Introduction
With today’s media-rich lifestyle, people are often dividing their attention and cognitive capacity into multiple tasks. For example, many students do their homework while chatting online, or people manipulate their smartphones while driving. While performing those tasks, the prefrontal cortex (PFC) is engaged in executing cognitive control, in storing task-relevant information in working memory, and in exercising inhibitory control as needed (
Baddeley, 1992;
Miller & Cohen, 2001;
Roberts et al., 1994). How such different cognitive functions are coordinated and executed is an open question in neuroscience. Related to this question is how different neural operations interact or even interfere with each other, increasing susceptibility to cognitive errors during multitasking.
We closely followed the experimental protocol by
Roberts et al. (
1994), and explored the interference effect between two cognitive tasks. One is an anti-saccade task (
Hallett, 1978;
Roberts et al., 1994), and the other is to remember a sequence of random digits for a few seconds.
A saccade is a rapid eye movement from one point of fixation to another, which brings the fovea—a small yet high-resolution part of the retina—into different regions of the visual field at roughly three times per second. Saccades can be triggered reflexively, in response to a salient visual feature (e.g., colors or textures that are distinct from the background) or a conspicuous movement in a visual scene. When a person is presented with a distinctive cue in the visual periphery, a natural, reflexive response is to make a “pro-saccade” by moving her gaze towards it. However, she can exercise inhibitory control over the saccadic reflex and to make an “anti-saccade,” or look towards the opposite side of the stimuli.
Because of the finite capacity of the neural hardware, overloading working memory leads to decreased inhibitory control. An analogy would be the sluggish performance of an old computer that is running a memory-intensive program. Patients with prefrontal lesions or dysfunctions, such as schizophrenia (Fukushima et al., 1990), Alzheimer’s disease (Fletcher & Sharpe, 1986), and attention deficit hyperactivity disorder (
Aman et al., 1998), tend to commit more errors in tasks that require inhibitory control or working memory.
Studies suggest that the superior colliculus (SC) of the brain is involved in the reflexive saccade (Schiller & Sandell, 1983) and it receives projections directly and indirectly from the dorsolateral PFC (
Goldman-Rakic, 1988). A number of event-related fMRI studies have shown the involvement of PFC during an anti-saccade task (
Ettinger et al., 2008;
Ford et al., 2005). We speculate that during an anti-saccade task, the PFC provides an inhibitory signal to the SC to prevent an unwanted reflexive saccade. However, if the PFC is occupied with another task, the inhibitory signal may not be sent, and the reflexive saccade would be more likely than an anti-saccade. Hence, it is our hypothesis that by overloading the capacity of the PFC with two tasks requiring inhibitory control and working memory, average performance would decrease.
Methods
College-aged subjects (N=11) participated in our study. The experiments were conducted under an Institutional Review Board (IRB) approved protocol. Each subject performed three types of tasks: pro-saccade with the working memory task (PM), and anti-saccade tasks with and without the working memory task (AM and A).
For each trial, subjects started by looking at a computer monitor with a central fixation point. A sequence of 8 random digits (e.g., “32021287”) was read out by a computer. (In a pilot study, it was observed that a short sequence like a 3-digit number did not require much effort to memorize perfectly, and did not produce any significant interference effect.) After a variable interval (between 1500 and 3500 ms), the fixation point was eliminated and a white square (cue) appeared on either the right or left side for 200 ms, during which the subjects were expected to make a saccadic eye movement towards or away from the cue, respectively for pro-saccade or anti-saccade tasks. Then, the cue disappeared, and a small arrow (target), pointing either up or down, was shown for 110 ms on the appropriate side of the monitor. The target was presented at 15 degrees of visual angle from the center of the screen. The size of the cue and masking squares was 1x1 degrees. The size of the arrow was 0.5 degree, fitting snugly inside the square. Given this eccentricity and size, the arrow was not clearly visible when subject’s gaze was on the fixation point.
The target appeared on the same location as the cue for the PM trials and on the opposite side of the cue for AM or A. Because the target arrow appeared only briefly and because it was too small to be seen clearly with peripheral vision, an incorrect eye movement (i.e., a pro-saccade during an anti-saccade trial) would not allow the subjects to identify the direction of the arrow. The target was then replaced by a gray square (mask) for another 200 ms, so that subjects would not be able to recognize the direction of the arrow by the persistence of vision.
Figure 1 shows the experimental design.
Subjects were instructed that the eye movement should be made as soon as the cue appeared. If a correct eye movement were made, the subjects would have been able to see the direction of the arrow, which appeared in a brief time interval between the cue and the mask. Following the visual task, subjects had to choose one of the following three choices with a keyboard about the direction of the arrow: Up, Down, or Unsure. The correctness of this keyboard response was used to measure the saccade performance. Subjects also had to type in the sequence of random digits, as best as their memory served. In trials without the memory task (A), the number sequences were still played, but subjects were instructed to ignore the audio, and they reported the direction of the arrow only.
Each subject performed 144 trials in 9 sets of 16, lasting a total of approximately one hour. Cue location (left or right) and arrow direction (up or down) were counter-balanced. Each subject performed three sets of each trial type in the following progression: PM > A > AM. Easier pro-saccades trials preceded less familiar anti-saccade trials, so that subjects could become acclimated to the experiments.
The stimuli were presented on a 17-inch computer monitor with a resolution of 1280 by 1024 pixels at 60 Hz. Subjects’ eyes were about 60 cm away (at about an arm’s length) from the monitor. Matlab R2012b was used to present both visual and auditory stimuli and to collect keyboard responses.
We note that in performing this experiment, there is no explicit need for an eye-tracker. By switching visual stimuli quickly enough and by ensuring that the target (i.e., arrows) cannot be seen with a peripheral vision, the success of subjects’ eye movements can be measured with a regular computer and a monitor.
Results
The average performance for pro-saccades was 94% (PM), while the average for anti-saccade was 43% (A) and 31% (AM), as shown in
Figure 2. The difference between A and AM conditions was significant (paired t-test,
p < 0.01). This is an expected trend, given the cognitive demand of keeping 8 digits in the working memory while inhibiting, rather than following, saccadic reflexes.
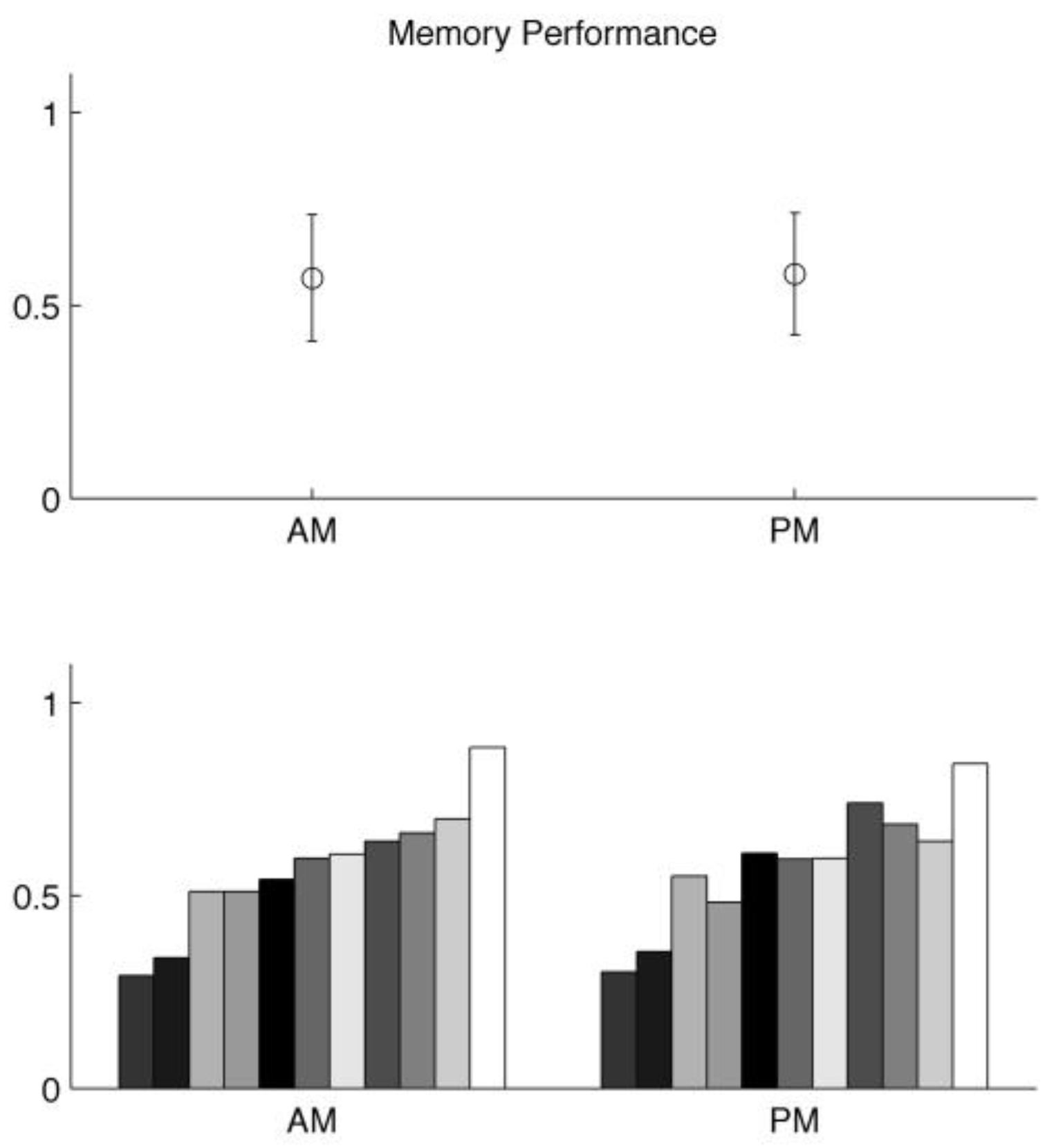
On the other hand, the average performances on the memory task were 57% (AM) and 58% (PM), as shown in
Figure 3, and they were not significantly different (paired t-test,
p > 0.1).
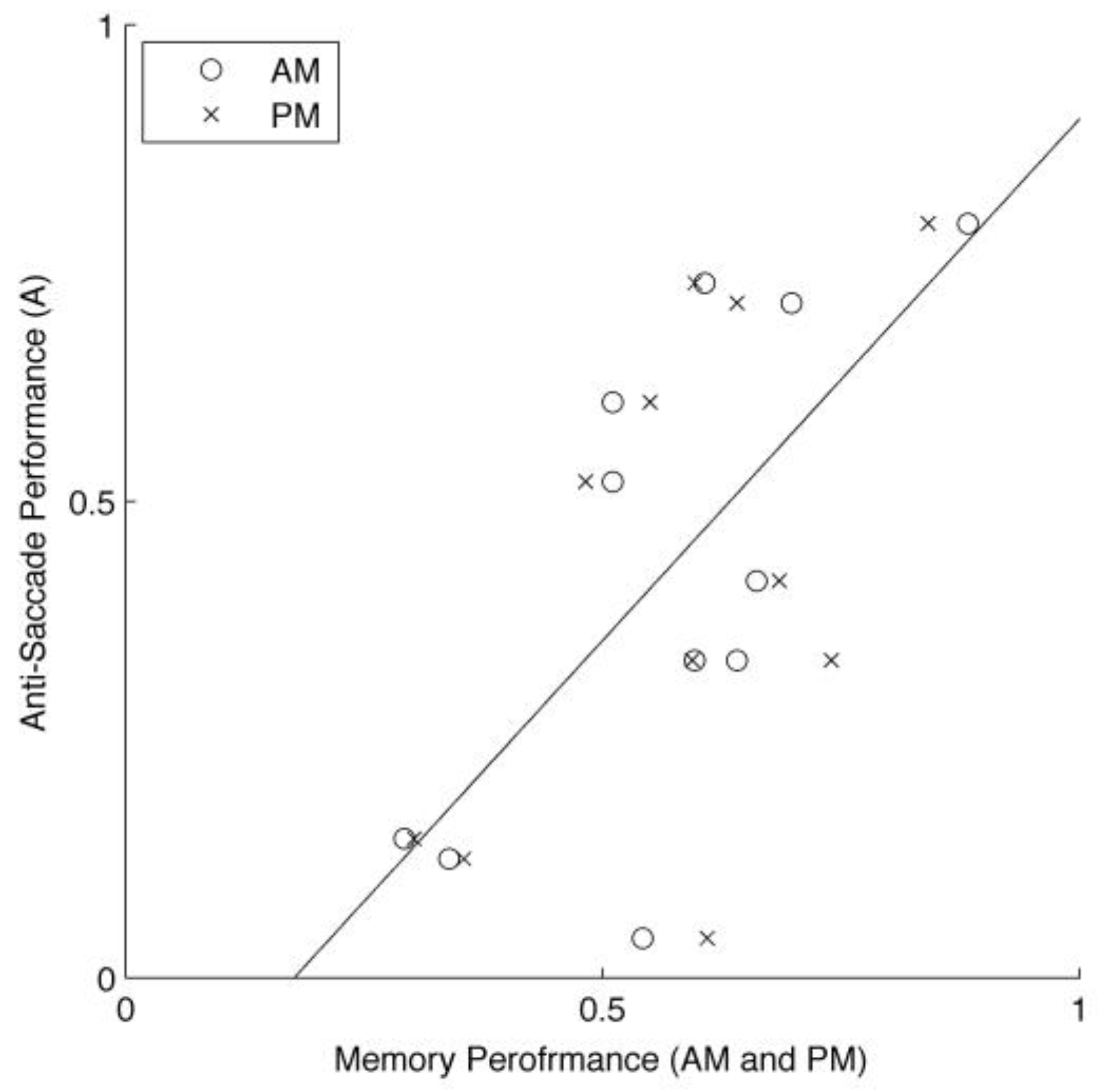
There was a high variability across subjects. For example, some subjects’ average performances on anti-saccade task were over 70%, whereas other subjects performed less than 10%. The memory performance was highly variable, too. Furthermore, these variabilities correlated with each other, as shown in
Figure 4.
Unsworth et al. (
2004) also reported that subjects with higher working memory capacity performed better in anti-saccade task.
Discussion
We presented a simple experiment that can measure the success of an eye movement without explicitly measuring the gaze location or requiring an eye tracker. In particular, this study explored how two concurrent tasks, involving inhibitory control and working memory in vision and audition, interacted with each other.
Our subjects’ performance was lower than usually reported (e.g., 10% error rate, as reported by Unsworth et al.), and this is likely due to a limitation of our approach of allowing only a narrow time-window (between 200 and 310 ms from the cue onset) for correctly looking at the target. If a subject had a slow reaction time, the mask would have replaced the target, even if the subject made the correct eye movement. Therefore, the observed performance based on the target identification is expected to be lower than what it would have been if the actual eye movements were measured, and the actual interference effect is likely to be more than the modest amount shown in
Figure 2.
We found an asymmetric interference effect between the two tasks. While the anti-saccade performance decreased significantly, the working memory performance was not affected. This asymmetry could be due to the fact that the anti-saccade is such an unnatural task that it is more sensitive to the capacity of the PFC. Also, while the working memory task might not require inhibitory control, the working memory could be a requisite of the anti-saccade task for which the subjects need to keep reminding themselves to look in the opposite direction of a stimulus. It will be interesting to see whether a different pair of tasks could produce an opposite trend (i.e., degraded performance on the working memory task without affecting the inhibitory control). Also,
Kirchner and Colonius (
2005) found that the anti-saccade performance could be facilitated, or impeded, by presenting an auditory stimulus either at the same or at the opposition location of a visual stimulus.
Another interesting result is the correlation between the anti-saccade and memory performances across the subjects (
Figure 4), which is consistent with the idea the degree of engagement or capacity of PFC’s executive control is important for the anti-saccade and working memory tasks (
Baddeley, 1992;
Miller & Cohen, 2001;
Roberts et al., 1994). At the same time, it has been reported that other factors, such as foreknowledge or task-switching (
Barton et al., 2006;
Unsworth et al., 2004), could influence anti-saccade performance.