Induction of Corneal Endothelial-like Cells from Mesenchymal Stem Cells of the Umbilical Cord
Abstract
:1. Introduction
2. Results
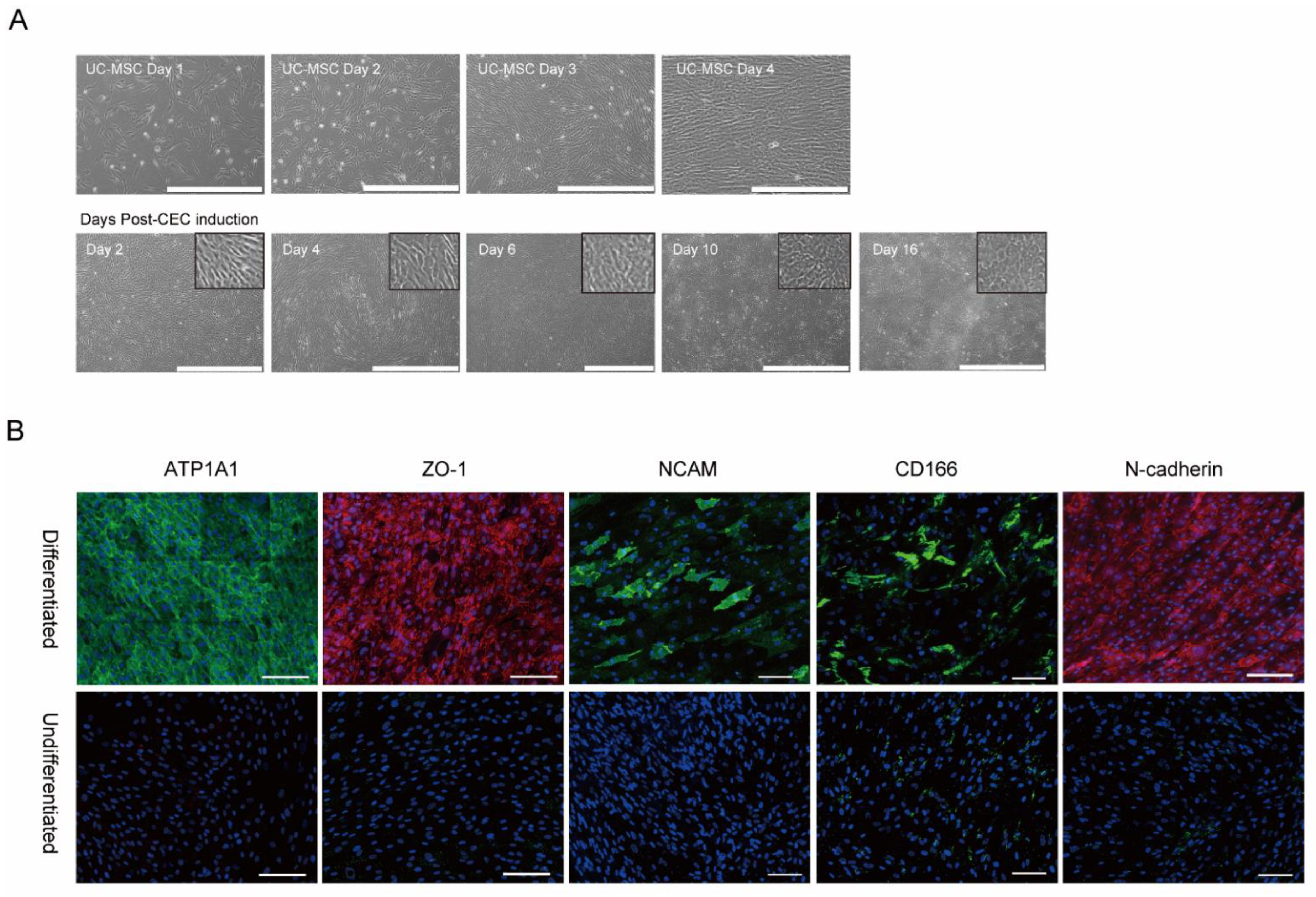
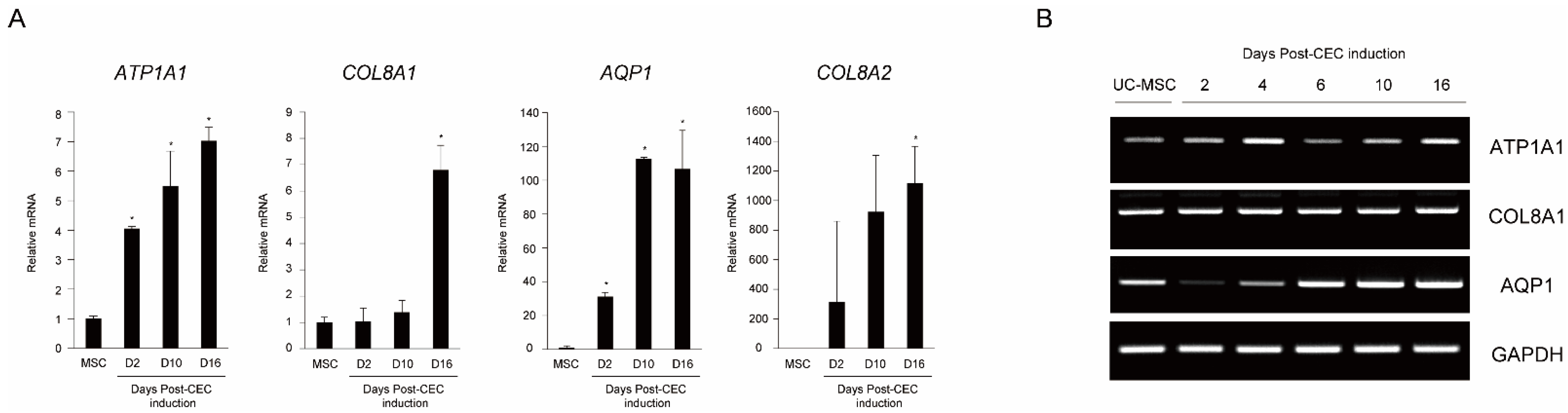
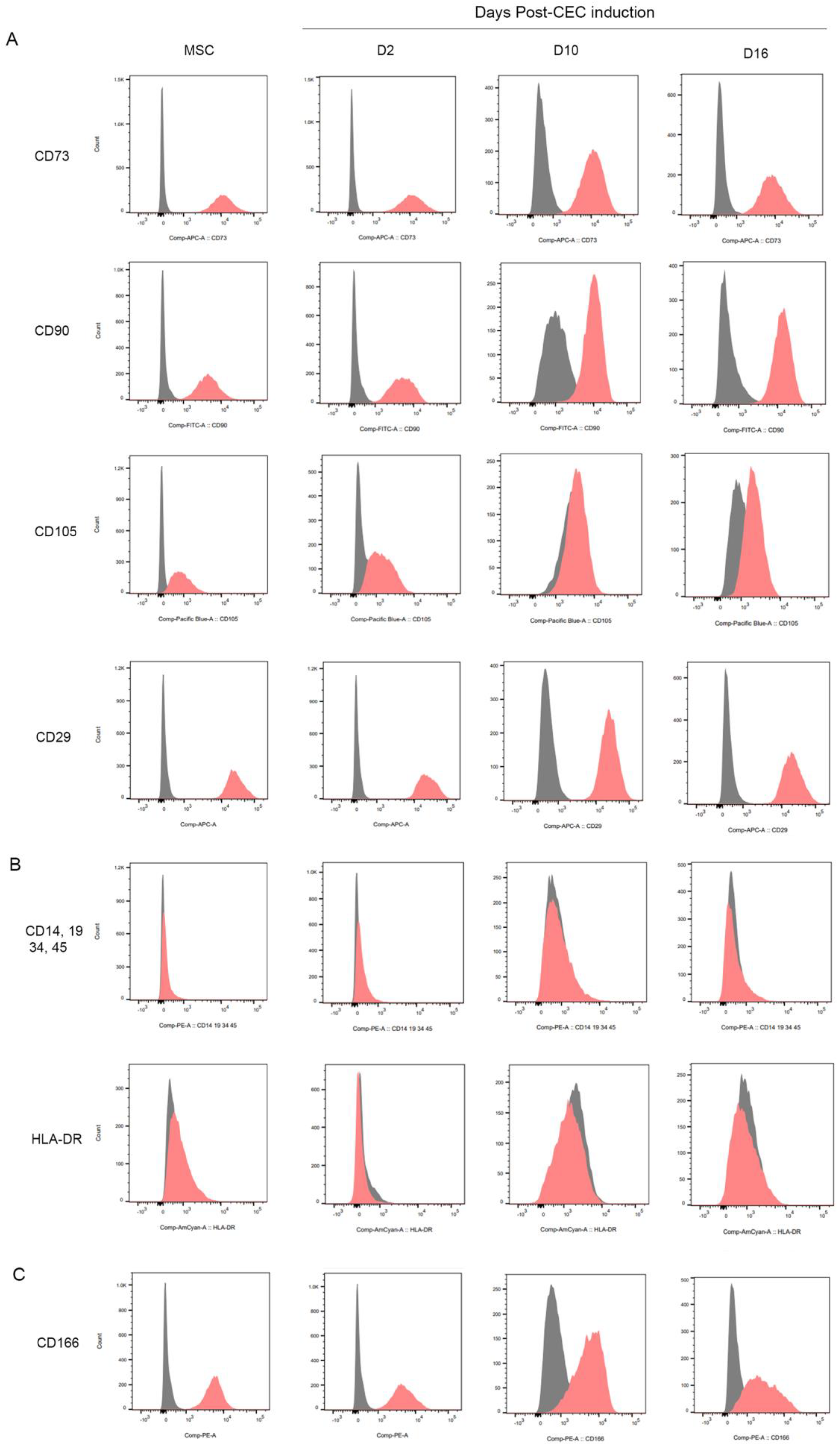
2.1. In vitro Differentiation of MSCs into Corneal Endothelial Cells
2.2. In Vivo Injection of MSCs and MSC-Induced CECs into a Rabbit Anterior Chamber
2.3. Engraft of Human Origin MSC-Induced Corneal Endothelial Cells
3. Discussion
4. Methods and Materials
4.1. Corneal Endothelial Cell Differentiation
4.2. Immunocytochemical Staining of MSC-Induced Corneal Endothelial Cells
4.3. RT-PCR and Quantitative RT-PCR (qRT-PCR)
4.4. Flow Cytometry Analysis
4.5. In Vivo Cell Transplantation Experiments
4.6. Human Mitochondrial DNA Detection
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahn, C.F.; Glassman, R.M.; MacCallum, D.K.; Lillie, J.H.; Meyer, R.F.; Robinson, B.J.; Rich, N.M. Postnatal development of corneal endothelium. Investig. Ophthalmol. Vis. Sci. 1986, 27, 44–51. [Google Scholar]
- Ali, M.; Khan, S.Y.; Gottsch, J.D.; Hutchinson, E.K.; Khan, A.; Riazuddin, S.A. Pluripotent stem cell-derived corneal endothelial cells as an alternative to donor corneal endothelium in keratoplasty. Stem Cell Rep. 2021, 16, 2320–2335. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wu, L.; Li, Z.; Dong, Y.; Pei, X.; Huang, Y.; Wang, L. Directed Differentiation of Human Corneal Endothelial Cells from Human Embryonic Stem Cells by Using Cell-Conditioned Culture Media. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3028–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronroos, P.; Ilmarinen, T.; Skottman, H. Directed Differentiation of Human Pluripotent Stem Cells towards Corneal Endothelial-Like Cells under Defined Conditions. Cells 2021, 10, 331. [Google Scholar] [CrossRef]
- Sun, B.; Bikkuzin, T.; Li, X.; Shi, Y.; Zhang, H. Human-Induced Pluripotent Stem Cells-Derived Corneal Endothelial-Like Cells Promote Corneal Transparency in a Rabbit Model of Bullous Keratopathy. Stem Cells Dev. 2021, 30, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Hatou, S.; Shimmura, S. Review: Corneal endothelial cell derivation methods from ES/iPS cells. Inflamm. Regen. 2019, 39, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pittenger, M.; Vanguri, P.; Simonetti, D.; Young, R. Adult mesenchymal stem cells: Potential for muscle and tendon regeneration and use in gene therapy. J. Musculoskelet. Neuronal. Interact. 2002, 2, 309–320. [Google Scholar]
- Deskins, D.L.; Bastakoty, D.; Saraswati, S.; Shinar, A.; Holt, G.E.; Young, P.P. Human mesenchymal stromal cells: Identifying assays to predict potency for therapeutic selection. Stem Cells Transl. Med. 2013, 2, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.J.; Heazlewood, C.F.; Munster, D.J.; Hutmacher, D.W.; Atkinson, K.; Harkin, D.G. Immunosuppressive properties of mesenchymal stromal cell cultures derived from the limbus of human and rabbit corneas. Cytotherapy 2014, 16, 64–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Roh, D.S.; Funderburgh, M.L.; Mann, M.M.; Marra, K.G.; Rubin, J.P.; Li, X.; Funderburgh, J.L. Adipose-derived stem cells differentiate to keratocytes in vitro. Mol. Vis. 2010, 16, 2680–2689. [Google Scholar]
- Garzon, I.; Martin-Piedra, M.A.; Alfonso-Rodriguez, C.; Gonzalez-Andrades, M.; Carriel, V.; Martinez-Gomez, C.; Campos, A.; Alaminos, M. Generation of a biomimetic human artificial cornea model using Wharton’s jelly mesenchymal stem cells. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4073–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, T.; Wang, W.; Zhang, J.; Chen, X.; Li, B.Z.; Li, L.S. Experimental study on repairing damage of corneal surface by mesenchymal stem cells transplantation. Zhonghua Yan Ke Za Zhi 2006, 42, 246–250. [Google Scholar] [PubMed]
- Liu, H.; Zhang, J.; Liu, C.Y.; Wang, I.J.; Sieber, M.; Chang, J.; Jester, J.V.; Kao, W.W. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: Lumican null mice. PLoS ONE 2010, 5, e10707. [Google Scholar] [CrossRef] [PubMed]
- Park, C.Y.; Lee, J.K.; Gore, P.K.; Lim, C.Y.; Chuck, R.S. Keratoplasty in the United States: A 10-Year Review from 2005 through 2014. Ophthalmology 2015, 122, 2432–2442. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C. Proliferative capacity of corneal endothelial cells. Exp. Eye Res. 2012, 95, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.O.; Jeon, H.S.; Hyon, J.Y.; Oh, Y.J.; Wee, W.R.; Chung, T.Y.; Shin, Y.J.; Kim, J.W. Recovery of Corneal Endothelial Cells from Periphery after Injury. PLoS ONE 2015, 10, e0138076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numa, K.; Imai, K.; Ueno, M.; Kitazawa, K.; Tanaka, H.; Bush, J.D.; Teramukai, S.; Okumura, N.; Koizumi, N.; Hamuro, J.; et al. Five-Year Follow-up of First 11 Patients Undergoing Injection of Cultured Corneal Endothelial Cells for Corneal Endothelial Failure. Ophthalmology 2021, 128, 504–514. [Google Scholar] [CrossRef]
- Kinoshita, S.; Koizumi, N.; Ueno, M.; Okumura, N.; Imai, K.; Tanaka, H.; Yamamoto, Y.; Nakamura, T.; Inatomi, T.; Bush, J.; et al. Injection of Cultured Cells with a ROCK Inhibitor for Bullous Keratopathy. N. Engl. J. Med. 2018, 378, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Faye, P.A.; Poumeaud, F.; Chazelas, P.; Duchesne, M.; Rassat, M.; Miressi, F.; Lia, A.S.; Sturtz, F.; Robert, P.Y.; Favreau, F.; et al. Focus on cell therapy to treat corneal endothelial diseases. Exp. Eye Res. 2021, 204, 108462. [Google Scholar] [CrossRef] [PubMed]
- Harkin, D.G.; Foyn, L.; Bray, L.J.; Sutherland, A.J.; Li, F.J.; Cronin, B.G. Concise reviews: Can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells 2015, 33, 785–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Q.; Liu, R.; Jiang, J.; Peng, J.; Yang, C.; Zhang, W.; Wang, S.; Song, J. What is the impact of human umbilical cord mesenchymal stem cell transplantation on clinical treatment? Stem Cell Res. Ther. 2020, 11, 519. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Ko, J.H.; Kim, M.K.; Wee, W.R. Effects of mesenchymal stem/stromal cells on cultures of corneal epithelial progenitor cells with ethanol injury. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7628–7635. [Google Scholar] [CrossRef] [PubMed]
- Kacham, S.; Bhure, T.S.; Eswaramoorthy, S.D.; Naik, G.; Rath, S.N.; Parcha, S.R.; Basu, S.; Sangwan, V.S.; Shukla, S. Human Umbilical Cord-Derived Mesenchymal Stem Cells Promote Corneal Epithelial Repair In Vitro. Cells 2021, 10, 1254. [Google Scholar] [CrossRef] [PubMed]
- Azmi, S.M.; Salih, M.; Abdelrazeg, S.; Roslan, F.F.; Mohamed, R.; Tan, J.J.; Shaharuddin, B. Human umbilical cord-mesenchymal stem cells: A promising strategy for corneal epithelial regeneration. Regen. Med. 2020, 15, 1381–1397. [Google Scholar] [CrossRef] [PubMed]
- Joyce, N.C.; Harris, D.L.; Markov, V.; Zhang, Z.; Saitta, B. Potential of human umbilical cord blood mesenchymal stem cells to heal damaged corneal endothelium. Mol. Vis. 2012, 18, 547–564. [Google Scholar] [PubMed]
- Liu, X.W.; Zhao, J.L. Transplantation of autologous bone marrow mesenchymal stem cells for the treatment of corneal endothelium damages in rabbits. Zhonghua Yan Ke Za Zhi 2007, 43, 540–545. [Google Scholar] [PubMed]
- Yamashita, K.; Inagaki, E.; Hatou, S.; Higa, K.; Ogawa, A.; Miyashita, H.; Tsubota, K.; Shimmura, S. Corneal Endothelial Regeneration Using Mesenchymal Stem Cells Derived from Human Umbilical Cord. Stem Cells Dev. 2018, 27, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, K.; Shetty, R.; Ghosh, A. Corneal cell therapy: With iPSCs, it is no more a far-sight. Stem Cell Res. Ther. 2018, 9, 287. [Google Scholar] [CrossRef] [Green Version]
- Hatou, S.; Yoshida, S.; Higa, K.; Miyashita, H.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. Functional corneal endothelium derived from corneal stroma stem cells of neural crest origin by retinoic acid and Wnt/beta-catenin signaling. Stem Cells Dev. 2013, 22, 828–839. [Google Scholar] [CrossRef]
- Inagaki, E.; Hatou, S.; Higa, K.; Yoshida, S.; Shibata, S.; Okano, H.; Tsubota, K.; Shimmura, S. Skin-Derived Precursors as a Source of Progenitors for Corneal Endothelial Regeneration. Stem Cells Transl. Med. 2017, 6, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Afshari, N.A. Generation of Human Corneal Endothelial Cells via In Vitro Ocular Lineage Restriction of Pluripotent Stem Cells. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6878–6884. [Google Scholar] [CrossRef] [Green Version]
- Okumura, N.; Koizumi, N. Regeneration of the Corneal Endothelium. Curr. Eye Res. 2020, 45, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Koizumi, N.; Ueno, M.; Sakamoto, Y.; Takahashi, H.; Tsuchiya, H.; Hamuro, J.; Kinoshita, S. ROCK inhibitor converts corneal endothelial cells into a phenotype capable of regenerating in vivo endothelial tissue. Am. J. Pathol. 2012, 181, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Okumura, N.; Sakamoto, Y.; Fujii, K.; Kitano, J.; Nakano, S.; Tsujimoto, Y.; Nakamura, S.; Ueno, M.; Hagiya, M.; Hamuro, J.; et al. Rho kinase inhibitor enables cell-based therapy for corneal endothelial dysfunction. Sci. Rep. 2016, 6, 26113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okumura, N.; Ueno, M.; Koizumi, N.; Sakamoto, Y.; Hirata, K.; Hamuro, J.; Kinoshita, S. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3680–3687. [Google Scholar] [CrossRef]
- Okumura, N.; Inoue, R.; Okazaki, Y.; Nakano, S.; Nakagawa, H.; Kinoshita, S.; Koizumi, N. Effect of the Rho Kinase Inhibitor Y-27632 on Corneal Endothelial Wound Healing. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6067–6074. [Google Scholar] [CrossRef]
- Meekins, L.C.; Rosado-Adames, N.; Maddala, R.; Zhao, J.J.; Rao, P.V.; Afshari, N.A. Corneal Endothelial Cell Migration and Proliferation Enhanced by Rho Kinase (ROCK) Inhibitors in In Vitro and In Vivo Models. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6731–6738. [Google Scholar] [CrossRef]
- Oikonomopoulos, A.; van Deen, W.K.; Manansala, A.R.; Lacey, P.N.; Tomakili, T.A.; Ziman, A.; Hommes, D.W. Optimization of human mesenchymal stem cell manufacturing: The effects of animal/xeno-free media. Sci. Rep. 2015, 5, 16570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, N.; Kasim, N.H.; Rahman, M.T. Optimization of pre-transplantation conditions to enhance the efficacy of mesenchymal stem cells. Int. J. Biol. Sci. 2015, 11, 324–334. [Google Scholar] [CrossRef] [Green Version]
- Rao, B.M.; Zandstra, P.W. Culture development for human embryonic stem cell propagation: Molecular aspects and challenges. Curr. Opin. Biotechnol. 2005, 16, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Chen, J.; Chen, P.; Zhu, M.; Yao, Q.; Gu, P.; Fu, Y.; Fan, X. Targeted transplantation of human umbilical cord blood endothelial progenitor cells with immunomagnetic nanoparticles to repair corneal endothelium defect. Stem Cells Dev. 2015, 24, 756–767. [Google Scholar] [CrossRef] [PubMed]
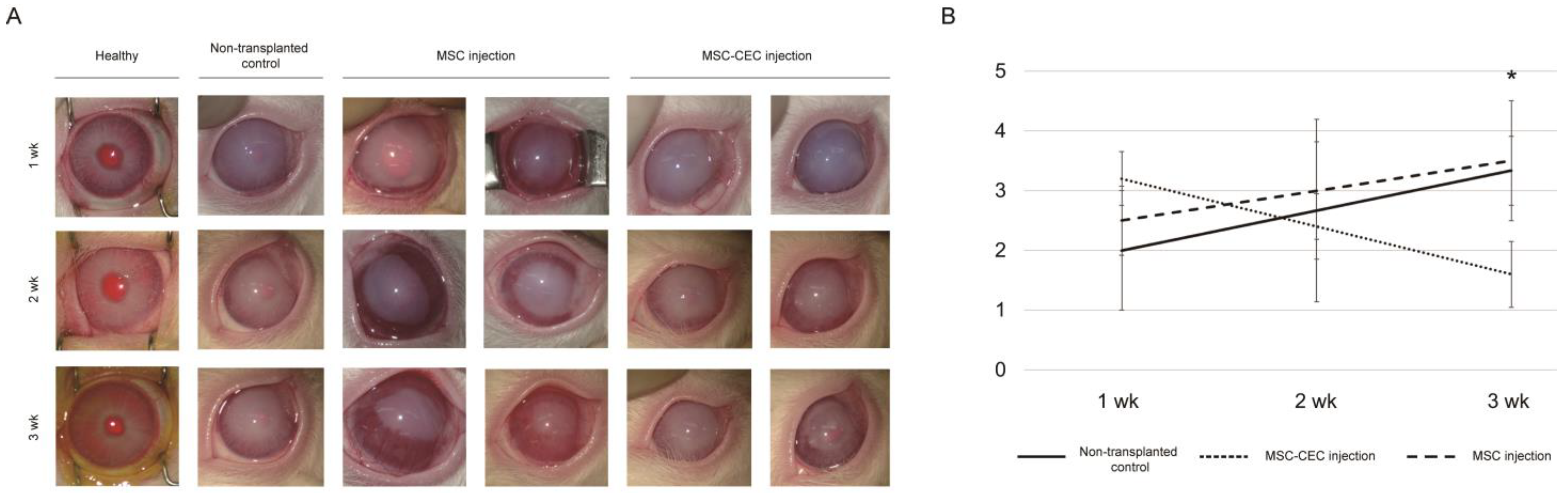

| Non-Transplanted Control (n = 4) | MSC Injection (n = 4) | MSC-Induced CEC Injection (n = 4) | |
|---|---|---|---|
| Week 1 | 2.0 ± 1.0 | 2.5 ± 0.6 | 3.2 ± 0.4 |
| Week 2 | 2.7 ± 1.5 | 3.0 ± 0.8 | 2.4 ± 0.5 |
| Week 3 | 3.3 ± 0.6 | 3.5 ± 1.0 | 1.6 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, E.A.; Chung, H.S.; Park, Y.; Sunwoo, J.H.; Lee, W.; Kim, J.; Tchah, H.; Lee, H.; Kim, J.Y. Induction of Corneal Endothelial-like Cells from Mesenchymal Stem Cells of the Umbilical Cord. Int. J. Mol. Sci. 2022, 23, 15408. https://doi.org/10.3390/ijms232315408
Ye EA, Chung HS, Park Y, Sunwoo JH, Lee W, Kim J, Tchah H, Lee H, Kim JY. Induction of Corneal Endothelial-like Cells from Mesenchymal Stem Cells of the Umbilical Cord. International Journal of Molecular Sciences. 2022; 23(23):15408. https://doi.org/10.3390/ijms232315408
Chicago/Turabian StyleYe, Eun Ah, Ho Seok Chung, Yoonkyung Park, Jeong Hye Sunwoo, Whanseo Lee, Jin Kim, Hungwon Tchah, Hun Lee, and Jae Yong Kim. 2022. "Induction of Corneal Endothelial-like Cells from Mesenchymal Stem Cells of the Umbilical Cord" International Journal of Molecular Sciences 23, no. 23: 15408. https://doi.org/10.3390/ijms232315408
APA StyleYe, E. A., Chung, H. S., Park, Y., Sunwoo, J. H., Lee, W., Kim, J., Tchah, H., Lee, H., & Kim, J. Y. (2022). Induction of Corneal Endothelial-like Cells from Mesenchymal Stem Cells of the Umbilical Cord. International Journal of Molecular Sciences, 23(23), 15408. https://doi.org/10.3390/ijms232315408






