Anticipation of Cognitive Conflict Is Reflected in Microsaccades: Evidence from a Cued-Flanker Task
Abstract
:Introduction
Methods
Participants
Apparatus
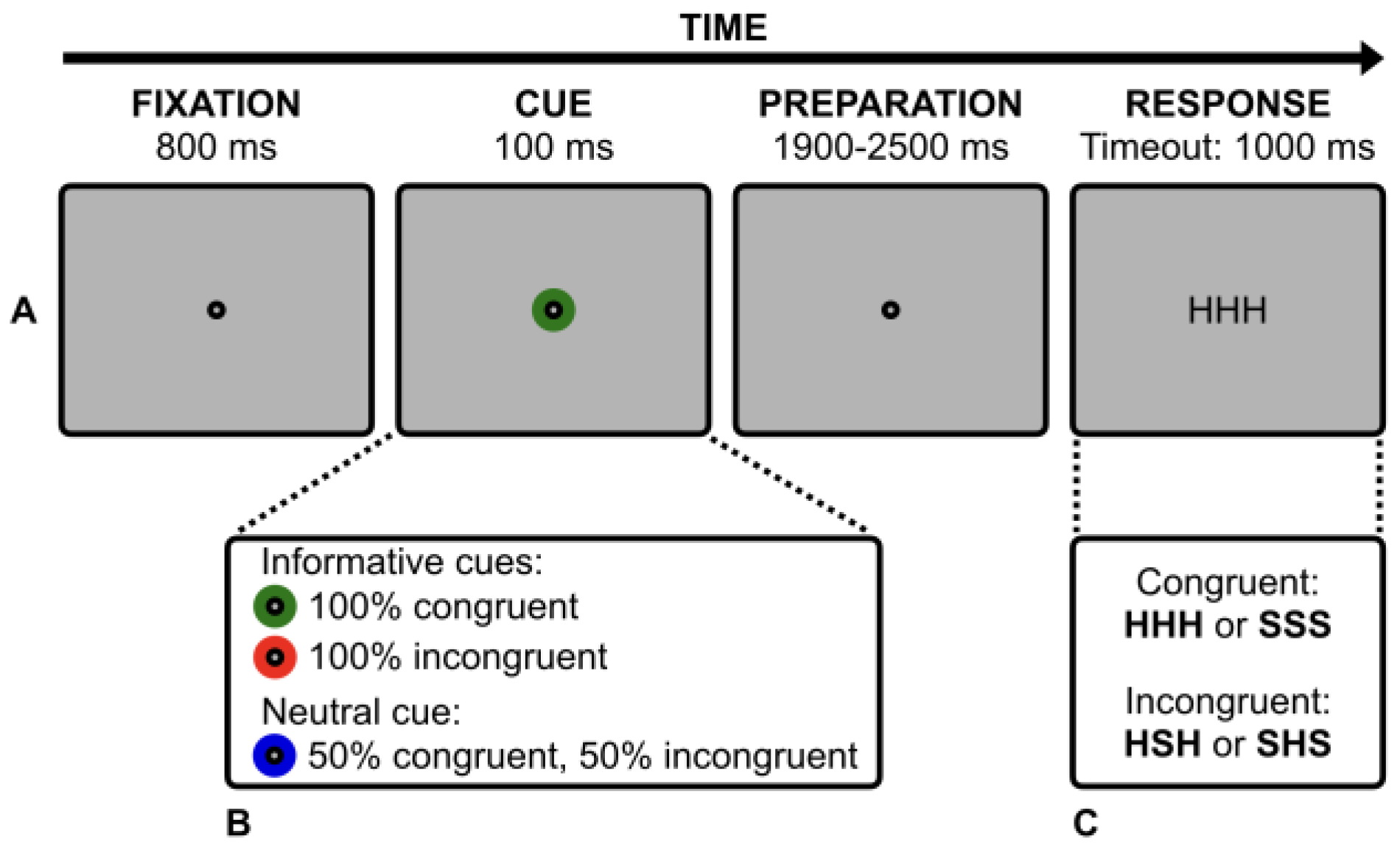
Stimuli and procedure
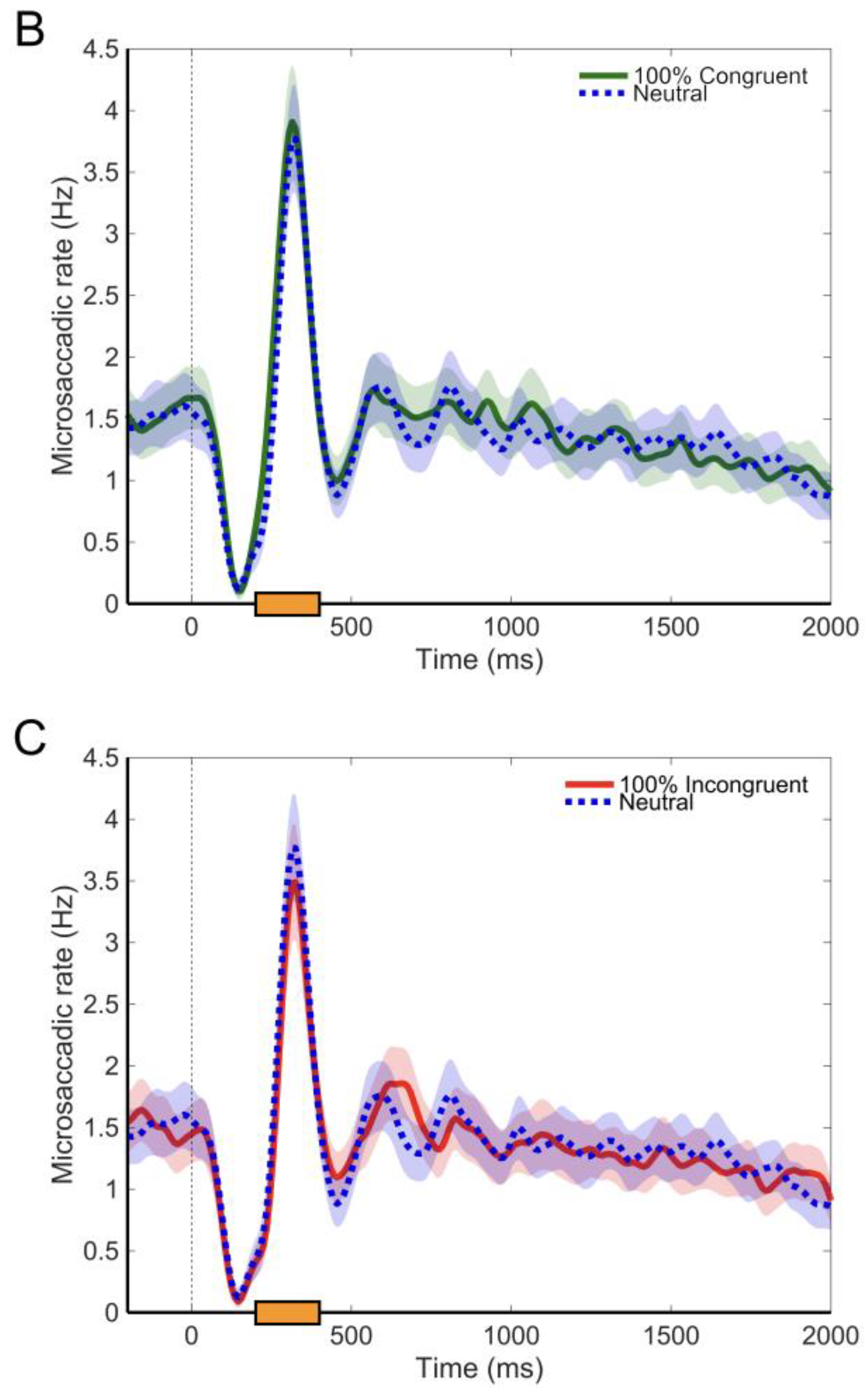
Results
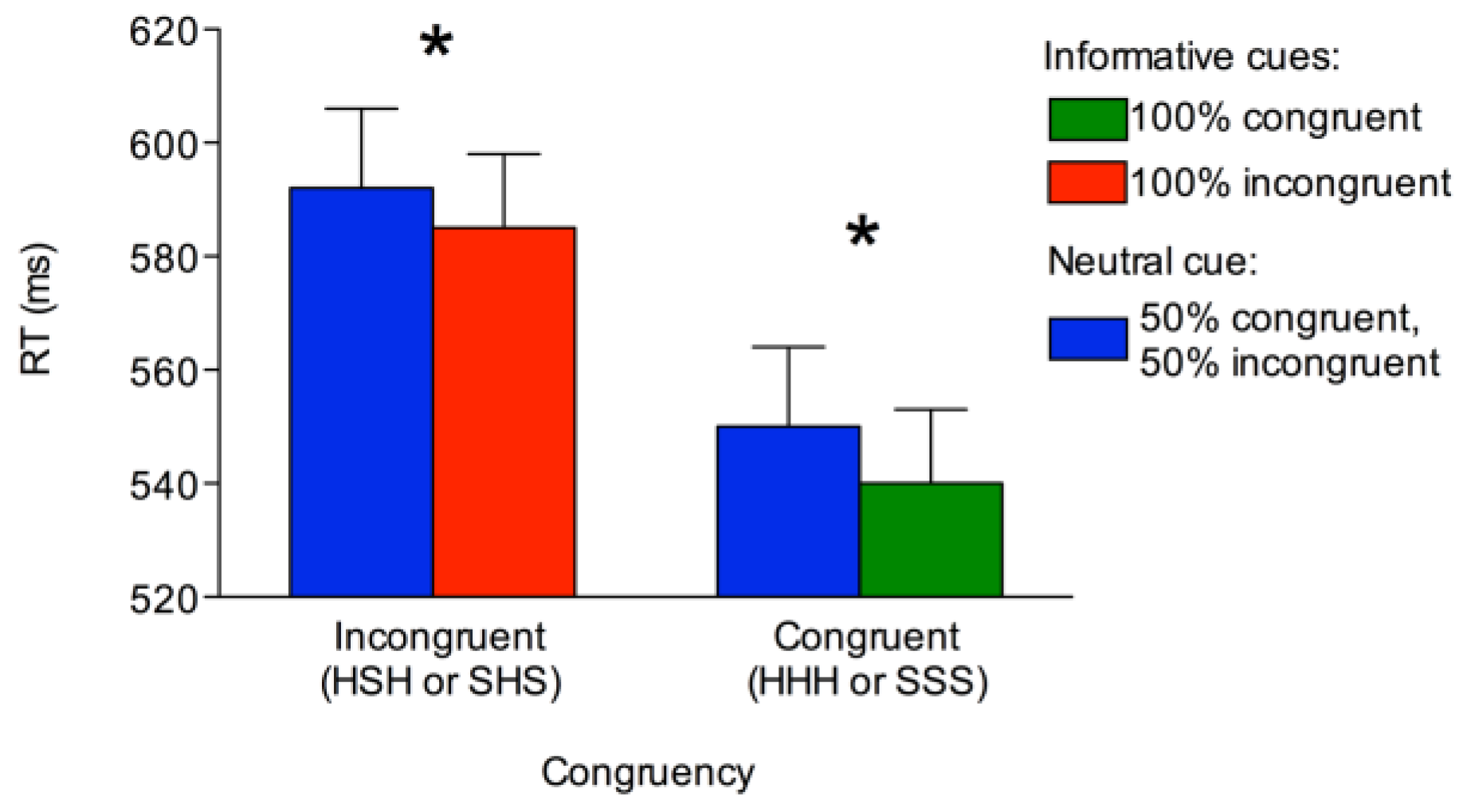
Manual responses
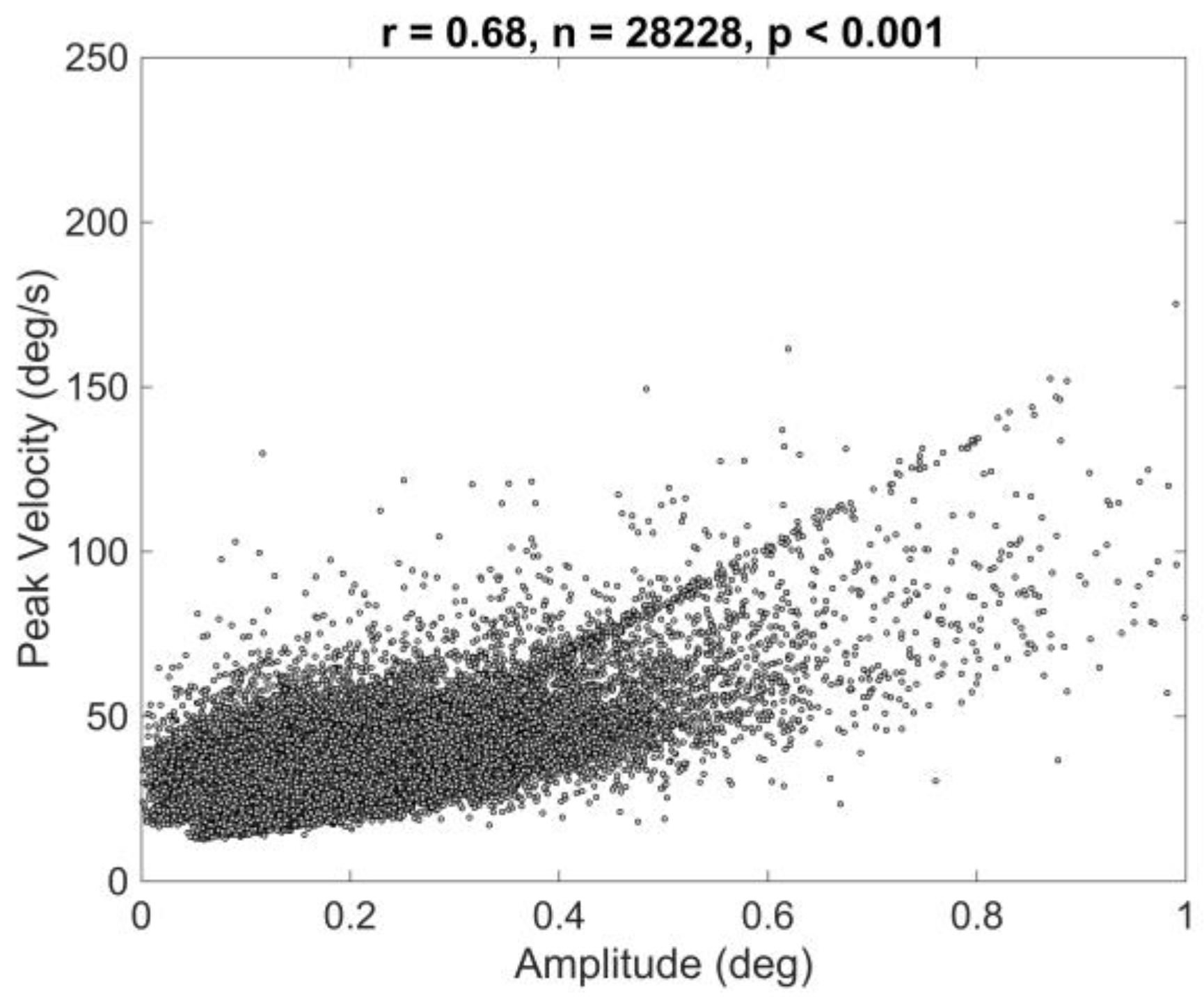
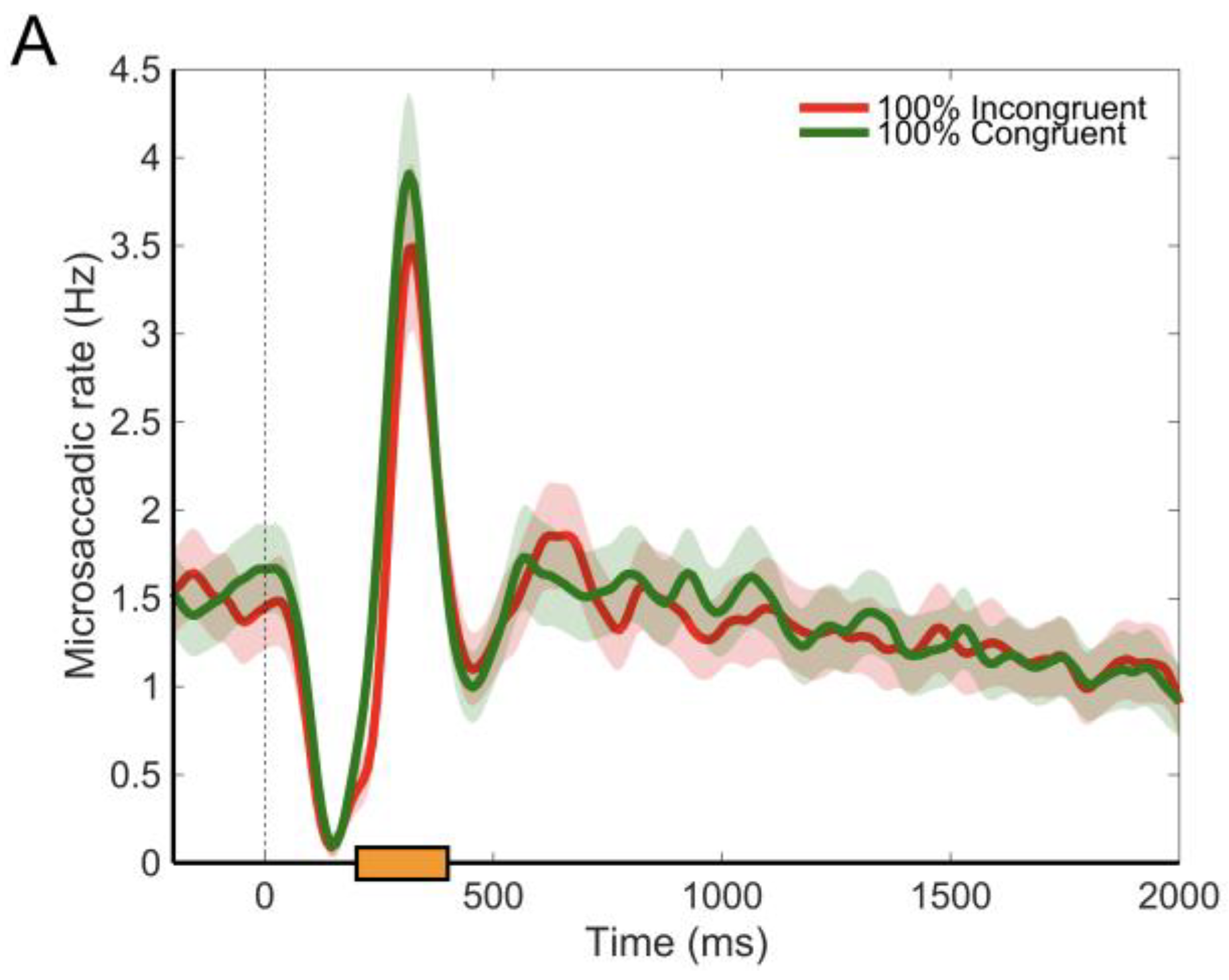
Microsaccades
Discussion
Ethics and Conflict of Interest
Acknowledgments
References
- Benedetto, S., M. Pedrotti, and B. Bridgeman. 2011. Microsaccades and exploratory saccades in a naturalistic environment. Journal of Eye Movement Research 4: 1–10. [Google Scholar]
- Betta, E., G. Galfano, and M. Turatto. 2007. Microsaccadic response during inhibition of return in a target-target paradigm. Vision Research 47: 428–436. [Google Scholar] [CrossRef] [PubMed]
- Betta, E., and M. Turatto. 2006. Are you ready? I can tell by looking at your microsaccades. Neuroreport 17: 1001–1004. [Google Scholar] [PubMed]
- Botvinick, M. M., T. S. Braver, D. M. Barch, C. S. Carter, and J. D. Cohen. 2001. Conflict monitoring and cognitive control. Psychological Review 108: 624–652. [Google Scholar] [CrossRef]
- Chen, Y., S. Martinez-Conde, S. L. Macknik, Y. Bereshpolova, H. A. Swadlow, and J. M. Alonso. 2008. Task difficulty modulates the activity of specific neuronal populations in primary visual cortex. Nature Neuroscience 11: 974–982. [Google Scholar]
- Collewijn, H., and E. Kowler. 2008. The significance of microsaccades for vision and oculomotor control. Journal of Vision 8: 1–21. [Google Scholar] [CrossRef]
- Correa, Á., A. Rao, and A. C. Nobre. 2009. Anticipating conflict facilitates controlled stimulus-response selection. Journal of Cognitive Neuroscience 21: 1461–1472. [Google Scholar]
- Dalmaso, M., L. Castelli, and G. Galfano. 2019. Microsaccadic rate and pupil size dynamics in pro-/antisaccade preparation: The impact of intermixed vs. blocked trial administration. Psychological Research. in press. [Google Scholar] [CrossRef]
- Dalmaso, M., L. Castelli, P. Scatturin, and G. Galfano. 2017. Working memory load modulates microsaccadic rate. Journal of Vision 17: 1–12. [Google Scholar] [CrossRef]
- Di Stasi, L. L., M. B. McCamy, A. Catena, S. L. Macknik, J. J. Canas, and S. Martinez-Conde. 2013. Microsaccade and drift dynamics reflect mental fatigue. European Journal of Neuroscience 38: 2389–2398. [Google Scholar] [CrossRef]
- Di Stasi, L. L., M. B. McCamy, S. Pannasch, R. Renner, A. Catena, J. J. Cañas, and S. Martinez-Conde. 2015. Effects of driving time on microsaccadic dynamics. Experimental Brain Research 233: 599–605. [Google Scholar] [PubMed]
- Engbert, R. 2006. Microsaccades: A microcosm for research on oculomotor control, attention, and visual perception. Progress in Brain Research 154: 177–192. [Google Scholar]
- Engbert, R. 2012. Computational modeling of collicular integration of perceptual responses and attention in microsaccades. Journal of Neuroscience 32: 8035–8039. [Google Scholar] [PubMed]
- Engbert, R., and R. Kliegl. 2003. Microsaccades uncover the orientation of covert attention. Vision Research 43: 1035–1045. [Google Scholar] [CrossRef]
- Eriksen, B. A., and C. W. Eriksen. 1974. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics 16: 143–149. [Google Scholar]
- Fan, J., B. D. McCandliss, J. Fossella, J. I. Flombaum, and M. I. Posner. 2005. The activation of attentional networks. Neuroimage 26: 471–479. [Google Scholar] [CrossRef]
- Fried, M., E. Tsitsiashvili, Y. S. Bonneh, A. Sterkin, T. Wygnanski-Jaffe, T. Epstein, and U. Polat. 2014. ADHD subjects fail to suppress eye blinks and microsaccades while anticipating visual stimuli but recover with medication. Vision Research 101: 62–72. [Google Scholar] [PubMed]
- Gao, X., H. Yan, and H.-J. Sun. 2015. Modulation of microsaccade rate by task difficulty revealed through between-and within-trial comparisons. Journal of Vision 15: 1–15. [Google Scholar]
- Gratton, G., M. G. Coles, and E. Donchin. 1992. Optimizing the use of information: strategic control of activation of responses. Journal of Experimental Psychology: General 121: 480–506. [Google Scholar]
- Hafed, Z. M., and J. J. Clark. 2002. Microsaccades as an overt measure of covert attention shifts. Vision Research 42: 2533–2545. [Google Scholar]
- Hafed, Z. M., L. Goffart, and R. J. Krauzlis. 2009. A neural mechanism for microsaccade generation in the primate superior colliculus. Science 323: 940–943. [Google Scholar] [PubMed]
- Hafed, Z. M., and A. Ignashchenkova. 2013. On the dissociation between microsaccade rate and direction after peripheral cues: Microsaccadic inhibition revisited. The Journal of Neuroscience 33: 16220–16235. [Google Scholar] [CrossRef]
- Hermens, F. 2015. Dummy eye measurements of microsaccades: Testing the influence of system noise and head movements on microsaccade detection in a popular video-based eye tracker. Journal of Eye Movement Research 8: 1–17. [Google Scholar] [CrossRef]
- Hermens, F., J. M. Zanker, and R. Walker. 2010. Microsaccades and preparatory set: a comparison between delayed and immediate, exogenous and endogenous pro-and anti-saccades. Experimental Brain Research 201: 489–498. [Google Scholar]
- Hutton, S. B. 2008. Cognitive control of saccadic eye movements. Brain and Cognition 68: 327–340. [Google Scholar] [CrossRef]
- Jeffreys, H. 1961. Theory of probability, 3rd ed. Oxford, UK: Oxford University Press. [Google Scholar]
- Krejtz, K., A. T. Duchowski, A. Niedzielska, C. Biele, and I. Krejtz. 2018. Eye tracking cognitive load using pupil diameter and microsaccades with fixed gaze. PLoS One 13: e0203629. [Google Scholar]
- Lange, E. B., F. Zweck, and P. Sinn. 2017. Microsaccade-rate indicates absorption by music listening. Consciousness and Cognition 55: 59–78. [Google Scholar] [PubMed]
- Lavie, N., A. Hirst, J. W. De Fockert, and E. Viding. 2004. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General 133: 339–354. [Google Scholar]
- Martinez-Conde, S., J. Otero-Millan, and S. L. Macknik. 2013. The impact of microsaccades on vision: Towards a unified theory of saccadic function. Nature Reviews Neuroscience 14: 83–96. [Google Scholar] [CrossRef]
- Miller, J. 1991. The flanker compatibility effect as a function of visual angle, attentional focus, visual transients, and perceptual load: A search for boundary conditions. Perception & Psychophysics 49: 270–288. [Google Scholar]
- Munoz, D. P., and S. Everling. 2004. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience 5: 218–228. [Google Scholar] [CrossRef] [PubMed]
- Pastukhov, A., and J. Braun. 2010. Rare but precious: microsaccades are highly informative about attentional allocation. Vision Research 50: 1173–1184. [Google Scholar] [CrossRef]
- Peel, T. R., Z. M. Hafed, S. Dash, S. G. Lomber, and B. D. Corneil. 2016. A causal role for the cortical frontal eye fields in microsaccade deployment. PLoS Biology 14: e1002531. [Google Scholar] [CrossRef] [PubMed]
- Poletti, M., and M. Rucci. 2016. A compact field guide to the study of microsaccades: Challenges and functions. Vision Research 118: 83–97. [Google Scholar] [CrossRef] [PubMed]
- Rolfs, M. 2009. Microsaccades: Small steps on a long way. Vision Research 49: 2415–2441. [Google Scholar] [CrossRef]
- Rolfs, M., R. Kliegl, and R. Engbert. 2008. Toward a model of microsaccade generation: The case of microsaccadic inhibition. Journal of Vision 8: 1–23. [Google Scholar] [CrossRef]
- Siegenthaler, E., F. M. Costela, M. B. McCamy, L. L. Di Stasi, J. Otero-Millan, A. Sonderegger, R. Groner, S. Macknik, and S. Martinez-Conde. 2014. Task difficulty in mental arithmetic affects microsaccadic rates and magnitudes. European Journal of Neuroscience 39: 287–294. [Google Scholar] [CrossRef]
- Valsecchi, M., E. Betta, and M. Turatto. 2007. Visual oddballs induce prolonged microsaccadic inhibition. Experimental Brain Research 177: 196–208. [Google Scholar] [CrossRef]
- Watanabe, M., Y. Matsuo, L. Zha, D. P. Munoz, and Y. Kobayashi. 2013. Fixational saccades reflect volitional action preparation. Journal of Neurophysiology 110: 522–535. [Google Scholar] [CrossRef]
- White, A. L., and M. Rolfs. 2016. Oculomotor inhibition covaries with conscious detection. Journal of Neurophysiology 116: 1507–1521. [Google Scholar] [CrossRef]
- Zuber, B. L., L. Stark, and G. Cook. 1965. Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 150: 1459–1460. [Google Scholar] [PubMed]
Copyright © 2019. This article is licensed under a Creative Commons Attribution 4.0 International License.
Share and Cite
Dalmaso, M.; Castelli, L.; Galfano, G. Anticipation of Cognitive Conflict Is Reflected in Microsaccades: Evidence from a Cued-Flanker Task. J. Eye Mov. Res. 2019, 12, 1-9. https://doi.org/10.16910/jemr.12.6.3
Dalmaso M, Castelli L, Galfano G. Anticipation of Cognitive Conflict Is Reflected in Microsaccades: Evidence from a Cued-Flanker Task. Journal of Eye Movement Research. 2019; 12(6):1-9. https://doi.org/10.16910/jemr.12.6.3
Chicago/Turabian StyleDalmaso, Mario, Luigi Castelli, and Giovanni Galfano. 2019. "Anticipation of Cognitive Conflict Is Reflected in Microsaccades: Evidence from a Cued-Flanker Task" Journal of Eye Movement Research 12, no. 6: 1-9. https://doi.org/10.16910/jemr.12.6.3
APA StyleDalmaso, M., Castelli, L., & Galfano, G. (2019). Anticipation of Cognitive Conflict Is Reflected in Microsaccades: Evidence from a Cued-Flanker Task. Journal of Eye Movement Research, 12(6), 1-9. https://doi.org/10.16910/jemr.12.6.3


