18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study
Abstract
1. Introduction
2. Results
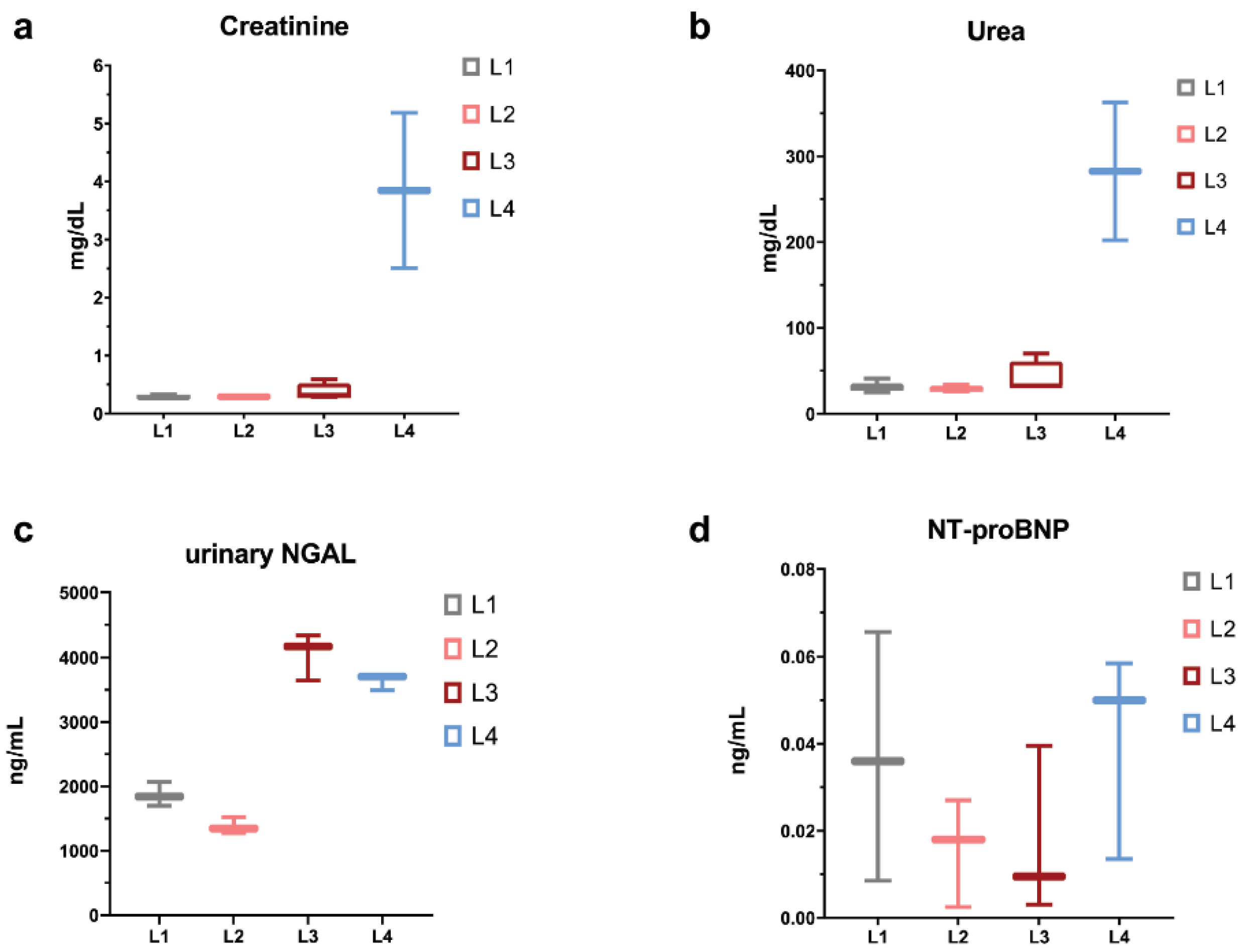
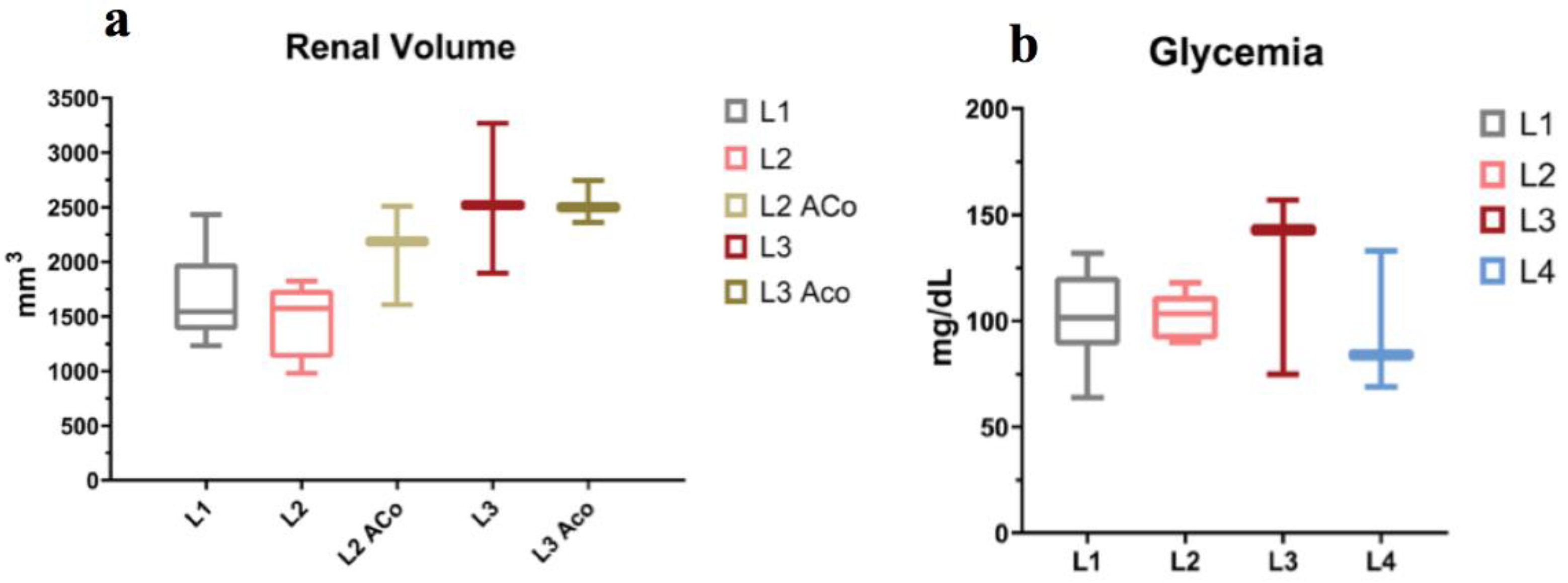
2.1. Clinical and Biochemical Characteristics
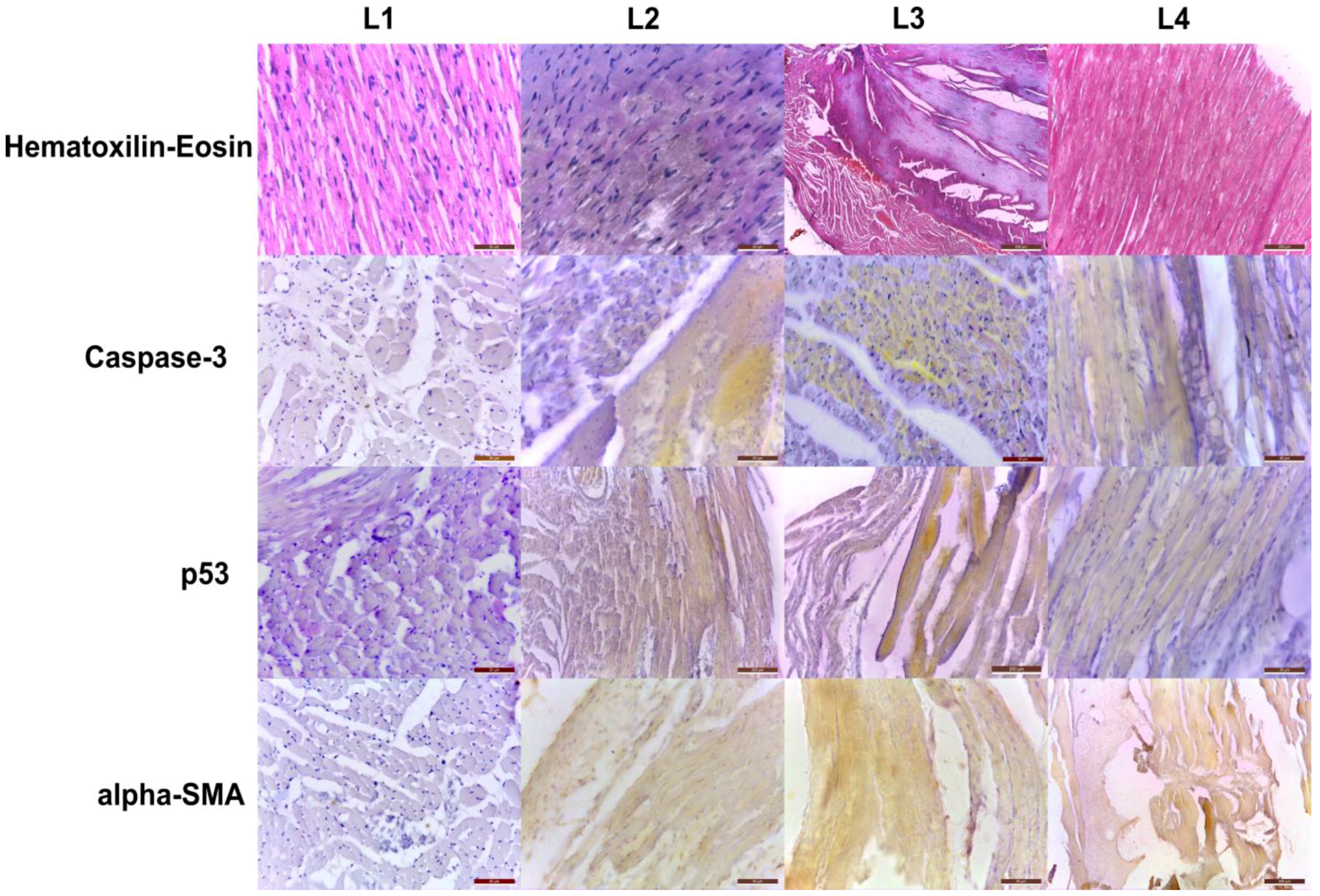
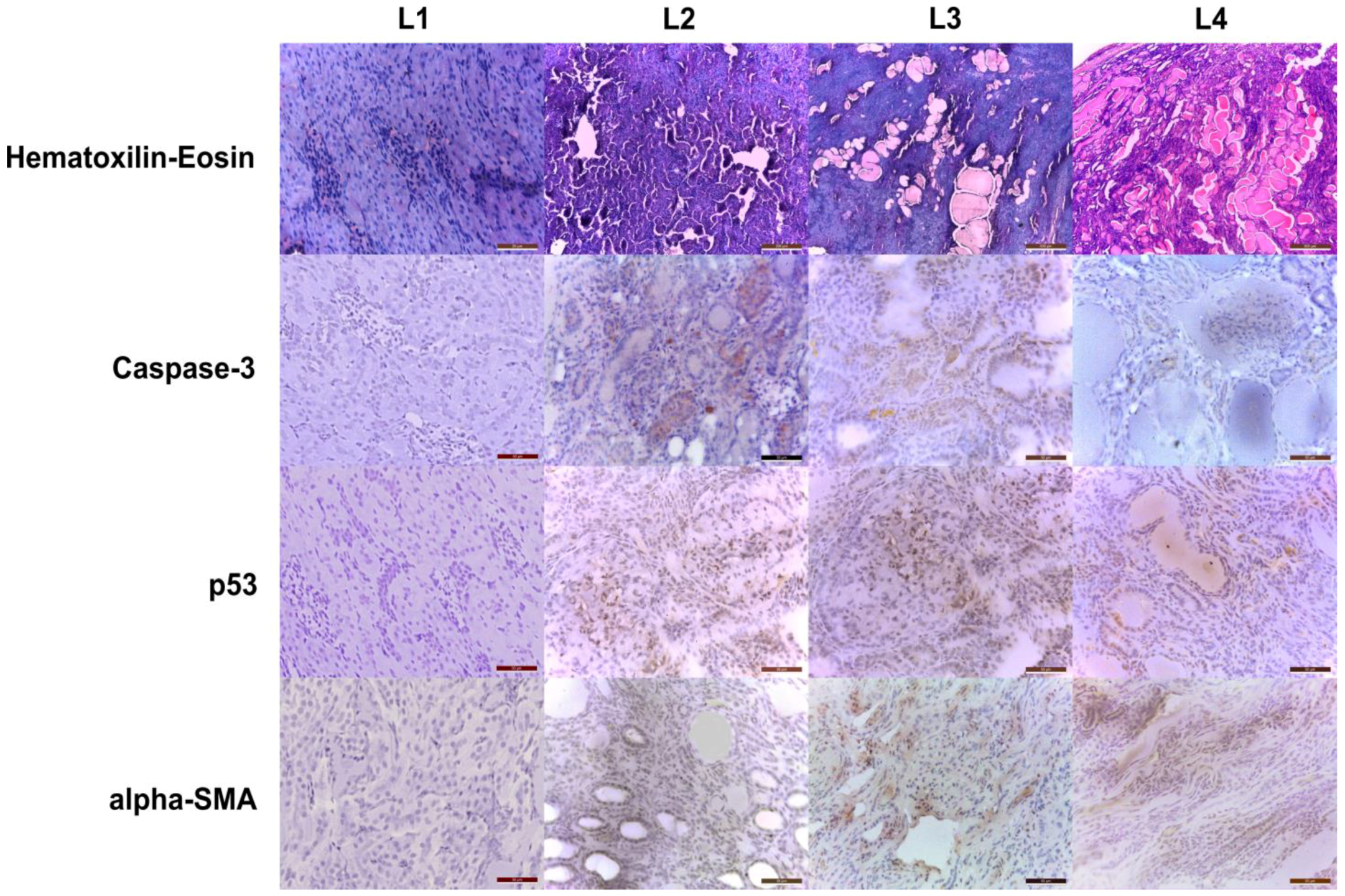
2.2. Histopathology and Immunohistochemistry Evaluation
2.3. MRI Organ Volumetry
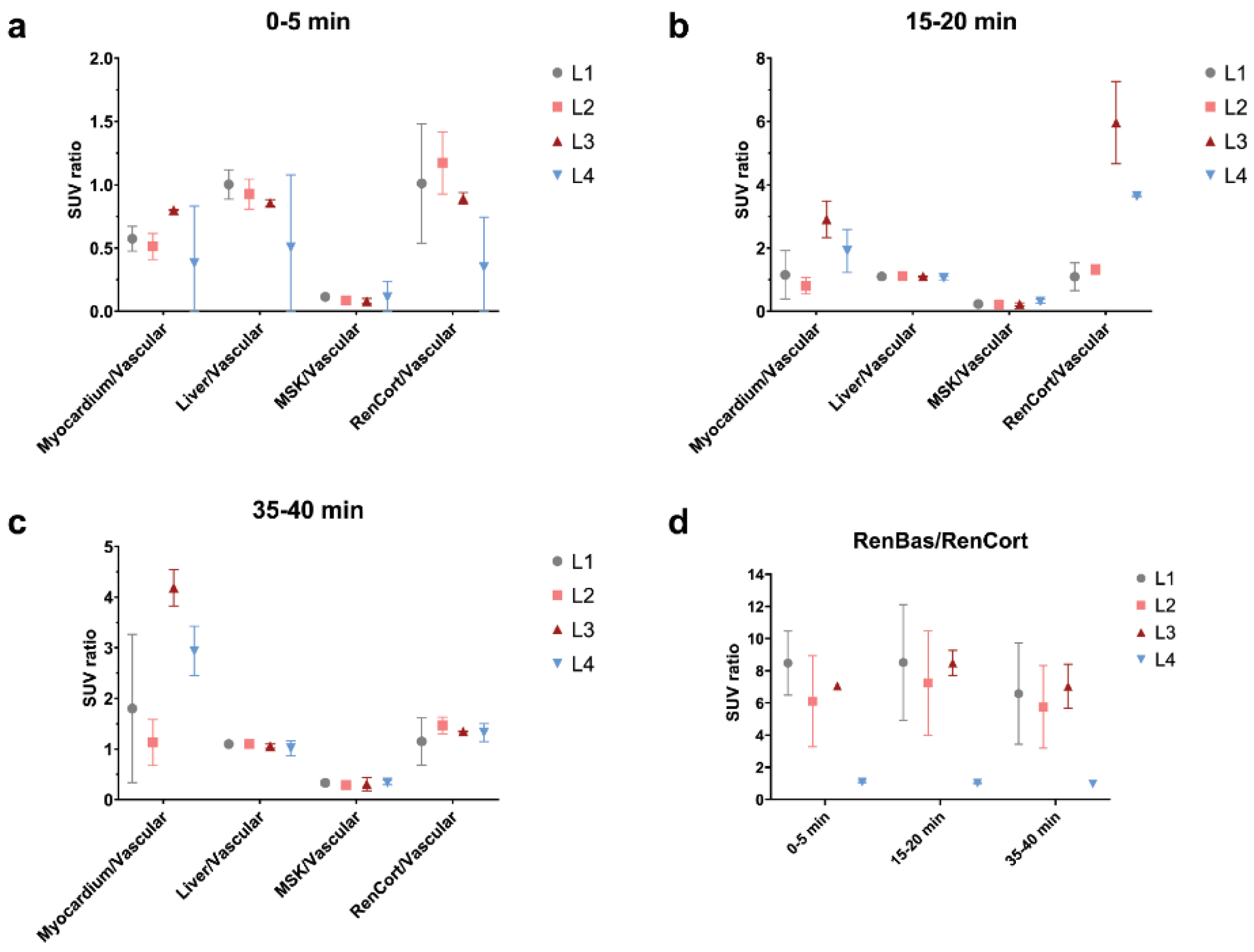
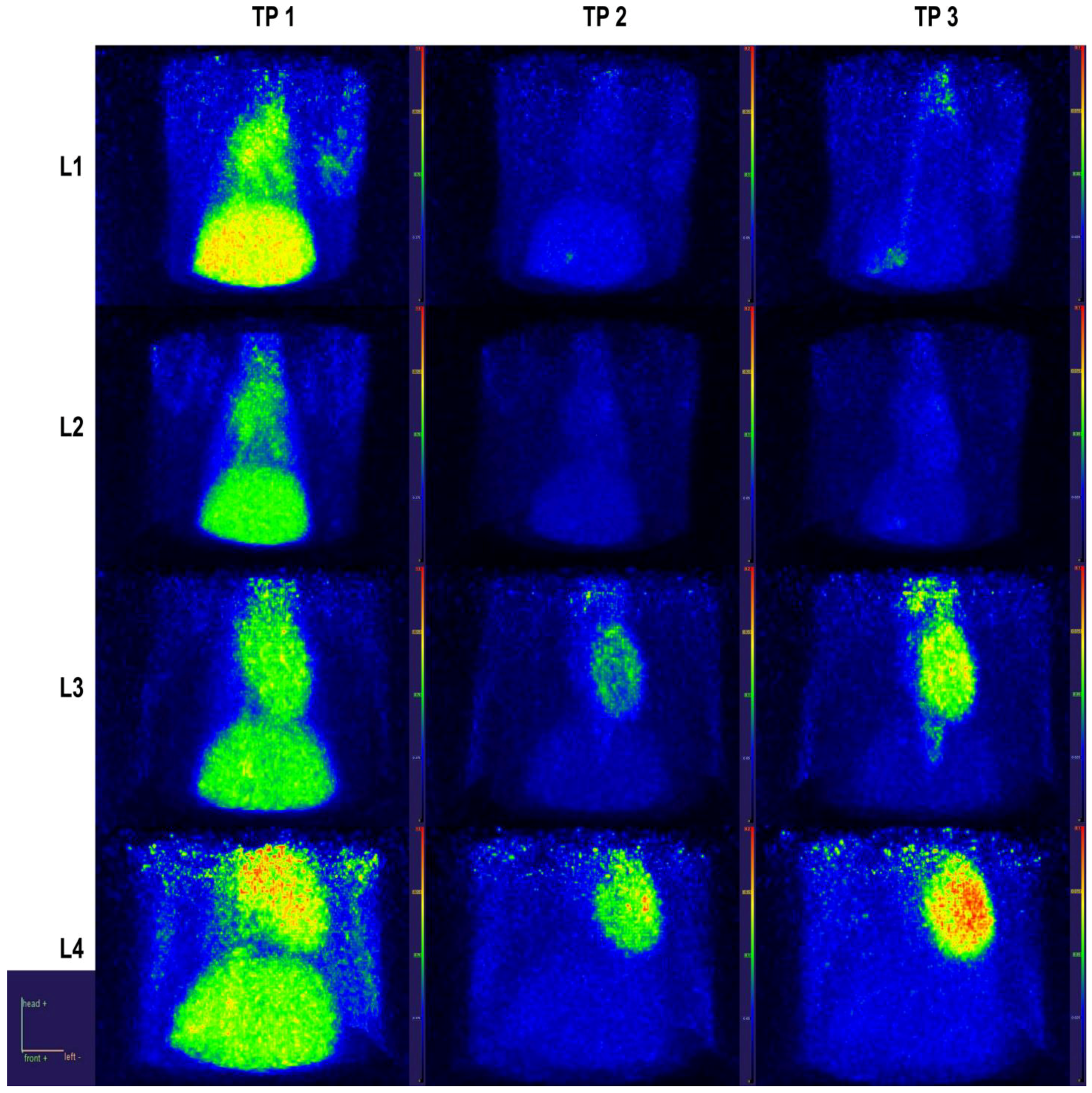
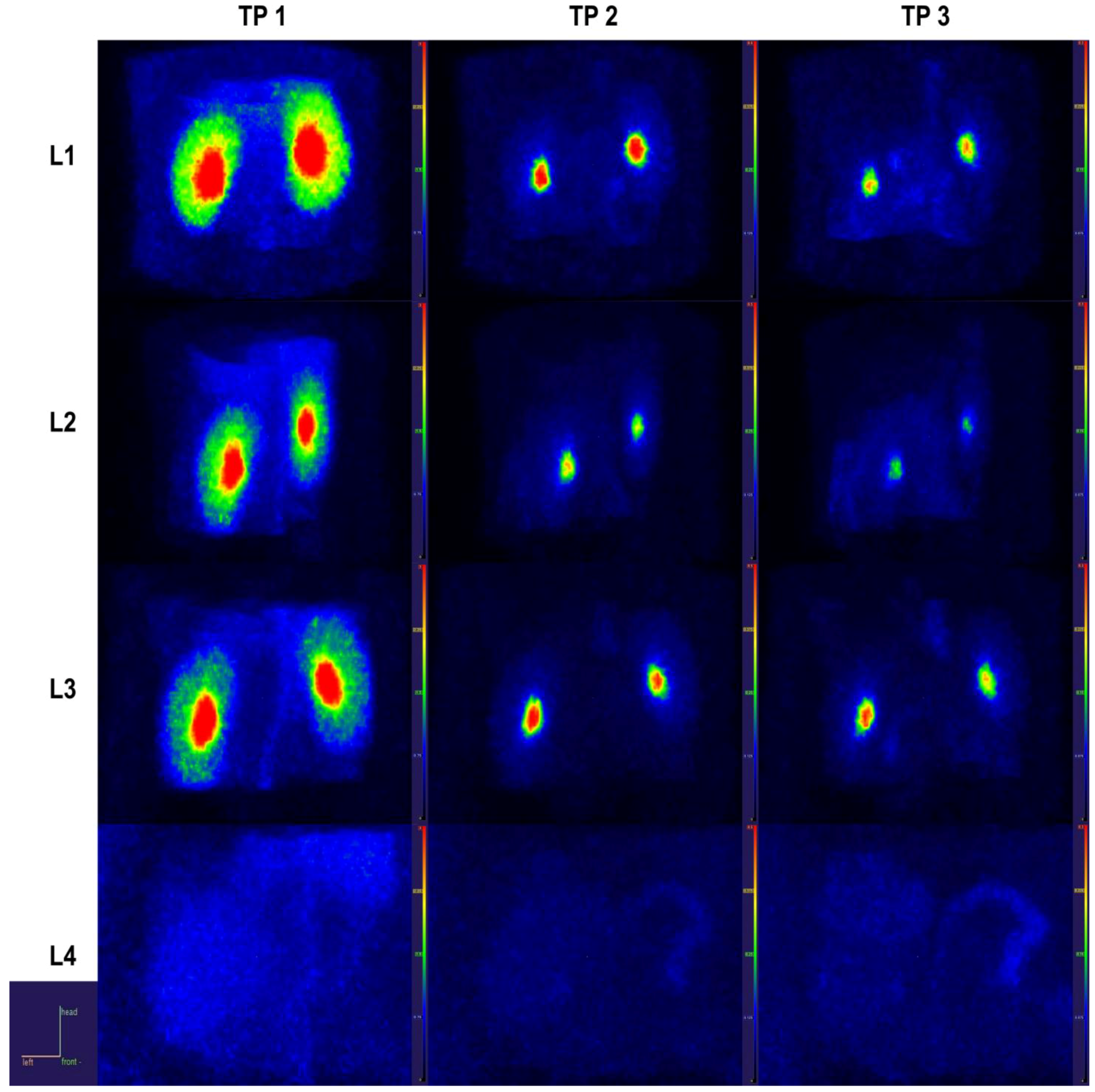
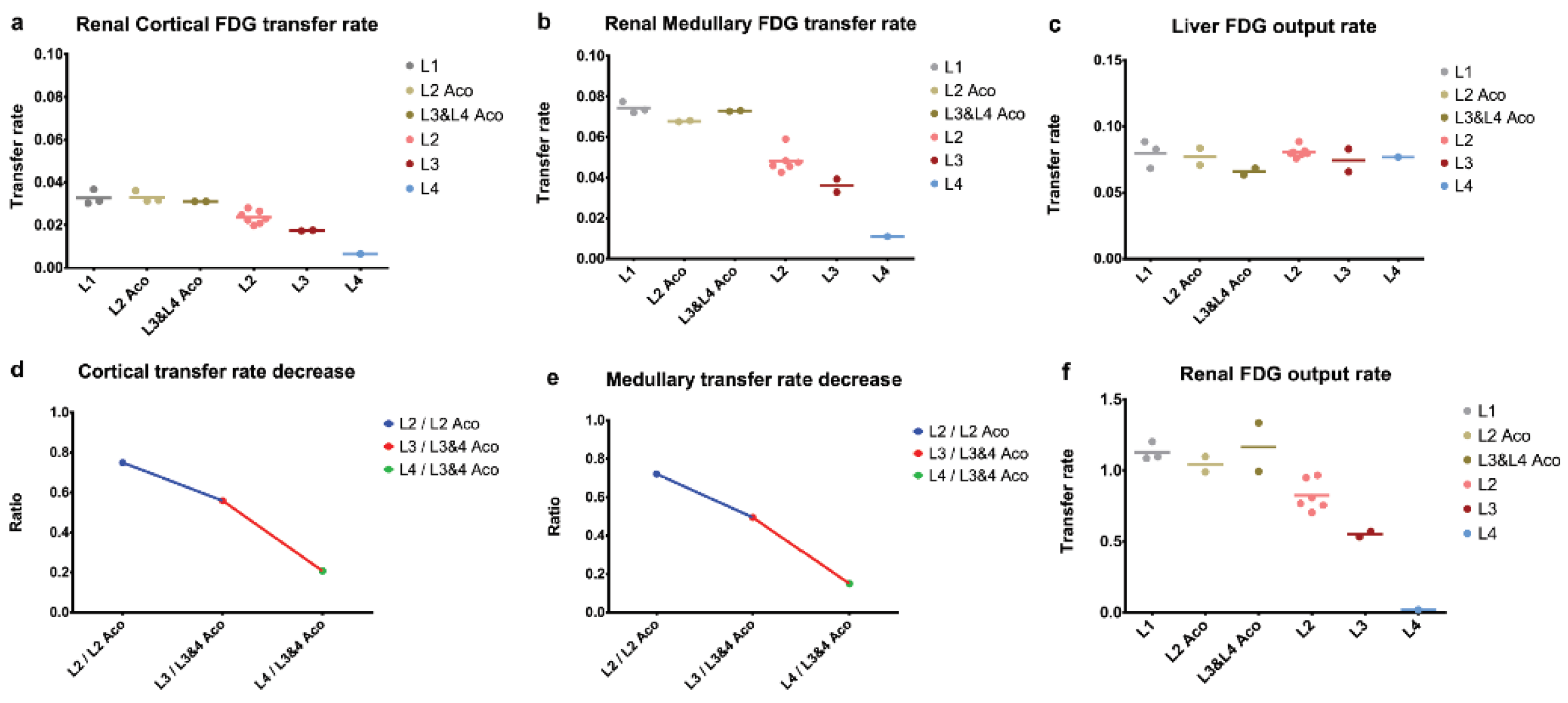
2.4. PET Data
2.4.1. Static Data
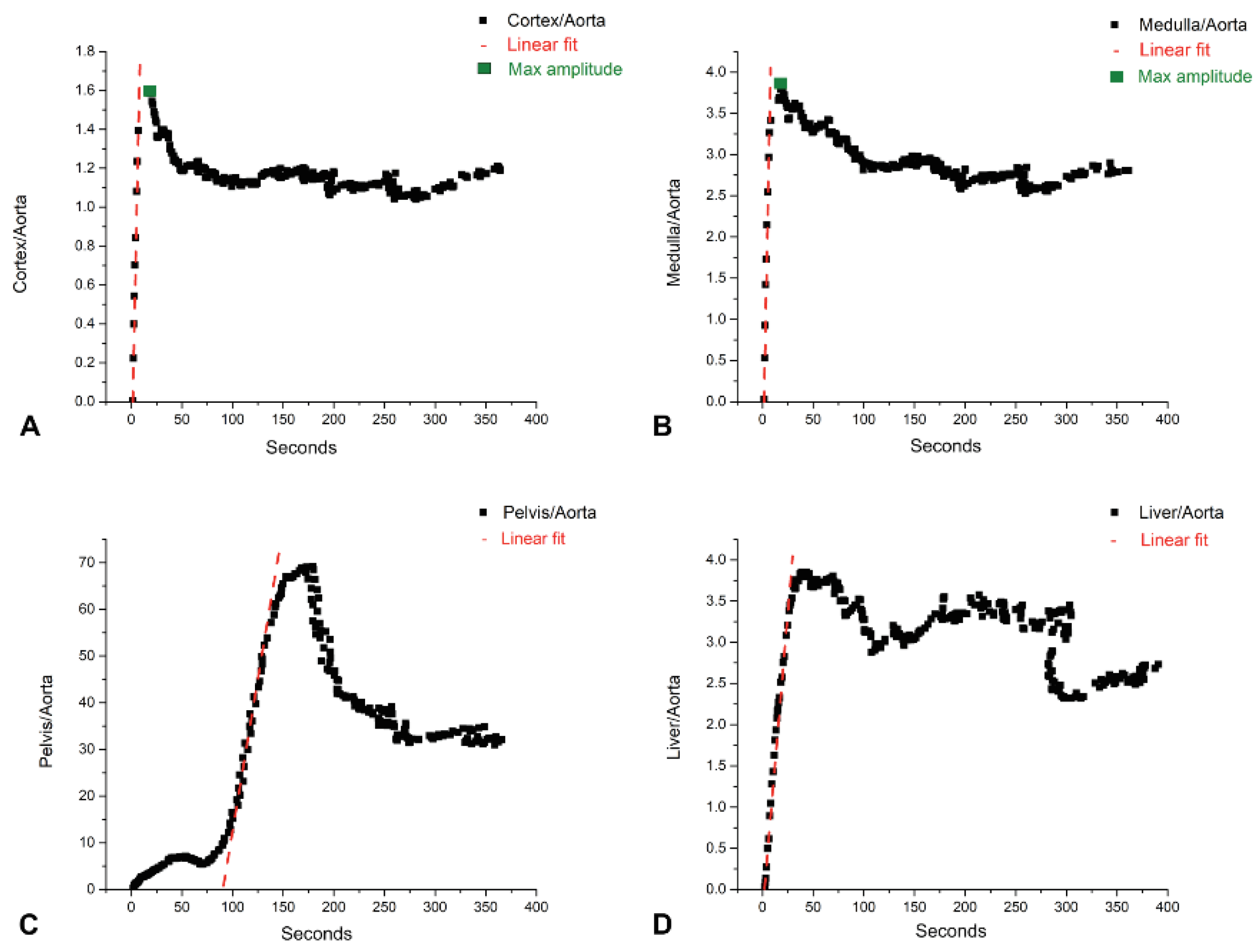
2.4.2. Dynamic Data, Early Patlak on 1 s Reconstruction
2.4.3. Dynamic Data and Compartmental Model
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Doxorubicin and Renal Mass Reduction Protocols
4.3. Clinical, Metabolic Cage Monitoring and Biochemistry
4.4. PET/MRI Data Acquisition
4.5. PET Data Extraction and Analysis
4.6. Histopathology and Immunohistochemistry Analysis
4.7. Statistical Analysis
5. Conclusions
6. Study Limitations
- -
- The high mortality rate among L3 and L4 individuals produced a small sample size and the inability to repeat the experiment if they exhibited tail radiotracer trapping; also, due to financial and bioethical restrictions, we did not have the opportunity to gain more insight into the timeline of model pathophysiology through a repeated sampling of the salient biochemical, histological or imaging markers, at multiple points in time, for the same group of rats (Figure S2).
- -
- Due to the small sample size in target groups (L3, L4) and a large number of target variables, neither the exploratory statistical technique nor hypothesis testing for effect size significance was performed.
- -
- From the perspective of PET/MRI imaging, a serious limitation was the lack of any modality to gate the PET and MR signal to cardiac or respiratory motion due to technical issues, which posed additional difficulties in image data extraction and interpretation. In addition, the limited bore size of the MRI RF antenna made it impossible to accommodate the oversized ascitic L4 rats for T1 anatomical sequencing.
- -
- Another restriction related to the PET/MRI system was an inaccuracy of the scanner bed positioning between the PET and MRI modalities due to the peculiar geometry of the bed, which generated significant challenges in fusing PET and MRI studies.
- -
- During data acquisition and analysis, about ¼ of the subject rats had almost complete tracer trapping in the tail vein upon injection, which led to the exclusion of these individuals from the PET analysis, and subsequently reduced our subject number.
- -
- Due to the aforementioned lack of respiratory gating, limited bore size, and the particular remodeling of the renal parenchyma for L4 rats after surgery meant that the delineation of the renal pelvic region was severely impaired or even impeded in some.
- -
- Regarding subject eligibility for analysis, some individuals exhibited bilateral renal pelvic abnormalities, leading to their exclusion from the PET analysis, the curves derived from this region being incomparable with the ones derived from the other subjects.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronco, C.; McCullough, P.; Anker, S.D.; Anand, I.; Aspromonte, N.; Bagshaw, S.M.; Bellomo, R.; Berl, T.; Bobek, I.; Cruz, D.N.; et al. Cardio-renal syndromes: Report from the consensus conference of the Acute Dialysis Quality Initiative. Eur. Heart J. 2009, 31, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Kumar, U.; Wettersten, N.; Garimella, P.S. Cardiorenal Syndrome. Cardiol. Clin. 2019, 37, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Grossman, R.C. Experimental Models of Renal Disease and the Cardiovascular System. Open Cardiovasc. Med. J. 2010, 4, 257–264. [Google Scholar]
- Chang, D.; Wang, Y.C.; Zhang, S.J.; Bai, Y.Y.; Liu, D.F.; Zang, F.C.; Wang, G.; Wang, B.; Ju, S. Visualizing myocardial inflammation in a rat model of type 4 cardiorenal syndrome by dual-modality molecular imaging. Biomaterials 2015, 68, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chuppa, S.; Liang, M.; Liu, P.; Liu, Y.; Casati, M.C.; Cowley, A.W.; Patullo, L.; Kriegel, A.J. MicroRNA-21 regulates peroxisome proliferator–activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4. Kidney Int. 2017, 93, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Cervenka, L.; Viklicky, O.; Franekova, J.; Havlenova, T.; Behounek, M.; Chmel, M.; Petrak, J. Kidney Response to Heart Failure: Proteomic Analysis of Cardiorenal Syndrome. Kidney Blood Press. Res. 2018, 43, 1437–1450. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Hong, X.; Miao, J.; Liao, Y.; Hou, F.F.; Zhou, L.; Liu, Y. Wnt/β-catenin signaling mediates both heart and kidney injury in type 2 cardiorenal syndrome. Kidney Int. 2019, 95, 815–829. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chen, Y.-T.; Wallace, C.G.; Chen, K.-H.; Cheng, B.-C.; Sung, P.-H.; Li, Y.-C.; Ko, S.-F.; Chang, H.-W.; Yip, H.-K. Early administration of empagliflozin preserved heart function in cardiorenal syndrome in rat. Biomed. Pharmacother. 2018, 109, 658–670. [Google Scholar] [CrossRef]
- Giam, B.; Kaye, D.M.; Rajapakse, N.W. Role of Renal Oxidative Stress in the Pathogenesis of the Cardiorenal Syndrome. Heart Lung Circ. 2016, 25, 874–880. [Google Scholar] [CrossRef]
- Chua, S.; Lee, F.-Y.; Chiang, H.-J.; Chen, K.-H.; Lu, H.-I.; Chen, Y.-T.; Yang, C.-C.; Lin, K.-C.; Chen, Y.-L.; Kao, G.-S.; et al. The cardioprotective effect of melatonin and exendin-4 treatment in a rat model of cardiorenal syndrome. J. Pineal Res. 2016, 61, 438–456. [Google Scholar] [CrossRef]
- Lahoti, T.S.; Patel, D.; Thekkemadom, V.; Beckett, R.; Ray, S.D. Doxorubicin-Induced In Vivo Nephrotoxicity Involves Oxidative Stress- Mediated Multiple Pro- and Anti-Apoptotic Signaling Pathways. Curr. Neurovasc. Res. 2012, 9, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Svíglerová, J.; Kuncová, J.; Nalos, L.; Tonar, Z.; Rajdl, D.; Stengl, M. Cardiovascular parameters in rat model of chronic renal failure induced by subtotal nephrectomy. Physiol. Res. 2010, 59, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Arrighi, J.A.; Diodati, J.G.; Quyyumi, A.A.; Alavi, K.; Bacharach, S.L.; Marin-Neto, J.A.; Katsiyiannis, P.T.; Bonow, R.O. Myocardial viability in patients with chronic coronary artery disease. Comparison of 99mTc-sestamibi with thallium reinjection and [18F]fluorodeoxyglucose. Circulation 1994, 89, 578–587. [Google Scholar] [CrossRef] [PubMed]
- Youssef, G.; Leung, E.; Mylonas, I.; Nery, P.; Williams, K.; Wisenberg, G.; Gulenchyn, K.Y.; Dekemp, R.A.; Dasilva, J.; Birnie, D.; et al. The Use of 18F-FDG PET in the Diagnosis of Cardiac Sarcoidosis: A Systematic Review and Metaanalysis Including the Ontario Experience. J. Nucl. Med. 2012, 53, 241–248. [Google Scholar] [CrossRef]
- Saby, L.; Laas, O.; Habib, G.; Cammilleri, S.; Mancini, J.; Tessonnier, L.; Casalta, J.-P.; Gouriet, F.; Riberi, A.; Avierinos, J.-F.; et al. Positron Emission Tomography/Computed Tomography for Diagnosis of Prosthetic Valve Endocarditis. J. Am. Coll. Cardiol. 2013, 61, 2374–2382. [Google Scholar] [CrossRef]
- Kandolin, R.M.; Wiefels, C.C.; Mesquita, C.T.; Chong, A.-Y.; Boland, P.; Glineur, D.; Sun, L.; Beanlands, R.S.; Mielniczuk, L.M. The Current Role of Viability Imaging to Guide Revascularization and Therapy Decisions in Patients with Heart Failure and Reduced Left Ventricular Function. Can. J. Cardiol. 2019, 35, 1015–1029. [Google Scholar] [CrossRef]
- Terrovitis, J.; Lautamäki, R.; Bonios, M.; Fox, J.; Engles, J.M.; Yu, J.; Leppo, M.K.; Pomper, M.G.; Wahl, R.L.; Seidel, J.; et al. Noninvasive Quantification and Optimization of Acute Cell Retention by In Vivo Positron Emission Tomography After Intramyocardial Cardiac-Derived Stem Cell Delivery. J. Am. Coll. Cardiol. 2009, 54, 1619–1626. [Google Scholar] [CrossRef]
- Geist, B.K.; Baltzer, P.; Fueger, B.; Hamboeck, M.; Nakuz, T.; Papp, L.; Rasul, S.; Sundar, L.K.S.; Hacker, M.; Staudenherz, A. Assessing the kidney function parameters glomerular filtration rate and effective renal plasma flow with dynamic FDG-PET/MRI in healthy subjects. EJNMMI Res. 2018, 8, 37. [Google Scholar] [CrossRef]
- Garbarino, S.; Caviglia, G.; Sambuceti, G.; Benvenuto, F.; Piana, M. A novel description of FDG excretion in the renal system: Application to metformin-treated models. Phys. Med. Biol. 2014, 59, 2469–2484. [Google Scholar] [CrossRef][Green Version]
- Shen, L.-j.; Lu, S.; Zhou, Y.-h.; Li, L.; Xing, Q.-m.; Xu, Y.-l. Developing a rat model of dilated cardiomyopathy with improved survival. J. Zhejiang Univ.-Sci. B 2016, 17, 975–983. [Google Scholar] [CrossRef]
- Zhang, Y.; Kompa, A.R. A practical guide to subtotal nephrectomy in the rat with subsequent methodology for assessing renal and cardiac function. Nephrology 2014, 19, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Nistiar, F.; Racz, O.; Lukacinova, A.; Hubkova, B.; Novakova, J.; Lovasova, E.; Sedlakova, E. Age dependency on some physiological and biochemical parameters of male Wistar rats in controlled environment. J. Environ. Sci. Health Part A 2012, 47, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Nagy, K.; Tóth, M.; Major, P.; Patay, G.; Egri, G.; Häggkvist, J.; Varrone, A.; Farde, L.; Halldin, C.; Gulyás, B. Performance Evaluation of the Small-Animal nanoScan PET/MRI System. J. Nucl. Med. 2013, 54, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Lanz, B.; Poitry-Yamate, C.; Gruetter, R. Image-Derived Input Function from the Vena Cava for 18F-FDG PET Studies in Rats and Mice. J. Nucl. Med. 2014, 55, 1380–1388. [Google Scholar] [CrossRef]
- Huang, Q.; Massey, J.C.; Mińczuk, K.; Li, J.; Kundu, B.K. Non-invasive determination of blood input function to compute rate of myocardial glucose uptake from dynamic FDG PET images of rat heart in vivo: Comparative study between the inferior vena cava and the left ventricular blood pool with spill over and partial volume corrections. Phys. Med. Biol. 2019, 64, 165010. [Google Scholar] [PubMed]
- Bertoldo, A.; Vicini, P.; Sambuceti, G.; Lammertsma, A.A.; Parodi, O.; Cobelli, C. Evaluation of compartmental and spectral analysis models of [18/F]FDG kinetics for heart and brain studies with PET. IEEE Trans. Biomed. Eng. 1998, 45, 1429–1448. [Google Scholar] [CrossRef]
- Kukreja, S.L.; Gunn, R.N. Bootstrapped DEPICT for error estimation in PET functional imaging. NeuroImage 2004, 21, 1096–1104. [Google Scholar] [CrossRef]
- Doenst, T. Complexities Underlying the Quantitative Determination of Myocardial Glucose Uptake with 2-Deoxyglucose. J. Mol. Cell Cardiol. 1998, 30, 1595–1604. [Google Scholar] [CrossRef]
- Werner, R.A.; Hess, A.; Koenig, T.; Diekmann, J.; Derlin, T.; Melk, A.; Thackeray, J.T.; Bauersachs, J.; Bengel, F.M. Molecular imaging of inflammation crosstalk along the cardio-renal axis following acute myocardial infarction. Theranostics 2021, 11, 7984–7994. [Google Scholar] [CrossRef]
- Sano, Y.; Ito, S.; Yoneda, M.; Nagasawa, K.; Matsuura, N.; Yamada, Y.; Uchinaka, A.; Bando, Y.K.; Murohara, T.; Nagata, K. Effects of various types of anesthesia on hemodynamics cardiac function, and glucose and lipid metabolism in rats. Am. J. Physiol.-Heart Circ. Physiol. 2016, 311, H1360–H1366. [Google Scholar] [CrossRef]
- Tse, F.L.S.; Chang, T.; Finkelstein, B.; Ballard, F.; Jaffe, J.M. Influence of Mode of Intravenous Administration and Blood Sample Collection on Rat Pharmacokinetic Data. J. Pharm. Sci. 1984, 73, 1599–1602. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Perry, J.B.; Allen, D.A.B.J.B.P.M.E.; Sabbah, H.N.; Stauffer, B.L.; Shaikh, S.R.; Cleland, J.G.F.; Colucci, W.S.; Butler, J.; Voors, A.A.; et al. Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2016, 14, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen, J.A.M.; Merritt, M.E.; Comment, A. Measuring changes in substrate utilization in the myocardium in response to fasting using hyperpolarized [1-13C] butyrate and [1-13C] pyruvate. Sci. Rep. 2016, 6, 25573. [Google Scholar] [CrossRef]
- Aubert, G.; Martin, O.J.; Horton, J.L.; Lai, L.; Vega, R.B.; Leone, T.C.; Koves, T.; Gardell, S.J.; Krüger, M.; Hoppel, C.L.; et al. The Failing Heart Relies on Ketone Bodies as a Fuel. Circulation 2016, 133, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Bedi, K.C., Jr.; Snyder, N.W.; Brandimarto, J.; Aziz, M.; Mesaros, C.; Worth, A.J.; Wang, L.L.; Javaheri, A.; Blair, I.A.; Margulies, K.B.; et al. Evidence for Intramyocardial Disruption of Lipid Metabolism and Increased Myocardial Ketone Utilization in Advanced Human Heart Failure. Circulation 2016, 133, 706–716. [Google Scholar] [CrossRef]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cel Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef]
- Raj, S.; Franco, V.I.; Lipshultz, S.E. Anthracycline-Induced Cardiotoxicity: A Review of Pathophysiology Diagnosis, and Treatment. Curr. Treat. Options Cardiovasc. Med. 2014, 16, 1–14. [Google Scholar] [CrossRef]
- Arai, M.; Yoguchi, A.; Takizawa, T.; Yokoyama, T.; Kanda, T.; Kurabayashi, M.; Nagai, R. Mechanism of Doxorubicin-Induced Inhibition of Sarcoplasmic Reticulum Ca2+ -ATPase Gene Transcription. Circ. Res. 2000, 86, 8–14. [Google Scholar] [CrossRef]
- Hayward, R.; Hydock, D.S. Doxorubicin cardiotoxicity in the rat: An in vivo characterization. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–32. [Google Scholar]
- Bárdi, E.; Bobok, I.; Oláh, A.V.; Kappelmayer, J.; Kiss, C. Anthracycline antibiotics induce acute renal tubular toxicity in children with cancer. Pathol. Oncol. Res. 2007, 13, 249–253. [Google Scholar] [CrossRef][Green Version]
- Kalender, Y.; Yel, M.; Kalender, S. Doxorubicin hepatotoxicity and hepatic free radical metabolism in rats. Toxicology 2005, 209, 39–45. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, H.; Ismail, M.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.G.; Ouhtit, A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Storm, G.; Hoesel, Q.G.C.M.; van Groot, G.; de Kop, W.; Steerenberg, P.A.; Hillen, F.C. A comparative study on the antitumor effect cardiotoxicity and nephrotoxicity of doxorubicin given as a bolus, continuous infusion or entrapped in liposomes in the Lou/M Wsl rat. Cancer Chemothe. Pharmacol. 1989, 24, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Zhang, Q.; Guo, J.; Zhang, T.; Zhao, J.; Li, J.; White, A.; Carmichael, P.L.; Westmoreland, C.; Peng, S. A PGC-1α-Mediated Transcriptional Network Maintains Mitochondrial Redox and Bioenergetic Homeostasis against Doxorubicin-Induced Toxicity in Human Cardiomyocytes: Implementation of TT21C. Toxicol. Sci. 2016, 150, 400–417. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Liu, X.; Bawa-Khalfe, T.; Lu, L.-S.; Lyu, Y.L.; Liu, L.F.; Yeh, E.T.H. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat. Med. 2012, 18, 1639–1642. [Google Scholar] [CrossRef]
- Luers, C.; Wachter, R.; Kleta, S.; Uhlir, M.; Koschack, J.; Scherer, M.; Binder, L.; Herrmann-Lingen, C.; Zapf, A.; Kulle, B.; et al. Natriuretic peptides in the detection of preclinical diastolic or systolic dysfunction. Clin. Res. Cardiol. 2010, 99, 217–226. [Google Scholar] [CrossRef]
- Ogawa, T.; Linz, W.; Stevenson, M.; Bruneau, B.G.; de Bold, M.L.K.; Chen, J.H.; Eid, H.; Schölkens, B.A.; de Bold, A.J. Evidence for Load-Dependent and Load-Independent Determinants of Cardiac Natriuretic Peptide Production. Circulation 1996, 93, 2059–2067. [Google Scholar] [CrossRef]
- Hallows, K.R.; Mount, P.F.; Pastor-Soler, N.M.; Power, D.A. Role of the energy sensor AMP-activated protein kinase in renal physiology and disease. Am. J. Physiol.-Renal. Physiol. 2010, 298, F1067–F1077. [Google Scholar] [CrossRef]
- Yin, X.-N.; Wang, J.; Cui, L.-F.; Fan, W.-X. Enhanced glycolysis in the process of renal fibrosis aggravated the development of chronic kidney disease. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4243–4251. [Google Scholar]
- de Boer, I.H. Vitamin D and glucose metabolism in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2008, 17, 566–572. [Google Scholar] [CrossRef]
- Li, A.; Zhang, W.; Zhang, L.; Liu, Y.; Li, K.; Du, G.; Qin, X. Elucidating the time-dependent changes in the urinary metabolome under doxorubicin-induced nephrotoxicity. Toxicol. Lett. 2020, 319, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.; Zárybnický, T.; Omi, T.; Shirai, Y.; Toyokuni, S.; Oda, S.; Yokoi, T. A scrutiny of circulating microRNA biomarkers for drug-induced tubular and glomerular injury in rats. Toxicology 2019, 415, 26–36. [Google Scholar] [CrossRef]
- Henneberry, H.P.; Aherne, G.W. Visualisation of doxorubicin in human and animal tissues and in cell cultures by immunogold-silver staining. Br. J. Cancer 1992, 65, 82–86. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Szalay, C.I.; Erdélyi, K.; Kökény, G.; Lajtár, E.; Godó, M.; Révész, C.; Kaucsár, T.; Kiss, N.; Sárközy, M.; Csont, T.; et al. Oxidative/Nitrative Stress and Inflammation Drive Progression of Doxorubicin-Induced Renal Fibrosis in Rats as Revealed by Comparing a Normal and a Fibrosis-Resistant Rat Strain. PLoS ONE 2015, 10, e0127090. [Google Scholar] [CrossRef]
- Brenner, B.M. Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int. 1983, 23, 647–655. [Google Scholar] [CrossRef]
- Correa-Rotter, R.; Hostetter, T.H.; Manivel, J.C.; Rosenberg, M.E. Renin expression in renal ablation. Hypertension 1992, 20, 483–490. [Google Scholar] [CrossRef]
- Hanifa, M.A.; Skott, M.; Maltesen, R.G.; Rasmussen, B.S.; Nielsen, S.; Frøkiær, J.; Ring, T.; Wimmer, R. Tissue urine and blood metabolite signatures of chronic kidney disease in the 5/6 nephrectomy rat model. Metabolomics 2019, 15, 112. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Chen, Y.T.; Chen, C.H.; Li, Y.C.; Shao, P.L.; Huang, T.H.; Chen, Y.L.; Sun, C.K.; Yip, H.K. The therapeutic impact of entresto on protecting against cardiorenal syndrome-associated renal damage in rats on high protein diet. Biomed. Pharmacother. 2019, 116, 108954. [Google Scholar] [CrossRef]
- Faleiro, L.; Kobayashi, R.; Fearnhead, H.; Lazebnik, Y. Multiple species of CPP32 and Mch2 are the major active caspases present in apoptotic cells. EMBO J. 1997, 16, 2271–2281. [Google Scholar] [CrossRef]
- Liu, H.-Z.; Gao, C.-Y.; Wang, X.-Q.; Fu, H.-X.; Yang, H.-H.; Wang, X.-P.; Liu, Y.-H.; Li, M.-W.; Niu, Z.-M.; Dai, G.-Y.; et al. Angiotensin(1-7) attenuates left ventricular dysfunction and myocardial apoptosis on rat model of adriamycin-induced dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi 2012, 40, 219–224. [Google Scholar]
- Gao, J.; Wei, L.; Song, J.; Jiang, H.; Gao, Y.; Wu, X.; Xu, S. In vitro and in vivo study of the expression of the Syk/Ras/c-Fos pathway in chronic glomerulonephritis. Mol. Med. Rep. 2018, 18, 3683–3690. [Google Scholar] [CrossRef]
- Ranjan, A.; Iwakuma, T. Emerging Non-Canonical Functions and Regulation of p53. Int. J. Mol. Sci. 2018, 19, 1015. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Lima, D.J.M.; Carvalho, J.J.; Tibirica, E.; Borges, J.P.; Matsuura, C. Time course of cardiomyopathy induced by doxorubicin in rats. Pharmacol. Rep. 2019, 71, 583–590. [Google Scholar] [CrossRef]
- Armutcu, F.; Demircan, K.; Yildirim, U.; Namuslu, M.; Yagmurca, M.; Celik, H.T. Hypoxia causes important changes of extracellular matrix biomarkers and ADAMTS proteinases in the adriamycin-induced renal fibrosis model. Nephrology 2019, 24, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Kliem, V.; Johnson, R.J.; Alpers, C.E.; Yoshimura, A.; Couser, W.G.; Koch, K.M.; Floege, J. Mechanisms involved in the pathogenesis of tubulointerstitial fibrosis in 5/6-nephrectomized rats. Kidney Int. 1996, 49, 666–678. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kompa, A.R.; Kumfu, S.; Nishijima, F.; Kelly, D.J.; Krum, H.; Wang, B.H. Subtotal nephrectomy accelerates pathological cardiac remodeling post-myocardial infarction: Implications for cardiorenal syndrome. Int. J. Cardiol. 2013, 168, 1866–1880. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Endo, S.; Fukuda, H.; Abe, Y.; Yoshioka, S.; Itoh, M.; Kubota, K.; Hatazawa, J.; Satoh, T.; Matsuzawa, T.; et al. Experimental studies on myocardial glucose metabolism of rats with 18F-2-fluoro-2-deoxy-d-glucose. Eur. J. Pediatr. 1985, 10, 341–345. [Google Scholar] [CrossRef]
- Chaudhari, U.; Ellis, J.K.; Wagh, V.; Nemade, H.; Hescheler, J.; Keun, H.C.; Sachinidis, A. Metabolite signatures of doxorubicin induced toxicity in human induced pluripotent stem cell-derived cardiomyocytes. Amino. Acids 2017, 49, 1955–1963. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shikano, N.; Nishii, R.; Kiyono, Y.; Araki, H.; Nishi, K.; Oh, M.; Okudaira, H.; Ogura, M.; Yoshimoto, M.; et al. Comparison of the transcellular transport of FDG and D-glucose by the kidney epithelial cell line, LLC-PK1. Nucl. Med. Commun. 2010, 31, 141–146. [Google Scholar] [CrossRef]
- Kriz, W.; Napiwotzky, P. Structural and Functional Aspects of the Renal Interstitium1. In Contributions to Nephrology; Karger, S.A.G., Ed.; Karger Publishers: Basel, Switzerland, 1979; pp. 104–108. [Google Scholar]
- Rodrigues, R.S.; Bozza, F.A.; Hanrahan, C.J.; Wang, L.-M.; Wu, Q.; Hoffman, J.M.; Zimmerman, G.A.; Morton, K.A. 18 F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury. Nucl. Med. Biol. 2017, 48, 52–62. [Google Scholar] [CrossRef]
- Gounden, V.; Bhatt, H.; Jialal, I. Renal Function Tests; Stat Pearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kety, S.S. The theory and applications of the exchange of inert gas at the lungs and tissues. Pharmacol. Rev. 1951, 3, 1–41. [Google Scholar] [PubMed]
- Chade, A.R. Renal vascular structure and rarefaction. Compr. Physiol. 2013, 3, 817–831. [Google Scholar] [PubMed]
- Potter, D.; Jarrah, A.; Sakai, T.; Harrah, J.; Holliday, M.A. Character of Function and Size in Kidney During Normal Growth of Rats. Pediatric. Res. 1969, 3, 51–59. [Google Scholar] [CrossRef] [PubMed][Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furcea, D.M.; Agrigoroaie, L.; Mihai, C.-T.; Gardikiotis, I.; Dodi, G.; Stanciu, G.D.; Solcan, C.; Beschea Chiriac, S.I.; Guțu, M.M.; Ștefănescu, C. 18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study. Int. J. Mol. Sci. 2022, 23, 15409. https://doi.org/10.3390/ijms232315409
Furcea DM, Agrigoroaie L, Mihai C-T, Gardikiotis I, Dodi G, Stanciu GD, Solcan C, Beschea Chiriac SI, Guțu MM, Ștefănescu C. 18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study. International Journal of Molecular Sciences. 2022; 23(23):15409. https://doi.org/10.3390/ijms232315409
Chicago/Turabian StyleFurcea, Dan Mihai, Laurențiu Agrigoroaie, Cosmin-T. Mihai, Ioannis Gardikiotis, Gianina Dodi, Gabriela D. Stanciu, Carmen Solcan, Sorin I. Beschea Chiriac, Mihai Marius Guțu, and Cipriana Ștefănescu. 2022. "18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study" International Journal of Molecular Sciences 23, no. 23: 15409. https://doi.org/10.3390/ijms232315409
APA StyleFurcea, D. M., Agrigoroaie, L., Mihai, C.-T., Gardikiotis, I., Dodi, G., Stanciu, G. D., Solcan, C., Beschea Chiriac, S. I., Guțu, M. M., & Ștefănescu, C. (2022). 18F-FDG PET/MRI Imaging in a Preclinical Rat Model of Cardiorenal Syndrome—An Exploratory Study. International Journal of Molecular Sciences, 23(23), 15409. https://doi.org/10.3390/ijms232315409







