Yield, Composition, and Property of Biochar Obtained from the Two-Step Pyrolysis of Rice Husk Impregnated with Boric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Impregnation Process
2.2.2. Pyrolysis Procedure
2.3. Characterizations
3. Results and Discussion
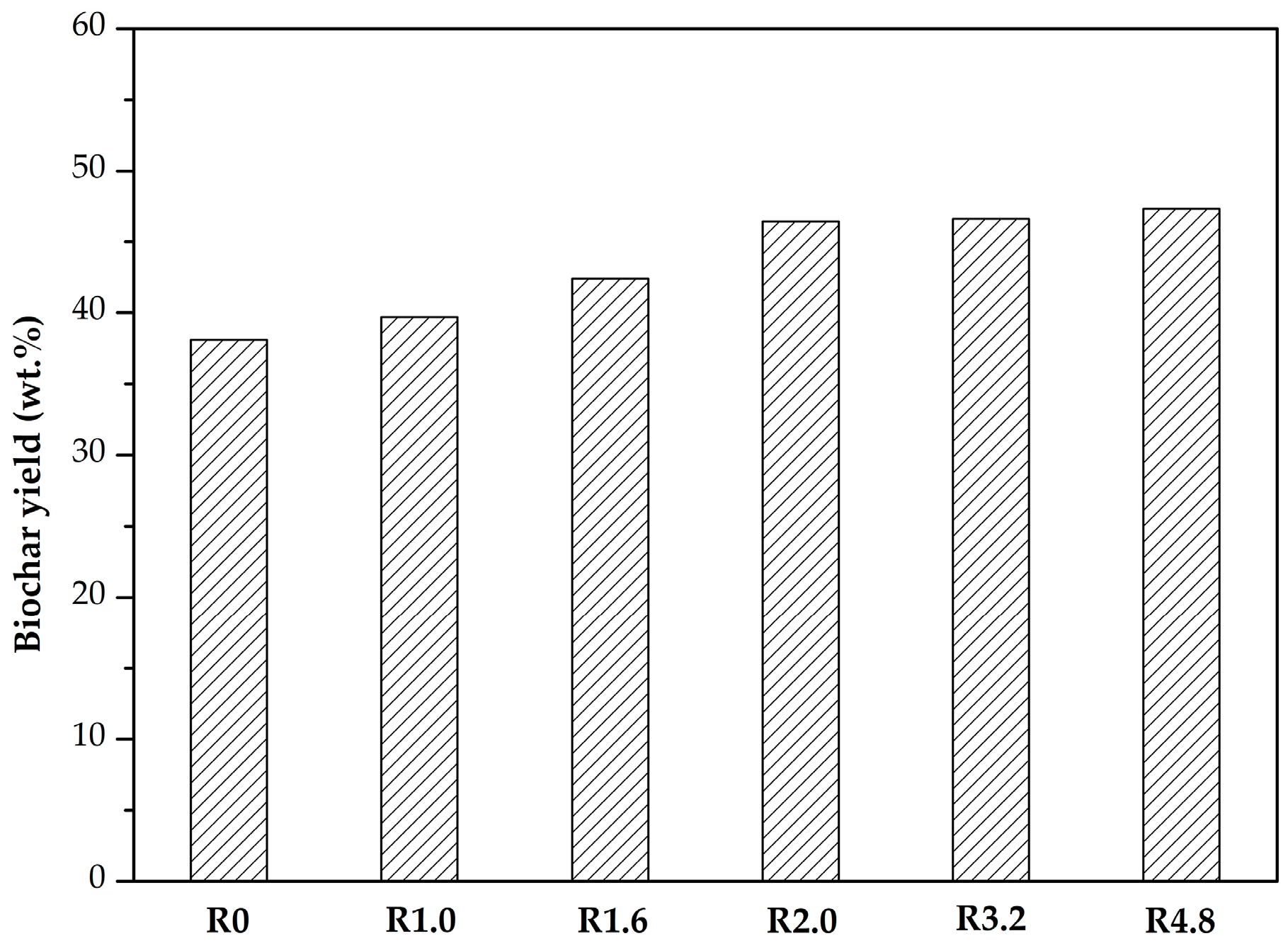
3.1. Biochar Yield
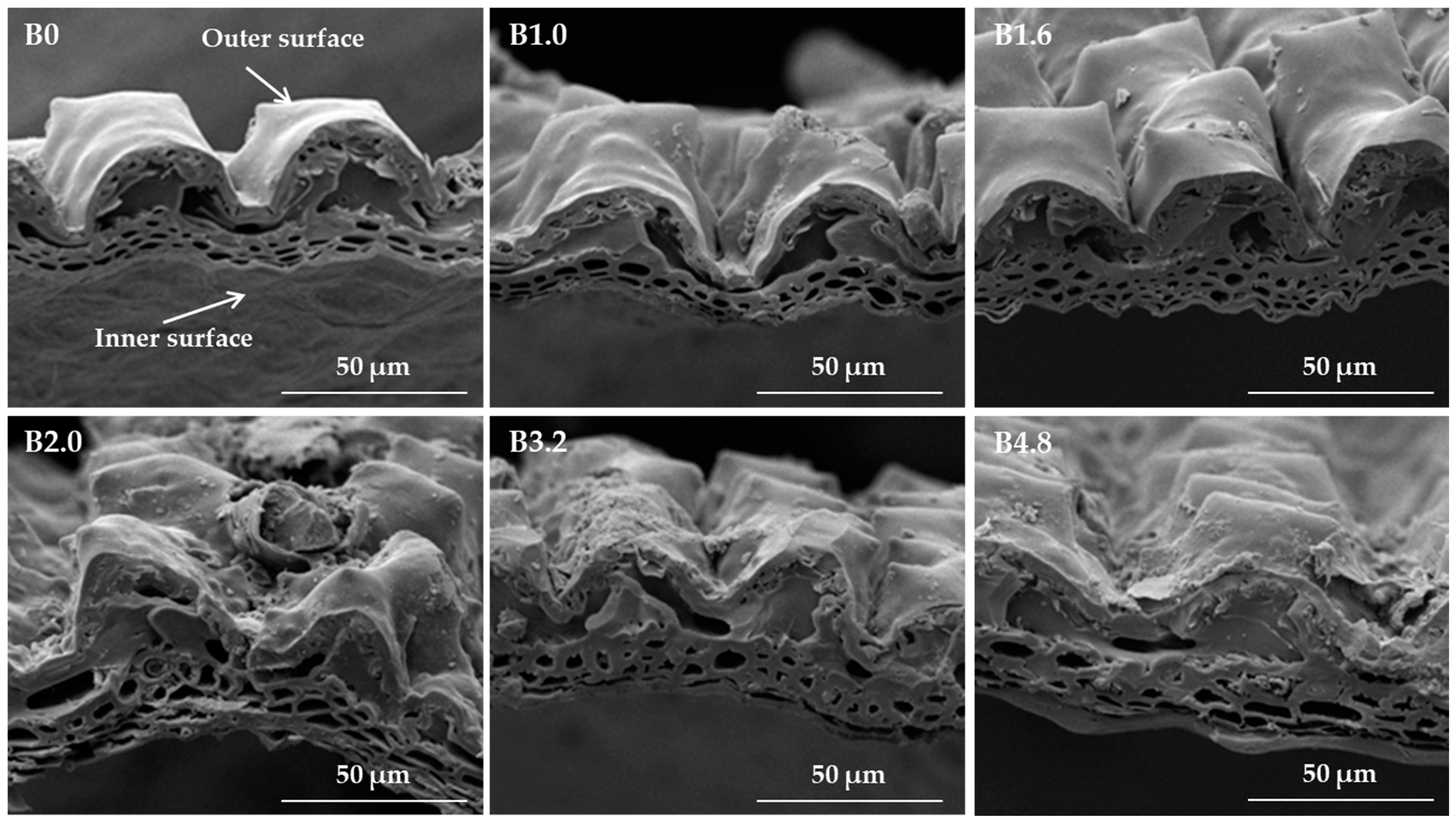
3.2. Biochar Composition
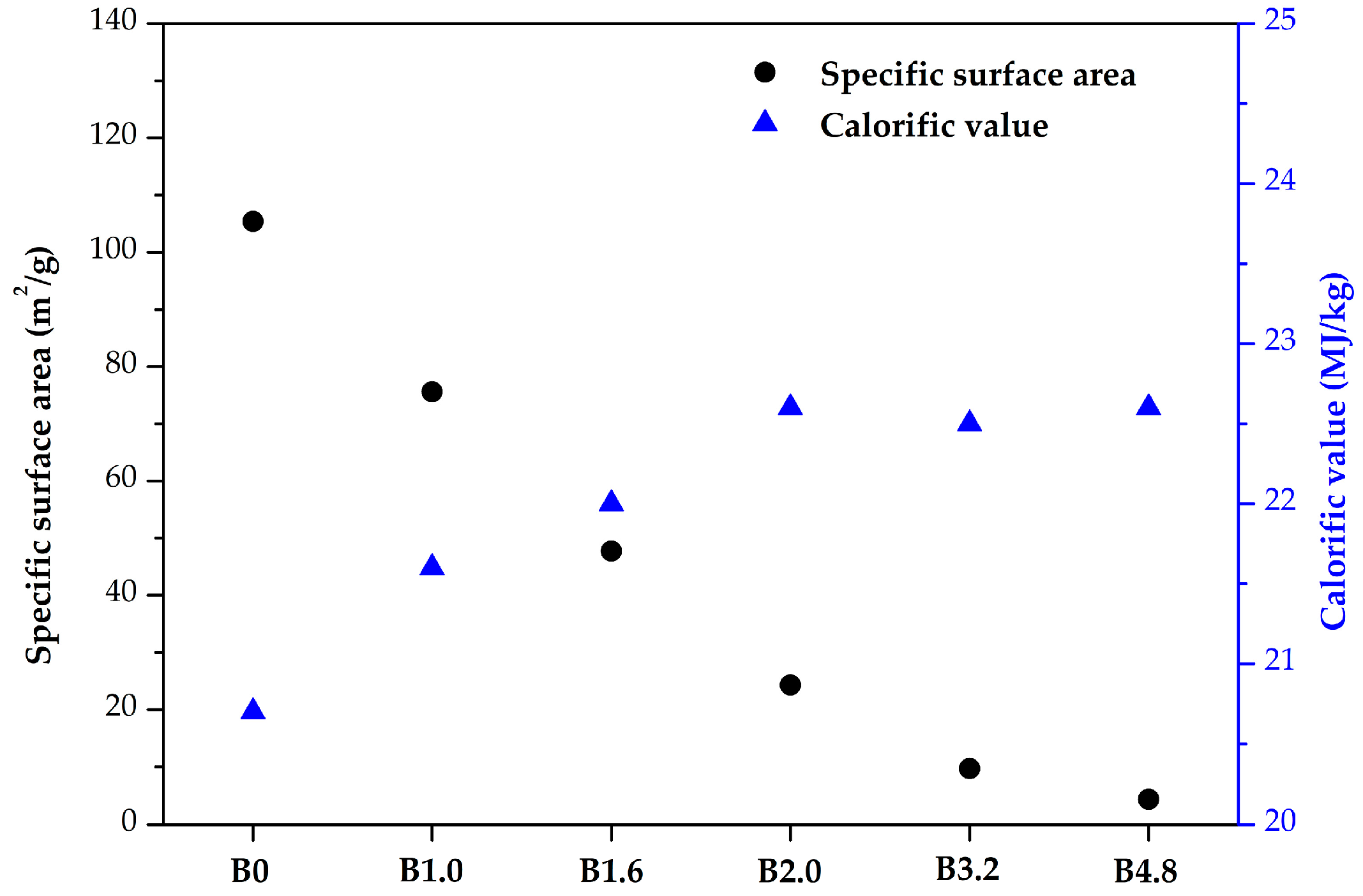
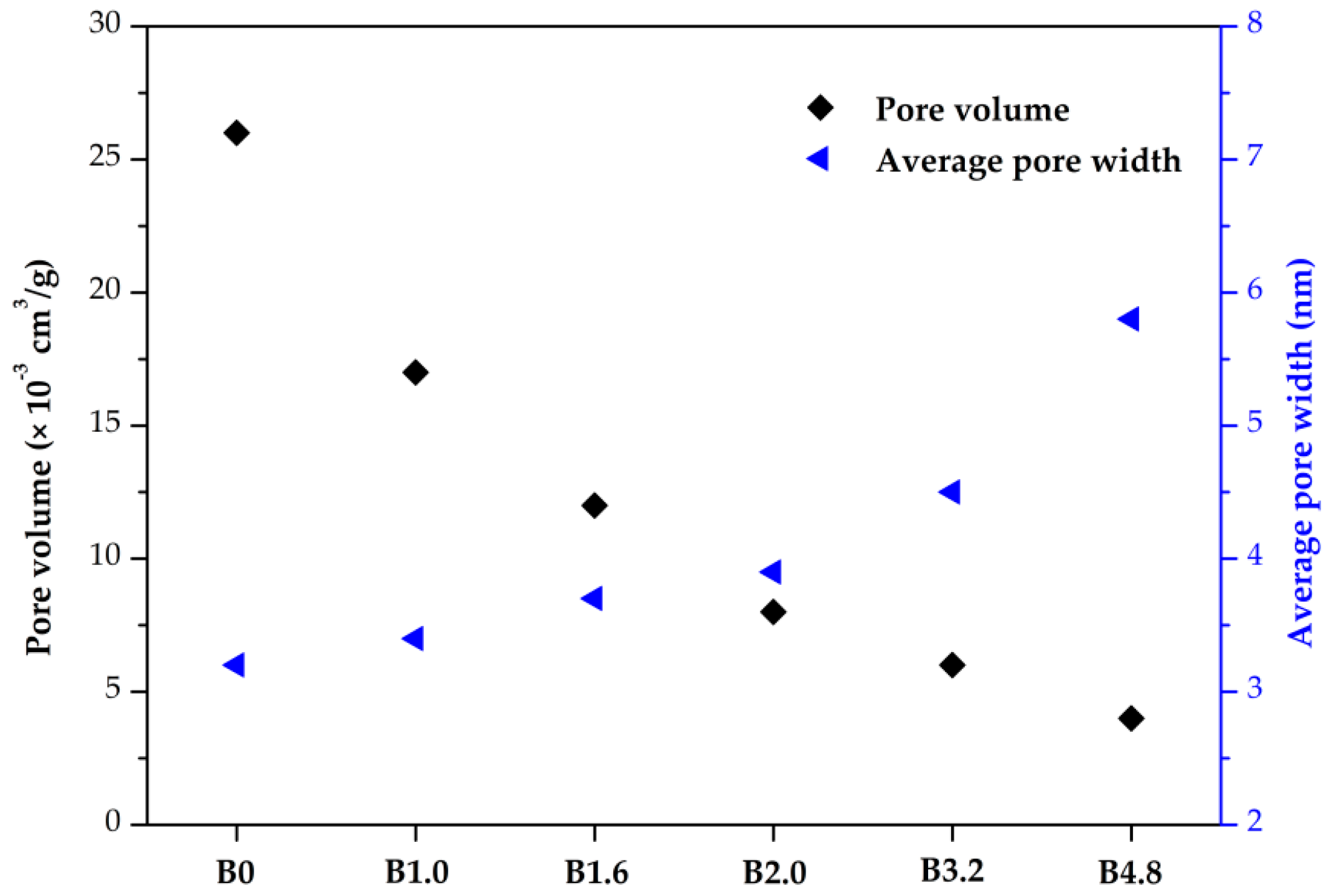
3.3. Calorific Value and Specific Surface Area of Biochar
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, J.L. Bio-oil from fast pyrolysis of rice husk: Yields and related properties and improvement of the pyrolysis system. J. Anal. Appl. Pyrolysis 2007, 80, 30–35. [Google Scholar] [CrossRef]
- Heo, H.S.; Park, H.J.; Dong, J.; Park, S.H.; Kim, S.; Suh, D.J.; Suh, Y.; Kim, S.; Park, Y. Fast pyrolysis of rice husk under different reaction conditions. J. Ind. Eng. Chem. 2010, 16, 27–31. [Google Scholar] [CrossRef]
- Manya, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Komnitsas, K.; Zaharaki, D.; Pyliotis, I.; Vamvuka, D.; Bartzas, G. Assessment of pistachio shell biochar quality and its potential for adsorption of heavy metals. Waste Biomass Valor. 2015, 6, 805–816. [Google Scholar] [CrossRef]
- Igalavithana, A.D.; Ok, Y.S.; Niazi, N.K.; Rizwan, M.; Al-Wabel, M.I.; Usman, A.R.A.; Moon, D.H.; Lee, S.S. Effect of corn residue biochar on the hydraulic properties of sandy loam soil. Sustainability 2017, 9, 266. [Google Scholar] [CrossRef]
- Santos, L.B.; Striebeck, M.V.; Crespi, M.S.; Capela, J.M.V.; Ribeiro, C.A.; Julio, M.D. Energy evaluation of biochar obtained from the pyrolysis of pine pellets. J. Therm. Anal. Calorim. 2016, 126, 1879–1887. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y.H.; Peng, J.F.; Liu, R.X.; Han, L. Synthesis of biomass-derived mesoporous carbon with super adsorption performance by an aqueous cooperative assemble route. ACS Sustain. Chem. Eng. 2017, 5, 2312–2319. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zhao, P.T.; Shao, Q.F.; Ma, D.C.; Takahashi, F.; Yoshikawa, K. In-situ catalytic conversion of tar using rice husk char-supported nickel-iron catalysts for biomass pyrolysis/gasification. Appl. Catal. B. 2014, 152–153, 140–151. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B. Effects of production conditions on yield and physicochemical properties of biochars produced from rice husk and oil palm empty fruit bunches. Environ. Sci. Pollut. Res. 2016, 23, 17928–17940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.P.; Hu, B.; Zhang, L.; Xiong, Y.Q. Effects of torrefaction on yield and quality of pyrolysis char and its application on preparation of activated carbon. J. Anal. Appl. Pyrolysis 2016, 119, 217–223. [Google Scholar] [CrossRef]
- Cheng, X.; Tang, Y.J.; Wang, B.; Jiang, J.M. Improvement of charcoal yield and quality by two-step pyrolysis on rice husks. Waste Biomass Valor. 2016, in press. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.X.; Chen, M.Q.; Min, F.F.; Zhang, S.P.; Ren, Z.W.; Yan, Y.J. Catalytic effects of six inorganic compounds on pyrolysis of three kinds of biomass. Thermochim. Acta 2006, 444, 110–114. [Google Scholar] [CrossRef]
- Wang, Q.W.; Li, J.; Winandy, J.E. Chemical mechanism of fire retardance of boric acid on wood. Wood Sci. Technol. 2004, 38, 375–389. [Google Scholar] [CrossRef]
- Griffin, G.J. The effect of fire retardants on combustion and pyrolysis of sugar-cane bagasse. Bioresour. Technol. 2011, 102, 8199–8204. [Google Scholar] [CrossRef] [PubMed]
- Kandola, B.K.; Horrocks, A.R.; Price, D.; Coleman, G.V. Flame-retardant treatments of cellulose and their influence on the mechanism of cellulose pyrolysis. J. Macromol. Sci. C Polym. Rev. 1996, 36, 721–794. [Google Scholar] [CrossRef]
- Di Blasi, C.; Branca, C.; Galgano, A. Flame retarding of wood by impregnation with boric acid—Pyrolysis products and char oxidation rates. Polym. Degrad. Stabil. 2007, 92, 752–764. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, X.Z.; Huang, H.J.; Wang, X.L.; Wang, H.; Zeng, G.M. Thermochemical liquefaction of rice husk for bio-oil production in mixed solvent (ethanol-water). Fuel Process. Technol. 2013, 112, 93–99. [Google Scholar] [CrossRef]
- Olsson, J.G.; Jaglid, U.; Pettersson, J.B.C. Alkali metal emission during pyrolysis of biomass. Energy Fuels 1997, 11, 779–784. [Google Scholar] [CrossRef]
- Jagtoyen, M.; Derbyshire, F. Activated carbons from yellow poplar and white oak by H3PO4 activation. Carbon 1998, 36, 1085–1097. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American lignocellulosic biomass and biochars in terms of their candidacy for alternate renewable fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Jimenez-Cordero, D.; Heras, F.; Alonso-Morales, N.; Gilarranz, M.A.; Rodriguez, J.J. Porous structure and morphology of granular chars from flash and conventional pyrolysis of grape seeds. Biomass Bioenergy 2013, 54, 123–132. [Google Scholar] [CrossRef]
| Analysis | Content | Value |
|---|---|---|
| Proximate analysis (wt %) | Ash | 15.0 |
| Volatiles | 73.8 | |
| Fixed-carbon | 11.2 | |
| Elemental analysis (wt %) | C | 42.5 |
| H | 5.7 | |
| O | 36.4 |
| Composition | B0 | B1.0 | B1.6 | B2.0 | B3.2 | B4.8 |
|---|---|---|---|---|---|---|
| Proximate analysis (wt %) | ||||||
| Ash | 32.2 | 30.8 | 30.4 | 29.9 | 30.0 | 29.9 |
| Volatiles | 12.5 | 13.9 | 14.2 | 14.7 | 14.6 | 14.7 |
| Fixed-carbon | 55.3 | 55.3 | 55.4 | 55.4 | 55.4 | 55.4 |
| Elemental analysis (wt %) | ||||||
| C | 55.2 | 57.5 | 58.7 | 59.3 | 59.4 | 59.5 |
| O | 9.9 | 8.7 | 8.5 | 7.8 | 7.7 | 7.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Wang, B. Yield, Composition, and Property of Biochar Obtained from the Two-Step Pyrolysis of Rice Husk Impregnated with Boric Acid. Energies 2017, 10, 1814. https://doi.org/10.3390/en10111814
Cheng X, Wang B. Yield, Composition, and Property of Biochar Obtained from the Two-Step Pyrolysis of Rice Husk Impregnated with Boric Acid. Energies. 2017; 10(11):1814. https://doi.org/10.3390/en10111814
Chicago/Turabian StyleCheng, Xu, and Biao Wang. 2017. "Yield, Composition, and Property of Biochar Obtained from the Two-Step Pyrolysis of Rice Husk Impregnated with Boric Acid" Energies 10, no. 11: 1814. https://doi.org/10.3390/en10111814
APA StyleCheng, X., & Wang, B. (2017). Yield, Composition, and Property of Biochar Obtained from the Two-Step Pyrolysis of Rice Husk Impregnated with Boric Acid. Energies, 10(11), 1814. https://doi.org/10.3390/en10111814





