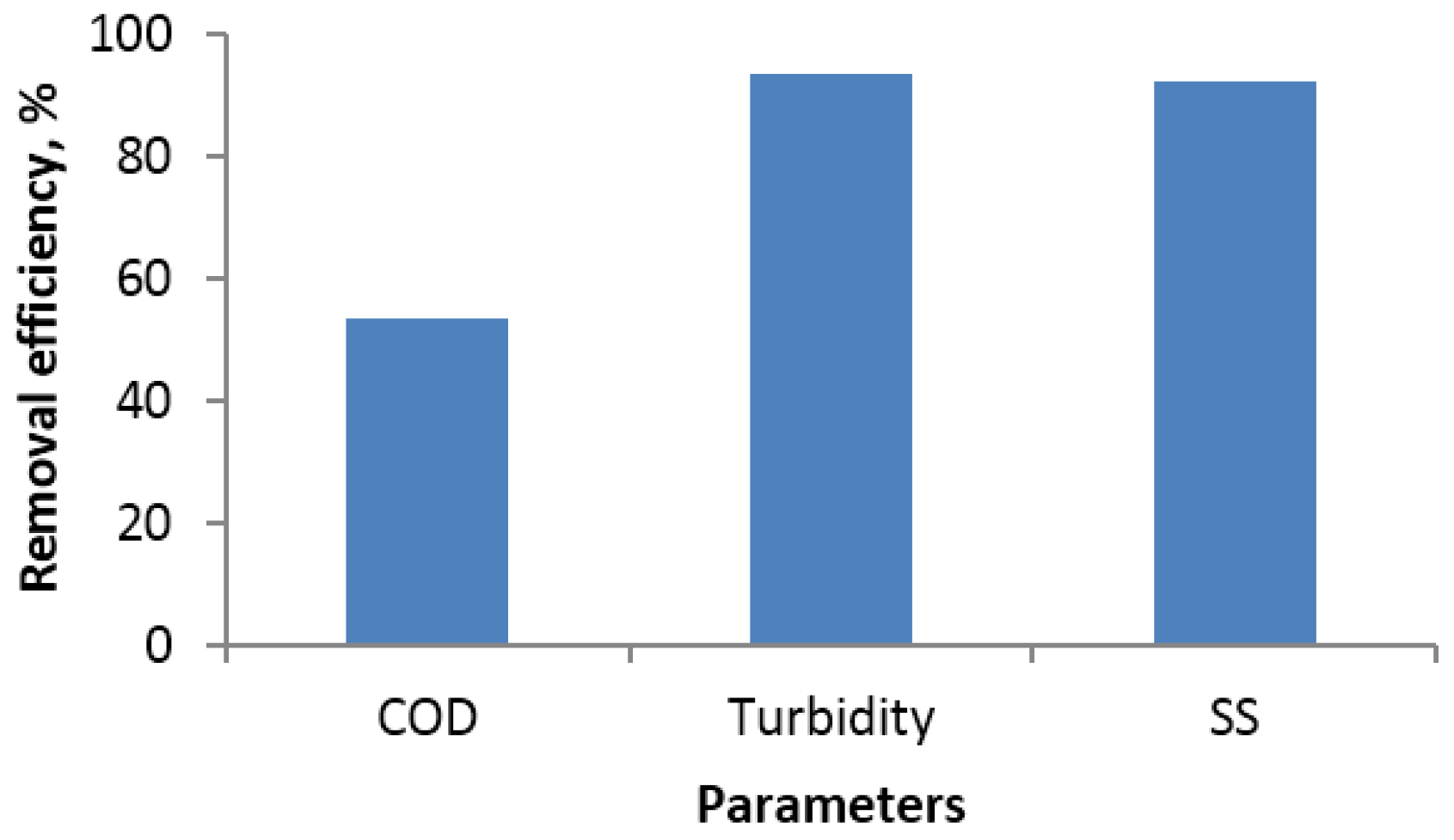
| Parameters | Influent (mg/L) | Reduction (%) |
|---|---|---|
| BOD5 | 3425 | 73 |
| TSS | 1230 | 76 |
| FOG | 1090 | 74 |
| TKN | 220 | 45 |
| TP | 64 | 27 |
| Chemicals | Dose (mg/L) | Dose (kg/m3) | Cost (AUD/t) | Cost (AUD/m3) | Consumed t/Year | Cost (AUD/Year) | Sludge (t/Year *) |
|---|---|---|---|---|---|---|---|
| Ferric chloride | 160 | 0.16 | 250 | 0.04 | 116 | 29,120 | 116 |
| Chitosan | 10 | 0.01 | 1500 | 0.015 | 7.3 | 10,920 | 7.3 |
| Total | - | - | - | 0.055 | 123.3 | 40,040 | 123.3 |
| Chemicals | Dose kg/m3 | Cost AUD/m3 | Sludge, kg/m3 | Efficiency COD % Removal | Impact |
|---|---|---|---|---|---|
| Alum | 0.75 | 0.11 | 0.75 | 65 | Alzheimer disease, rise wastewater pH, negative impact on the AD and high sludge volume |
| Ferric sulphate | 0.75 | 0.19 | 0.75 | 65 | Positive impact on the AD |
| Ferric sulphate + Anionic polymer (AP) | 0.375 | 0.34 * | 0.375 | 46–87 | Polymer has toxic impact on AD, low sludge volume |
| 0.012 | 0.069 * | 0.012 | |||
| Ferric chloride + Chitosan | 0.16 | 0.04 | 0.16 | 53.7 | Positive impact on the AD, low sludge volume |
| 0.01 | 0.015 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamawand, I.; Ghadouani, A.; Bundschuh, J.; Hamawand, S.; Al Juboori, R.A.; Chakrabarty, S.; Yusaf, T. A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry. Energies 2017, 10, 731. https://doi.org/10.3390/en10050731
Hamawand I, Ghadouani A, Bundschuh J, Hamawand S, Al Juboori RA, Chakrabarty S, Yusaf T. A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry. Energies. 2017; 10(5):731. https://doi.org/10.3390/en10050731
Chicago/Turabian StyleHamawand, Ihsan, Anas Ghadouani, Jochen Bundschuh, Sara Hamawand, Raed A. Al Juboori, Sayan Chakrabarty, and Talal Yusaf. 2017. "A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry" Energies 10, no. 5: 731. https://doi.org/10.3390/en10050731
APA StyleHamawand, I., Ghadouani, A., Bundschuh, J., Hamawand, S., Al Juboori, R. A., Chakrabarty, S., & Yusaf, T. (2017). A Critical Review on Processes and Energy Profile of the Australian Meat Processing Industry. Energies, 10(5), 731. https://doi.org/10.3390/en10050731








