Performance and Stability Enhancement of Perovskite-Type Nanomaterials Applied for Carbon Capture Utilizing Oxyfuel Combustion
Abstract
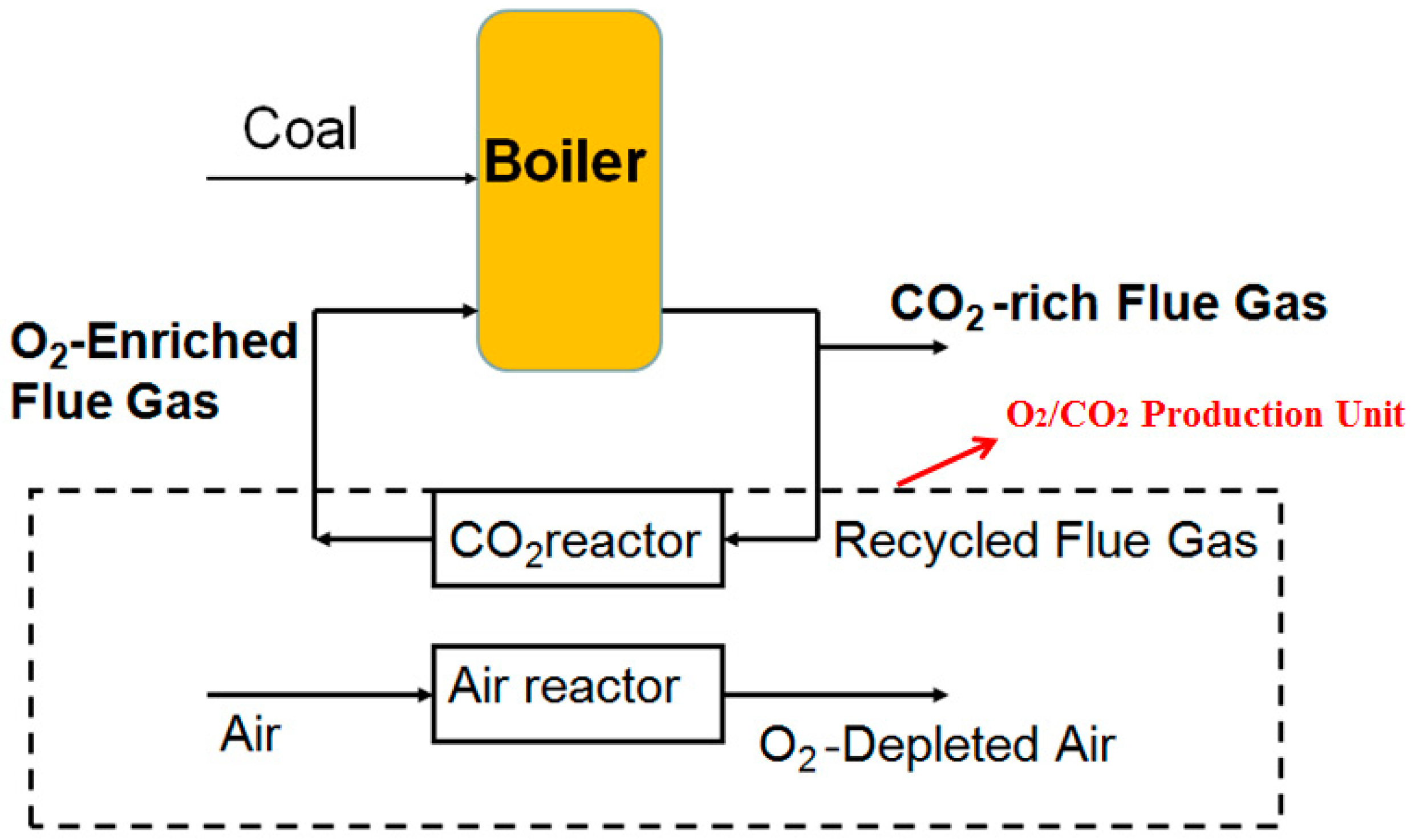
:1. Introduction
2. Experiments
2.1. Powder Synthesis
2.2. Fixed-Bed Experiments
3. Results and Discussion
3.1. Effects of A-Site Substitution on Oxygen Desorption Performance
3.2. Effects of B-Site Substitution on Oxygen Desorption Performance
3.3. Effects of Adsorption Time
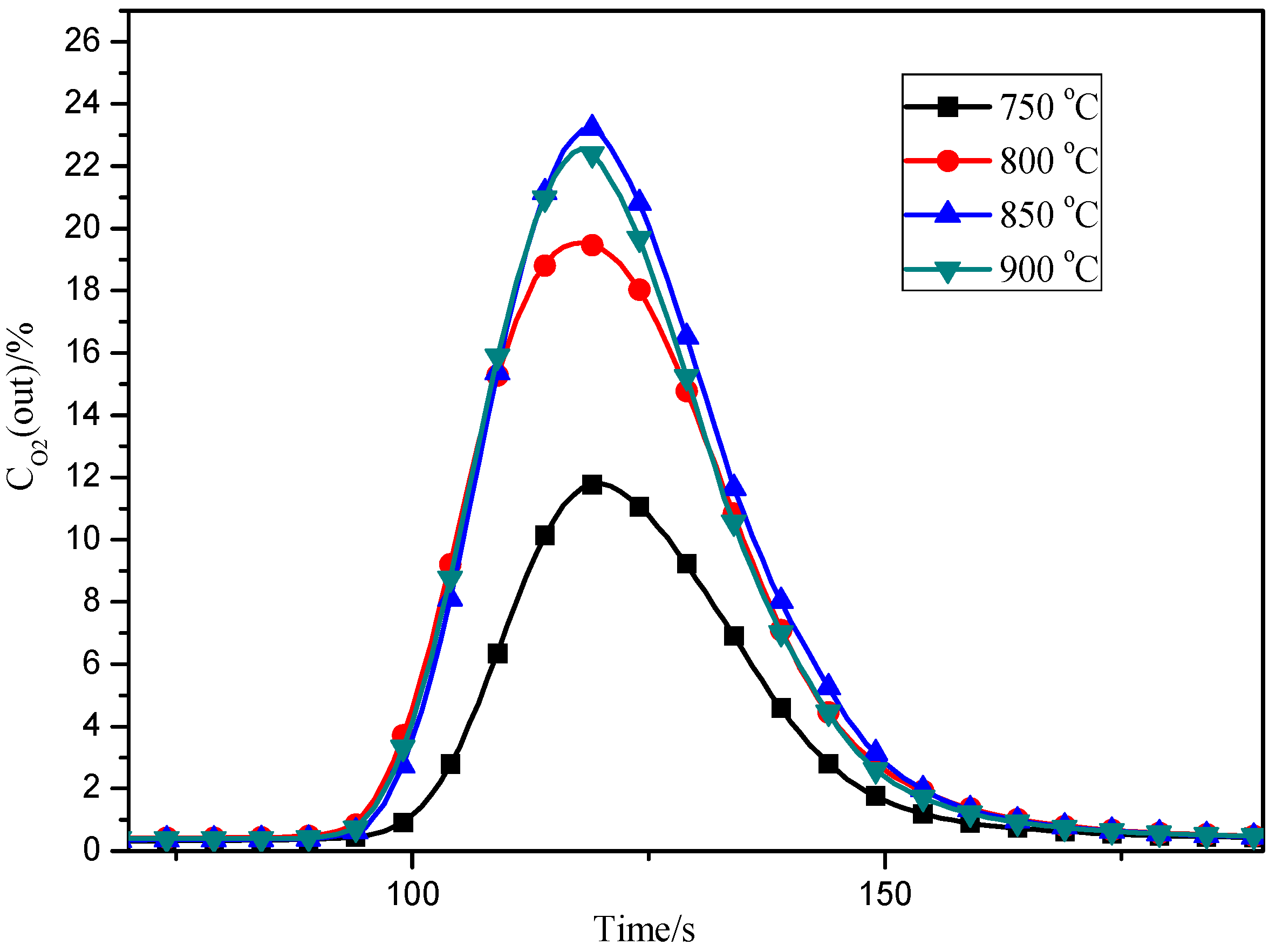
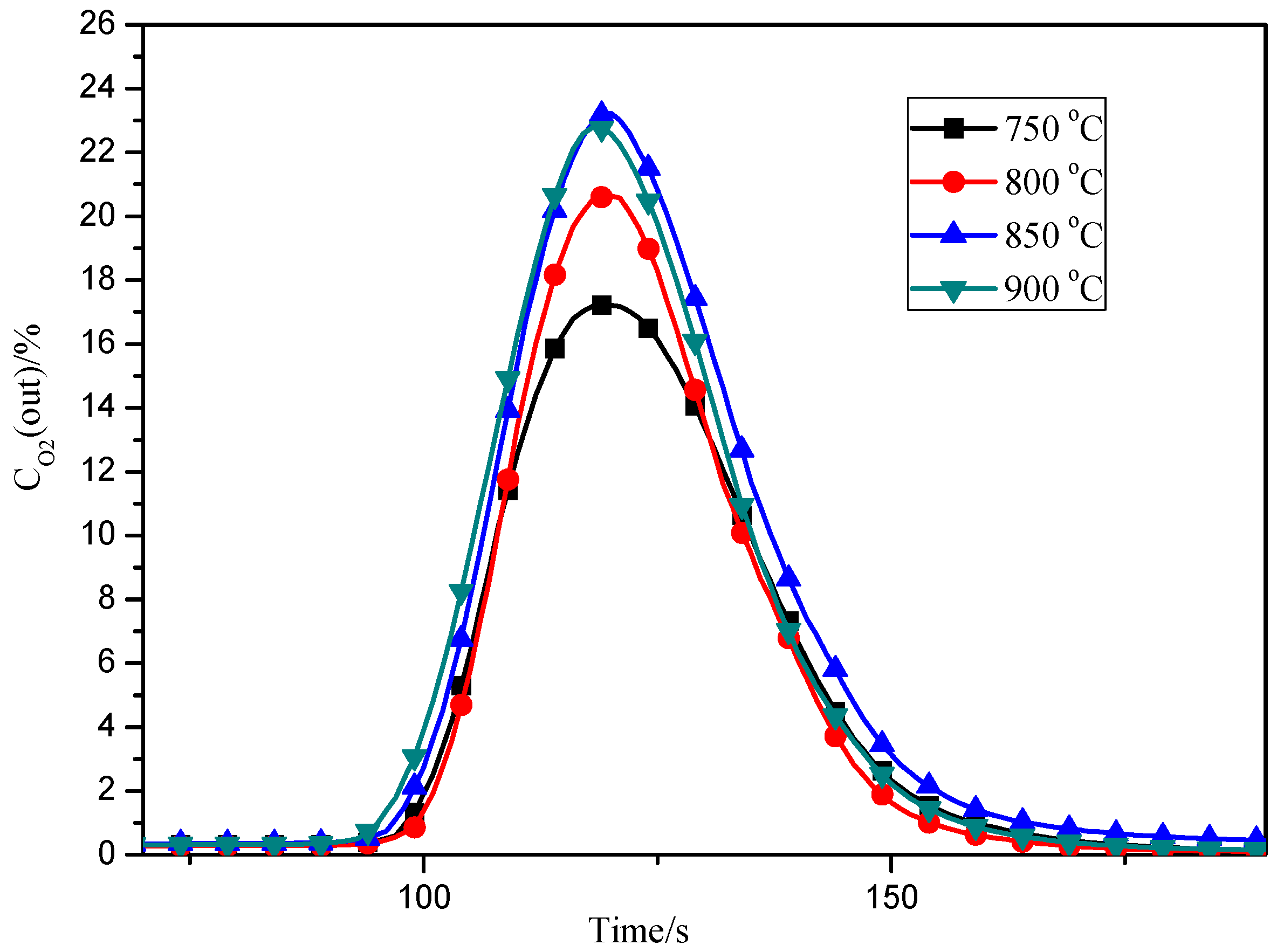
3.4. Effects of Adsorption Temperature
3.5. Effects of Desorption Temperature
3.6. Microstructure Analysis
4. Conclusions
- (1)
- The results showed that the oxygen desorption amount of A-site substitution was in the order: BaCoO3-δ > BaSrCoO3-δ > BaCaCoO3-δ >SrCoO3-δ > BaMgCoO3-δ > CaCoO3-δ > MgCoO3-δ. The substitution of Ba2+ with Sr2+/Ca2+/Mg2+ reduced the oxygen desorption amount for BaCoO3-δ.
- (2)
- The substitution of Co in the B-site with different transition metal ions had more significant effects on the oxygen desorption performance compared with A-site substitution. The B-site Co ion substituted by Cr, Cu, Fe, Mn, Ni, Zn, Zr reduced the oxygen desorption performance of BaCoO3-δ. It is indicated that BaCoO3-δ had the best oxygen desorption performance among the above A/B-site–substituted ACoO3-δ and BaBO3-δ.
- (3)
- The effects of the operation parameters on the oxygen desorption performance of BaCoO3-δ were investigated in detail. It was found that the optimal adsorption time, adsorption temperature and desorption temperature for BaCoO3-δ were determined to be 20 min, 850 °C and 850 °C, respectively, in this specific case.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, Q.W.; Zheng, Y.; Luo, C.; Zheng, C.G. Development and characterization of Ba1-xSrxCo0.8Fe0.2O3-δ perovskite for oxygen production in oxyfuel combustion system. Chem. Eng. J. 2014, 225, 462–470. [Google Scholar] [CrossRef]
- Wang, B.W.; Xiao, G.; Song, X.Y.; Zhao, H.B.; Zheng, C.G. Chemical looping combustion of high-sulfur coal with NiFe2O4-combined oxygen carrier. J. Therm. Anal. Calorim. 2014, 118, 1593–1602. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, Y.; Yin, J.J.; Qin, C.L.; Ding, N.; Zheng, C.G.; Feng, B. Effect of sulfation during oxy-fuel calcination stage in calcium looping on CO2 capture performance of CaO-based sorbent. Energy Fuels 2013, 27, 1008–1014. [Google Scholar] [CrossRef]
- Croiset, E.; Thambimuthu, K.V. NOx and SO2 emissions from O2/CO2 recycle coal combustión. Fuel 2001, 80, 2117–2121. [Google Scholar] [CrossRef]
- Buhre, B.J.P.; Elliott, L.K.; Sheng, C.D.; Gupta, R.P.; Wall, T.F. Oxy-fuel combustion technology for coal-fired power generation. Prog. Energy Combust. Sci. 2005, 31, 283–307. [Google Scholar] [CrossRef]
- Sun, M.; Chen, X.W.; Hong, L. Leveraging the A-site Ba2+–Sr2+ ratio in the designated perovskite to enhance oxygen transport and structural/interfacial stability. RSC Adv. 2014, 4, 5618–5625. [Google Scholar] [CrossRef]
- Liu, D.Y.; Gangishetty, M.K.; Kelly, T.L. Effect of CH3NH3PbI3 thickness on device efficiency in planar heterojunction perovskite solar cells. J. Mater. Chem. A 2014, 2, 19873–19881. [Google Scholar] [CrossRef]
- Yang, M.J.; Guo, R.; Kadel, K.; Liu, Y.Y.; O’Shea, K.; Bone, R.; Wang, X.W.; He, J.; Li, W.Z. Improved charge transport of Nb-doped TiO2 nanorods in methylammonium lead iodide bromide perovskite solar cells. J. Mater. Chem. 2014, 2, 19616–19622. [Google Scholar] [CrossRef]
- Sun, J.C.; Li, Y.; Ji, S.J.; Wen, Z.S. The performance of cathode material of perovskite-like La-Ni-M-O for low temperature soli d oxide fuel cell. Int. J. Energy Res. 2011, 35, 1032–1036. [Google Scholar] [CrossRef]
- Duan, L.Q.; Sun, S.Y.; Qu, W.J.; Bian, J. Study on different zero CO2 emission IGCC systems with CO2 capture by integrating OTM. Int. J. Energy Res. 2016, 40, 1410–1427. [Google Scholar] [CrossRef]
- Yang, Z.; Lin, Y.S. High-temperature sorption process for air separation and oxygen removal. Ind. Eng. Chem. Res. 2002, 41, 2775–2784. [Google Scholar] [CrossRef]
- Yang, Q.; Lin, Y.S. Improved sorbent for high-temperature production of oxygen-enriched carbon dioxide stream. Ind. Eng. Chem. Res. 2007, 46, 6025–6031. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.M.; Furukawa, S.; Yamazoe, N. Oxygen permeation through perovskite-type oxides. Chem. Lett. 1985, 11, 1743–1746. [Google Scholar] [CrossRef]
- Rui, Z.B.; Ding, J.J.; Li, Y.D. SrCo0.8Fe0.2O3-δsorbent for high-temperature production of oxygen-enriched carbon dioxide atream. Fuel 2010, 89, 1429–1434. [Google Scholar] [CrossRef]
- Guntuka, S.; Banerjee, S.; Farooq, S.; Srinivasan, M.P. A- and B-site substituted lanthanum cobaltite perovskite as high temperature oxygen sorbent. Ind. Eng. Chem. Res. 2008, 47, 154–162. [Google Scholar] [CrossRef]
- Shen, Q.W.; Zheng, Y.; Li, S.A.; Ding, H.R.; Xu, Y.Q.; Zheng, C.G.; Thern, M. Optimize process parameters of microwave-assisted EDTA method using orthogonal experiment for novel BaCoO3-δ perovskite. J. Alloys Compd. 2016, 658, 125–131. [Google Scholar] [CrossRef]
- Zheng, J.Q.; Zhu, Y.J.; Xu, J.S.; Lu, B.Q.; Qi, C.; Chen, F.; Wu, J. Microwave-assisted rapid synthesis and photocatalytic activity of mesoporous Nd-doped SrTiO3 nanospheres and nanoplates. Mater. Lett. 2013, 100, 62–65. [Google Scholar] [CrossRef]
- Lin, S.L.; Fuh, M.R. Orthogonal array optimization of ultrasound-assisted emulsification–microextraction for the determination of chlorinated phenoxyacetic acids in river water. J. Chromatogr. A 2010, 1217, 3467–3472. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, S.; Mortalo, C.; Fasolin, S. Influence of Microwave-Assisted Pechini Method on La0.80Sr0.20Ga0.83Mg0.17O3–δ Ionic Conductivity. Fuel Cells 2012, 12, 54–60. [Google Scholar] [CrossRef]
- Farahmand, F.; Moradkhani, D.; Safarzadeh, M.S.; Rashchi, F.; Farahmand, F.; Moradkhani, D.; Safarzadeh, M.S.; Rashchi, F. Brine leaching of lead-bearing zinc plant residues: Process optimization using orthogonal array design methodology. Hydrometallurgy 2009, 95, 316–324. [Google Scholar] [CrossRef]
- Yang, F.; Huang, J.L.; Wuban, T.O.; Hong, Y.L.; Du, M.M.; Sun, D.H.; Jia, L.S.; Li, Q.B. Efficient Ag/CeO2 catalysts for CO oxidation prepared with microwave-assisted biosynthesis. Chem. Eng. J. 2015, 269, 105–112. [Google Scholar] [CrossRef]
- Zhou, W.; Shao, Z.; Ran, R.; Chen, Z. High performance electrode for electrochemical oxygen generator cell based on solid electrolyte ion transport membrane. Electrochim. Acta 2007, 52, 6297–6303. [Google Scholar] [CrossRef]










© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Q.; Zhang, Y.; Ding, H.; Wu, L.; Xu, Y.; Shi, B.; Zheng, Y.; Yuan, J. Performance and Stability Enhancement of Perovskite-Type Nanomaterials Applied for Carbon Capture Utilizing Oxyfuel Combustion. Energies 2017, 10, 164. https://doi.org/10.3390/en10020164
Shen Q, Zhang Y, Ding H, Wu L, Xu Y, Shi B, Zheng Y, Yuan J. Performance and Stability Enhancement of Perovskite-Type Nanomaterials Applied for Carbon Capture Utilizing Oxyfuel Combustion. Energies. 2017; 10(2):164. https://doi.org/10.3390/en10020164
Chicago/Turabian StyleShen, Qiuwan, Yindi Zhang, Haoran Ding, Lijuan Wu, Yongqing Xu, Baocheng Shi, Ying Zheng, and Jinliang Yuan. 2017. "Performance and Stability Enhancement of Perovskite-Type Nanomaterials Applied for Carbon Capture Utilizing Oxyfuel Combustion" Energies 10, no. 2: 164. https://doi.org/10.3390/en10020164
APA StyleShen, Q., Zhang, Y., Ding, H., Wu, L., Xu, Y., Shi, B., Zheng, Y., & Yuan, J. (2017). Performance and Stability Enhancement of Perovskite-Type Nanomaterials Applied for Carbon Capture Utilizing Oxyfuel Combustion. Energies, 10(2), 164. https://doi.org/10.3390/en10020164





