Characterization of PLA/PCL/Green Mussel Shells Hydroxyapatite (HA) Biocomposites Prepared by Chemical Blending Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
| Parameters | Materials | |
|---|---|---|
| PLA [30] | PCL [31] | |
| Wire diameter (mm) | 1.75 | 1.75 |
| Print temperature (°C) | 180–200 | 70–100 |
| Melting point (°C) | 165–180 | 58–62 |
| Density (g/cm3) | 1.24 | 1.28 |
| Elongation at break (%) | 4 | 560 |
| Melt flow index (g/10 min) | 7–9 | 3–5 |
| Pull force (Kgf) | 11–16 | 2 |
| Water absorption (%) | 0.5 | 0.2 |
2.2. Fabrication of Specimen Test and Testing
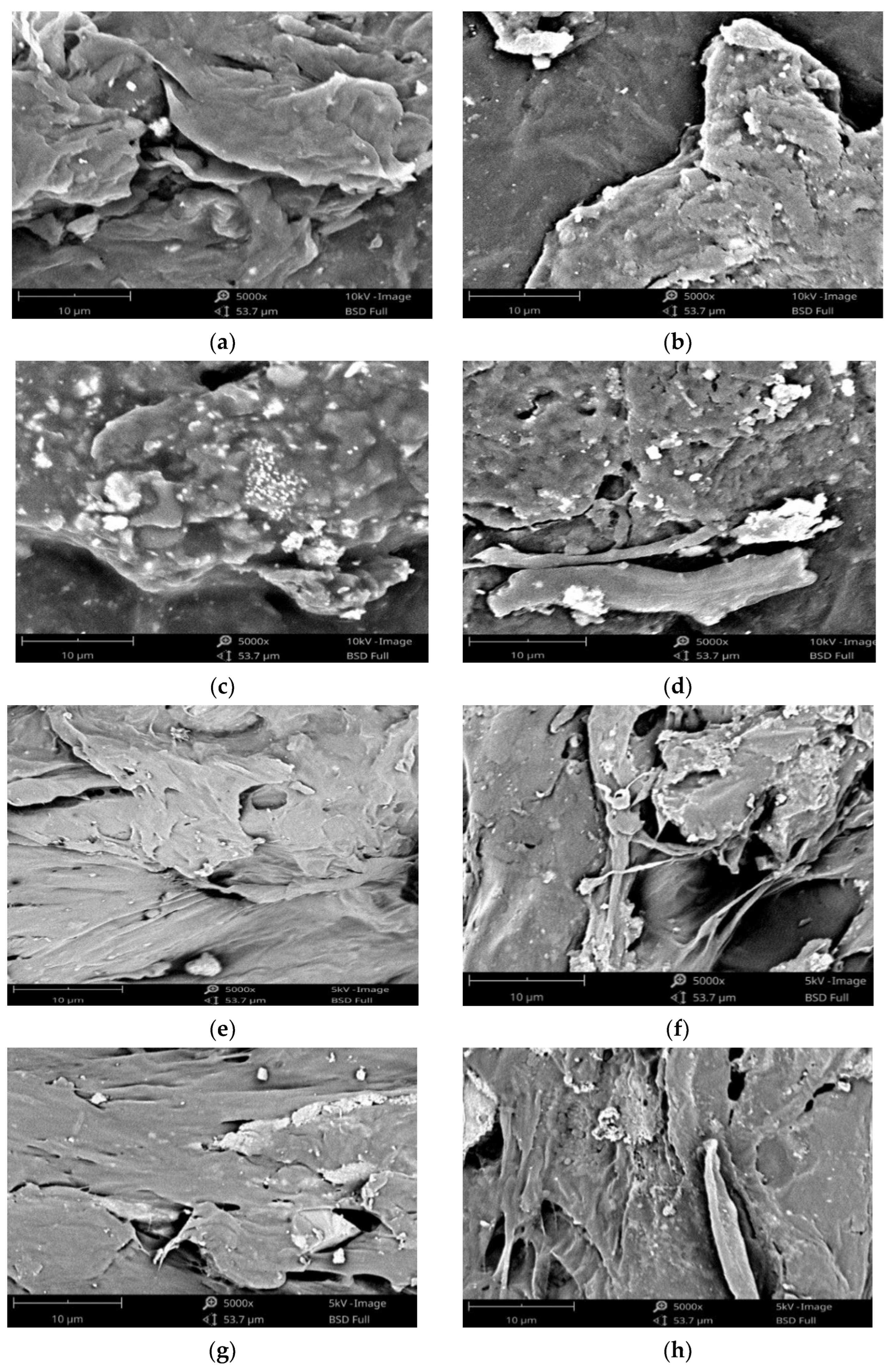
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statistics Indonesia Traffic Accident, Killed Person, Seriously Injured, Slight Injured and Expected of Material Losses Value. Available online: https://www.bps.go.id/indicator/17/513/1/jumlah-kecelakaan-korban-mati-luka-berat-luka-ringan-dan-kerugian-materi.html (accessed on 31 December 2021).
- Budijanto, D. Health Statistics (Health Information System); Ministry of Health Indonesia: Jakarta, Indonesia, 2020; ISBN 9786024169770. [Google Scholar]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Ma’ruf, M.T.; Siswomihardjo, W.; Soesatyo, M.H.N.E.; Tontowi, A. Effect of glutaraldehyde as a crosslinker on mechanical characteristics of catgut reinforced polyvinyl alcohol-hydroxyapatite composite as bone-fracture fixation material. ARPN J. Eng. Appl. Sci. 2015, 10, 6359–6364. [Google Scholar]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 120, 1473–1530. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Katti, K.S.; Verma, D.; Katti, D.R. Materials for joint replacement. In Joint Replacement Technology; Woodhead Publishing Series in Biomaterials; Revell, P.A., Ed.; Woodhead Publishing: Cambridge, UK, 2008; Chapter 4; pp. 81–104. ISBN 978-1-84569-245-2. [Google Scholar]
- Darsan, R.S.; Retnam, B.S.J.; Sivapragash, M. Material Characteristic study and fabrication of Hydroxyapatite (HA) with Poly (Lactide/Lactic) Acids (PLA) for orthopaedic implants. Middle-East J. Sci. Res. 2017, 25, 1491–1500. [Google Scholar] [CrossRef]
- Washington, K.E.; Kularatne, R.N.; Karmegam, V.; Biewer, M.C.; Stefan, M.C. Recent advances in aliphatic polyesters for drug delivery applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1446. [Google Scholar] [CrossRef]
- Moura, N.K.; Siqueira, I.A.W.B.; Machado, J.P.; Kido, H.W.; Avanzi, I.R.; Rennó, A.C.; Trichês, E.D.; Passador, F.R. Production and characterization of porous polymeric membranes of PLA/PCL blends with the addition of hydroxyapatite. J. Compos. Sci. 2019, 3, 45. [Google Scholar] [CrossRef] [Green Version]
- Dehghani Firoozabadi, F.; Ramazani Saadatabadi, A.; Asefnejad, A. In Vitro Studies and Evaluation of Antibacterial Properties of Biodegradable Bone Joints Based on PLA/PCL/HA. J. Clin. Res. Paramed. Sci. 2022, 11, e124080. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Torres, E.; Dominguez-Candela, I.; Castello-Palacios, S.; Vallés-Lluch, A.; Fombuena, V. Development and characterization of polyester and acrylate-based composites with hydroxyapatite and halloysite nanotubes for medical applications. Polymers 2020, 12, 1703. [Google Scholar] [CrossRef] [PubMed]
- Pitjamit, S.; Thunsiri, K.; Nakkiew, W.; Wongwichai, T. The possibility of interlocking nail fabrication from FFF 3D printing PLA/PCL/HA composites coated by local silk fibroin for canine bone fracture treatment. Materials 2020, 13, 1564. [Google Scholar] [CrossRef] [PubMed]
- Azzaoui, K.; Mejdoubi, E.; Lamhamdi, A.; Hammouti, B.; Akartasse, N.; Berrabah, M.; Elidrissi, A.; Jodeh, S.; Hamed, O.; Abidi, N. Novel tricomponenets composites films from polylactic acid/hydroxyapatite/poly-caprolactone suitable for biomedical applications. J. Mater. Environ. Sci. 2016, 7, 761–769. [Google Scholar]
- Fitriyana, D.F.; Ismail, R.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Syahreza Al Mulqi, M. Hydroxyapatite synthesis from clam shell using hydrothermal method: A review. In Proceedings of the 2019 International Biomedical Instrumentation and Technology Conference, IBITeC 2019, Yogyakarta, Indonesia, 23–24 October 2019; pp. 7–11. [Google Scholar]
- Lin, K.; Chang, J. Structure and properties of hydroxyapatite for biomedical applications. In Hydroxyapatite (HAp) for Biomedical Applications; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2015; pp. 3–19. ISBN 9781782420415. [Google Scholar]
- Abdul Halim, N.A.; Hussein, M.Z.; Kandar, M.K. Nanomaterials-upconverted hydroxyapatite for bone tissue engineering and a platform for drug delivery. Int. J. Nanomed. 2021, 16, 6477–6496. [Google Scholar] [CrossRef]
- Ielo, I.; Calabrese, G.; de Luca, G.; Conoci, S. Recent advances in hydroxyapatite-based biocomposites for bone tissue regeneration in orthopedics. Int. J. Mol. Sci. 2022, 23, 9721. [Google Scholar] [CrossRef] [PubMed]
- Raucci, M.G.; D’Antò, V.; Guarino, V.; Sardella, E.; Zeppetelli, S.; Favia, P.; Ambrosio, L. Biomineralized porous composite scaffolds prepared by chemical synthesis for bone tissue regeneration. Acta Biomater. 2010, 6, 4090–4099. [Google Scholar] [CrossRef]
- Fong, M.K.; Rahman, M.N.A.; Arifin, A.M.T.; Haq, R.H.A.; Hassan, M.F.; Taib, I. Characterization, thermal and biological properties of PCL/PLA/PEG/N-HA composites. Biointerface Res. Appl. Chem. 2021, 11, 9017–9026. [Google Scholar] [CrossRef]
- Heimbach, B.; Tonyali, B.; Zhang, D.; Wei, M. High performance resorbable composites for load-bearing bone fixation devices. J. Mech. Behav. Biomed. Mater. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Azmi, A.M.R.; Sultan, M.T.H.; Jawaid, M.; Nor, A.F.M. A newly developed bulletproof vest using kenaf–X-ray film hybrid composites. In Mechanical and Physical Testing of Biocomposites, Fibre-Reinforced Composites and Hybrid Composites; Woodhead Publishing Series in Composites Science and Engineering; Woodhead Publishing: Cambridge, UK, 2019; Chapter 9; pp. 157–169. ISBN 978-0-08-102292-4. [Google Scholar]
- Ferri, J.M.; Jordá, J.; Montanes, N.; Fenollar, O.; Balart, R. Manufacturing and characterization of poly(lactic acid) composites with hydroxyapatite. J. Thermoplast. Compos. Mater. 2017, 31, 865–881. [Google Scholar] [CrossRef] [Green Version]
- Rezania, N.; Asadi-Eydivand, M.; Abolfathi, N.; Bonakdar, S.; Mehrjoo, M.; Solati-Hashjin, M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J. Mater. Sci. Mater. Med. 2022, 33, 31. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Hassanajili, S.; Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and characterization of PLA/PCL/HA composite scaffolds using indirect 3D printing for bone tissue engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef] [PubMed]
- Fitriyana, D.F.; Nugraha, F.W.; Laroybafih, M.B.; Ismail, R.; Bayuseno, A.P.; Muhamadin, R.C.; Ramadan, M.B.; Qudus, A.R.A.; Siregar, J.P. The effect of hydroxyapatite concentration on the mechanical properties and degradation rate of biocomposite for biomedical applications. IOP Conf. Ser. Earth Environ. Sci. 2022, 969, 012045. [Google Scholar] [CrossRef]
- Ismail, R.; Fitriyana, D.F.; Santosa, Y.I.; Nugroho, S.; Hakim, A.J.; Al Mulqi, M.S.; Jamari, J.; Bayuseno, A.P. The potential use of green mussel (Perna Viridis) shells for synthetic calcium carbonate polymorphs in biomaterials. J. Cryst. Growth 2021, 572, 126282. [Google Scholar] [CrossRef]
- PLA 3D Printing Filament. Available online: http://www.sunlugw.com/en/PLA-PG7007757 (accessed on 5 September 2022).
- PCL 3D Printing Filament. Available online: http://www.sunlugw.com/en/PCL-PG7008429 (accessed on 5 September 2022).
- Ismail, R.; Laroybafih, M.B.; Fitriyana, D.F.; Nugroho, S.; Santoso, Y.I.; Hakim, A.J.; Mulqi, M.S.A.; Priharyoto, A. The effect of hydrothermal holding time on the characterization of hydroxyapatite synthesized from green mussel shells. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 80, 84–93. [Google Scholar] [CrossRef]
- Arifvianto, B.; Suyitno; Mahardika, M. Degradation of mechanically surface treated AZ31B magnesium alloy in 3.5 wt.% NaCl solution. Mater. Sci. Forum 2019, 948, 237–242. [Google Scholar] [CrossRef]
- Marco, I.; Feyerabend, F.; Willumeit-Römer, R.; van der Biest, O. Influence of testing environment on the degradation behavior of magnesium alloys for bioabsorbable implants. In TMS2015 Supplemental Proceedings; Springer: Cham, Switzerland, 2015; pp. 497–506. [Google Scholar] [CrossRef]
- Mei, D.; Lamaka, S.V.; Lu, X.; Zheludkevich, M.L. Selecting medium for corrosion testing of bioabsorbable magnesium and other metals—A critical review. Corros. Sci. 2020, 171, 108722. [Google Scholar] [CrossRef]
- Mushtaq, A.; Mukhtar, H.B.; Shariff, A.M. FTIR study of enhanced polymeric blend membrane with amines. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 1811–1820. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Some general aspects of the application of IR spectroscopy to the investigation of minerals. In Springer Mineralogy; Springer: Cham, Switzerland, 2016; pp. 1–49. ISBN 9783319253497. [Google Scholar]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef] [Green Version]
- Shojaei, S.; Nikuei, M.; Goodarzi, V.; Hakani, M.; Khonakdar, H.A.; Saeb, M.R. Disclosing the role of surface and bulk erosion on the viscoelastic behavior of biodegradable poly(ε-caprolactone)/poly(lactic acid)/hydroxyapatite nanocomposites. J. Appl. Polym. Sci. 2019, 136, 47151. [Google Scholar] [CrossRef]
- Ferati, F. Structural information from ratio bands in the FTIR Spectra of long chain and branched alkanes in petrodiesel samples. J. Environ. Treat. Tech. 2020, 8, 1140–1143. [Google Scholar]
- Lu, Y.; Chen, Y.-C.; Zhang, P.-H. Preparation and characterisation of Polylactic acid (PLA)/Polycaprolactone (PCL) composite microfibre membranes. Fibres Text. East. Eur. 2016, 24, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Haq, R.H.A.; Rahman, M.N.A.; Ariffin, A.M.T.; Hassan, M.F.; Yunos, M.Z.; Adzila, S. Characterization and Mechanical Analysis of PCL/PLA composites for FDM feedstock filament. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012038. [Google Scholar] [CrossRef]
- Kim, M.-J.; Koh, Y.-H. Synthesis of aligned porous poly(ε-caprolactone) (PCL)/hydroxyapatite (HA) composite microspheres. Mater. Sci. Eng. C 2013, 33, 2266–2272. [Google Scholar] [CrossRef]
- Jing, X.; Mi, H.-Y.; Turng, L.-S. Comparison between PCL/hydroxyapatite (HA) and PCL/halloysite nanotube (HNT) composite scaffolds prepared by co-extrusion and gas foaming. Mater. Sci. Eng. C 2017, 72, 53–61. [Google Scholar] [CrossRef]
- Åkerlund, E.; Diez-escudero, A.; Grzeszczak, A. The effect of PCL addition on 3D-printable PLA/HA composite filaments for the treatment of bone defects. Polymers 2022, 14, 3305. [Google Scholar] [CrossRef] [PubMed]
- Priselac, D.; Mahović Poljaček, S.; Tomašegović, T.; Leskovac, M. Blends based on poly(ε-Caprolactone) with addition of poly(lactic acid) and coconut fibers: Thermal analysis, ageing behavior and application for embossing process. Polymers 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Ferri, J.M.; Fenollar, O.; Jorda-Vilaplana, A.; García-Sanoguera, D.; Balart, R. Effect of miscibility on mechanical and thermal properties of poly(lactic acid)/polycaprolactone blends. Polym. Int. 2016, 65, 453–463. [Google Scholar] [CrossRef]
- Pires, L.S.O.; Fernandes, M.H.F.V.; de Oliveira, J.M.M. Crystallization kinetics of PCL and PCL-glass composites for additive manufacturing. J. Therm. Anal. Calorim. 2018, 134, 2115–2125. [Google Scholar] [CrossRef]
- Charles, L.F.; Shaw, M.T.; Olson, J.R.; Wei, M. Fabrication and mechanical properties of PLLA/PCL/HA composites via a biomimetic, dip coating, and hot compression procedure. J. Mater. Sci. Mater. Med. 2010, 21, 1845–1854. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Wu, X.; Wu, X.; Zhang, Y.; Li, B. Microstructure and Mechanical Properties of Li2Si2O5 Whisker-Reinforced Glass-Ceramics. Front. Mater. 2022, 9, 849601. [Google Scholar] [CrossRef]
- Terohid, S.A.A.; Heidari, S.; Jafari, A.; Asgary, S. Effect of growth time on structural, morphological and electrical properties of tungsten oxide nanowire. Appl. Phys. A Mater. Sci. Process. 2018, 124, 567. [Google Scholar] [CrossRef] [Green Version]
- Katogi, H.; Takemura, K. The effect of crystallinity on the mechanical properties of plain woven carbon reinforced composites using polypropylene. WIT Trans. Built Environ. 2014, 137, 301–310. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofac. Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Altuntas, E.; Aydemir, D. Effects of Wood Flour on the Mechanical, Thermal and Morphological Properties of Poly (L-Lactic Acid)-Chitosan Biopolymer Composites. Maderas Cienc. y Tecnol. 2019, 21, 611–618. [Google Scholar] [CrossRef]
- Sadudeethanakul, S.; Wattanutchariya, W.; Nakkiew, W.; Chaijaruwanich, A.; Pitjamit, S. Bending Strength and Biological Properties of PLA-HA Composites for Femoral Canine Bone Fixation Plate. IOP Conf. Ser. Mater. Sci. Eng. 2019, 635, 012004. [Google Scholar] [CrossRef] [Green Version]
- Ko, H.S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef]
- Verma, N.; Zafar, S.; Talha, M. Influence of Nano-Hydroxyapatite on Mechanical Behavior of Microwave Processed Polycaprolactone Composite Foams. Mater. Res. Express 2019, 6, 085336. [Google Scholar] [CrossRef]
- Hench, L. An Introduction to Bioceramics, 2nd ed.; World scientific: Hackensack, NJ, USA, 2013; ISBN 978-1-908977-15-1. [Google Scholar]
- Ascenzi, A.; Baschieri, P.; Benvenuti, A. The Bending Properties of Single Osteons. J. Biomech. 1990, 23, 763–771. [Google Scholar] [CrossRef]
- Aldabib, J.M.; Ishak, Z.A.M. Effect of Hydroxyapatite Filler Concentration on Mechanical Properties of Poly (Methyl Methacrylate) Denture Base. SN Appl. Sci. 2020, 2, 732. [Google Scholar] [CrossRef] [Green Version]
- Aydın, M.S. Fabrication and Characterization of PCL-nHA Composite Scaffolds by Using Non-Solvent Induced Phase Separation Technique in Bone Tissue Engineering. Ph.D. Thesis, Sabanci University, Istanbul, Turkey, 2019. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications —A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Mir, S.M.; Aldulijan, I.; Mahajan, A.; Anwar, A.; Leon, C.H.; Terracciano, A.; Zhao, X.; Su, T.L.; Kalyon, D.M.; et al. Load-bearing biodegradable PCL-PGA-beta TCP scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, K.H.E.; Kroemer, H.J.; Kroemer-Elbert, K.E. Engineering Physiology: Bases of Human Factors Engineering/Ergonomics, 4th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Can, E.; Udenir, G.; Kanneci, A.I.; Kose, G.; Bucak, S. Investigation of PLLA/PCL blends and paclitaxel release profiles. AAPS PharmSciTech 2011, 12, 1442–1453. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.C.; Reis, R.L.; Claase, M.B.; Grijpma, D.W.; Feijen, J. Development and properties of polycaprolactone/hydroxyapatite composite biomaterials. J. Mater. Sci. Mater. Med. 2003, 14, 103–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, L.; Zhu, C.; Wu, J.; Liu, X. Hybrid Composites of phosphate glass fibre/nano-hydroxyapatite/polylactide: Effects of nano-hydroxyapatite on mechanical properties and degradation behaviour. J. Mater. Sci. Chem. Eng. 2018, 6, 13–31. [Google Scholar] [CrossRef] [Green Version]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E. Additive manufacturing of PLA-based scaffolds intended for bone regeneration and strategies to improve their biological properties. e-Polymers 2020, 20, 571–599. [Google Scholar] [CrossRef]
- Guo, M.; Chu, Z.; Yao, J.; Feng, W.; Wang, Y.; Wang, L.; Fan, Y. The effects of tensile stress on degradation of biodegradable PLGA membranes: A quantitative study. Polym. Degrad. Stab. 2016, 124, 95–100. [Google Scholar] [CrossRef]
- Lakshmipriya, S.; Imayathamizhan, N.M. Development of silk/polycaprolactone biocomposite for internal bone plate application. J. Ind. Text. 2021, 51, 3–23. [Google Scholar] [CrossRef]
- Whulanza, Y.; Azadi, A.; Supriadi, S.; Rahman, S.F.; Chalid, M.; Irsyad, M.; Nadhif, M.H.; Kreshanti, P. Tailoring mechanical properties and degradation rate of maxillofacial implant based on sago starch/polylactid acid blend. Heliyon 2022, 8, e08600. [Google Scholar] [CrossRef]
- Vieira, A.C.; Vieira, J.C.; Ferra, J.M.; Magalhães, F.D.; Guedes, R.M.; Marques, A.T. Mechanical study of PLA-PCL fibers during in vitro degradation. J. Mech. Behav. Biomed. Mater. 2011, 4, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Prakasam, M.; Locs, J.; Salma-Ancane, K.; Loca, D.; Largeteau, A.; Berzina-Cimdina, L. Biodegradable materials and metallic implants—A review. J. Funct. Biomater. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Smeltzer, S.C.; Bare, B.G.; Hinkle, J.L.; Cheever, K.H. Brunner & Suddarth’s Textbook of Medical Surgical Nursing, 11th ed.; Lippincot Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 14, ISBN 9780781785921. [Google Scholar]

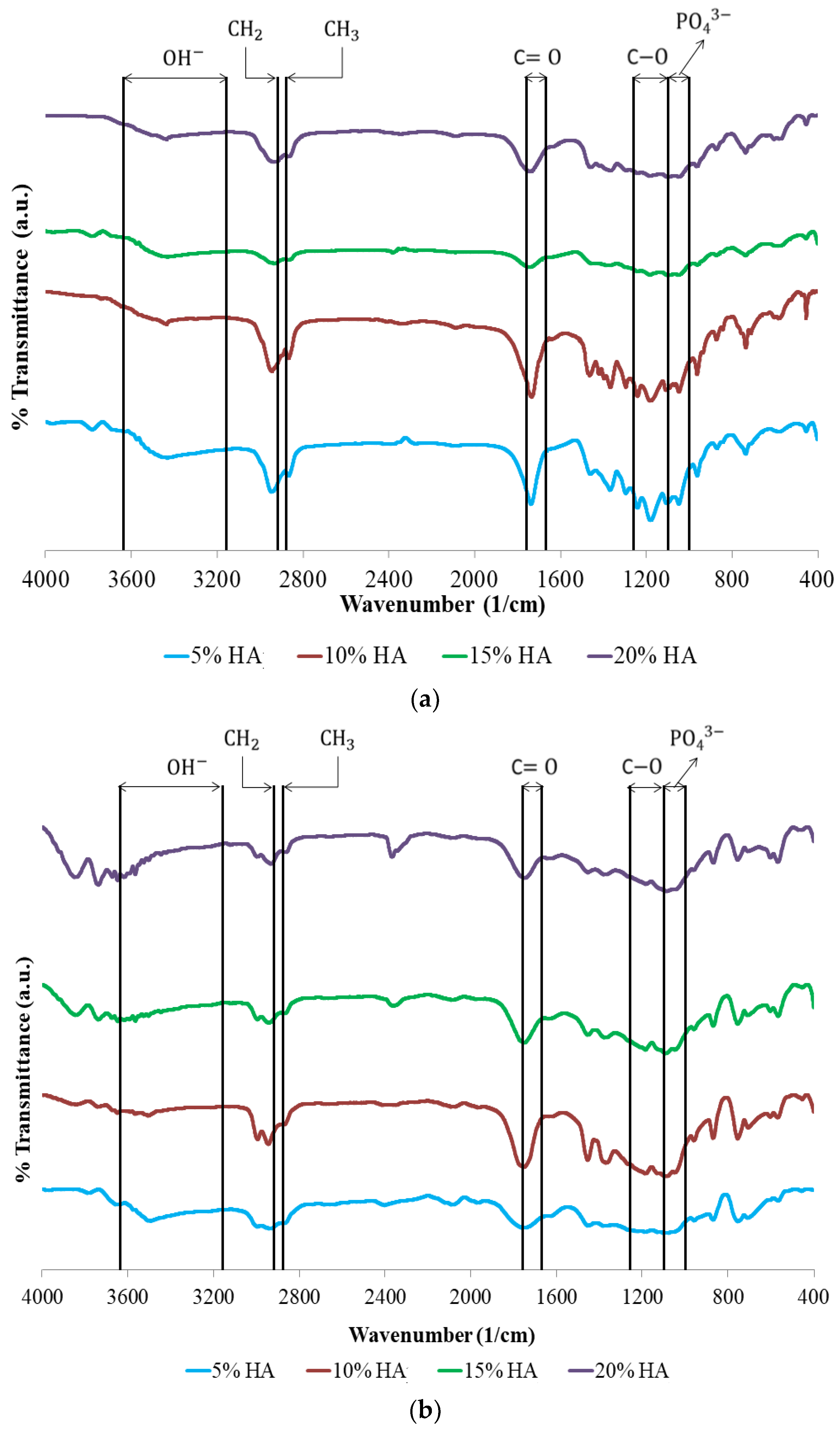



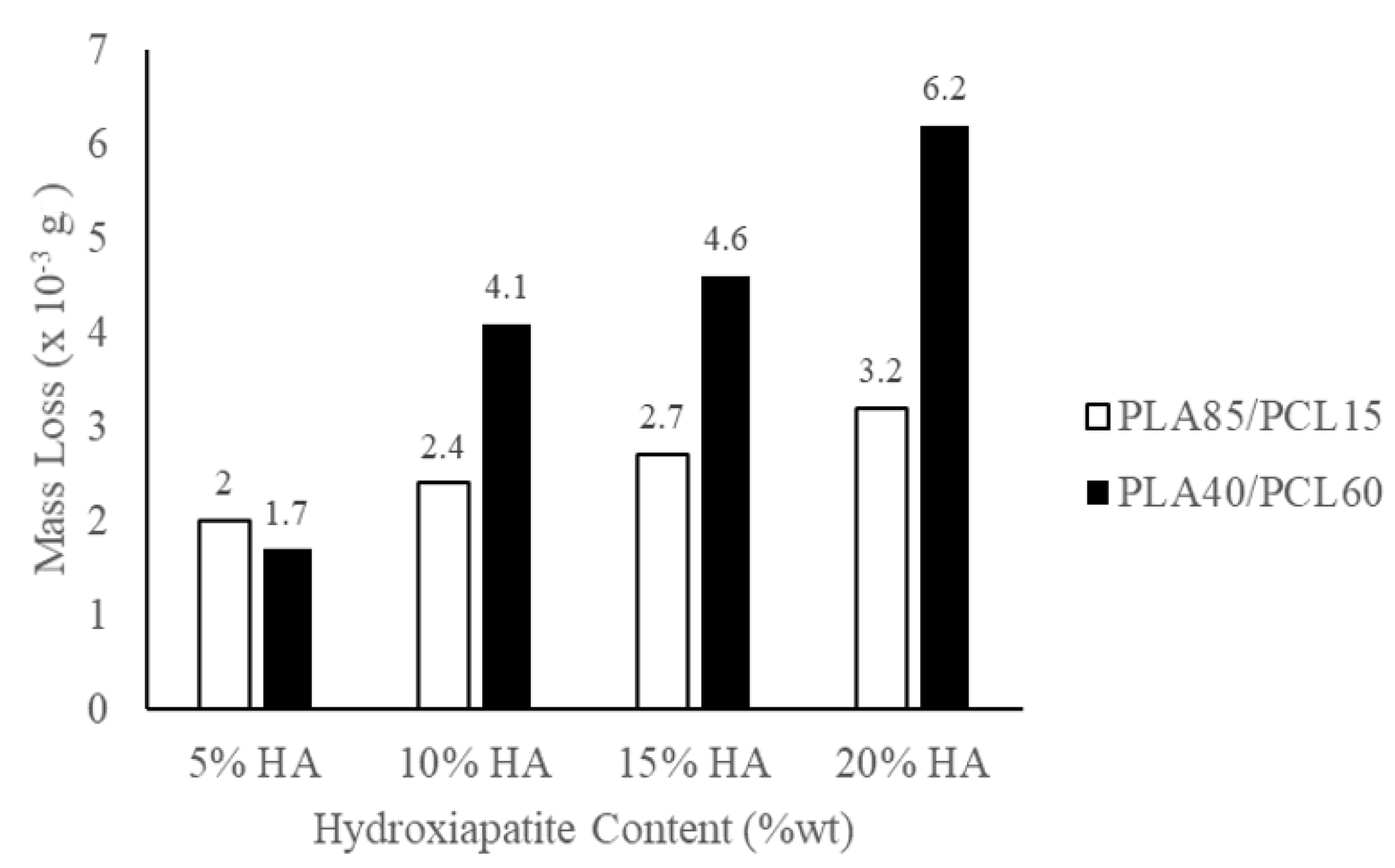
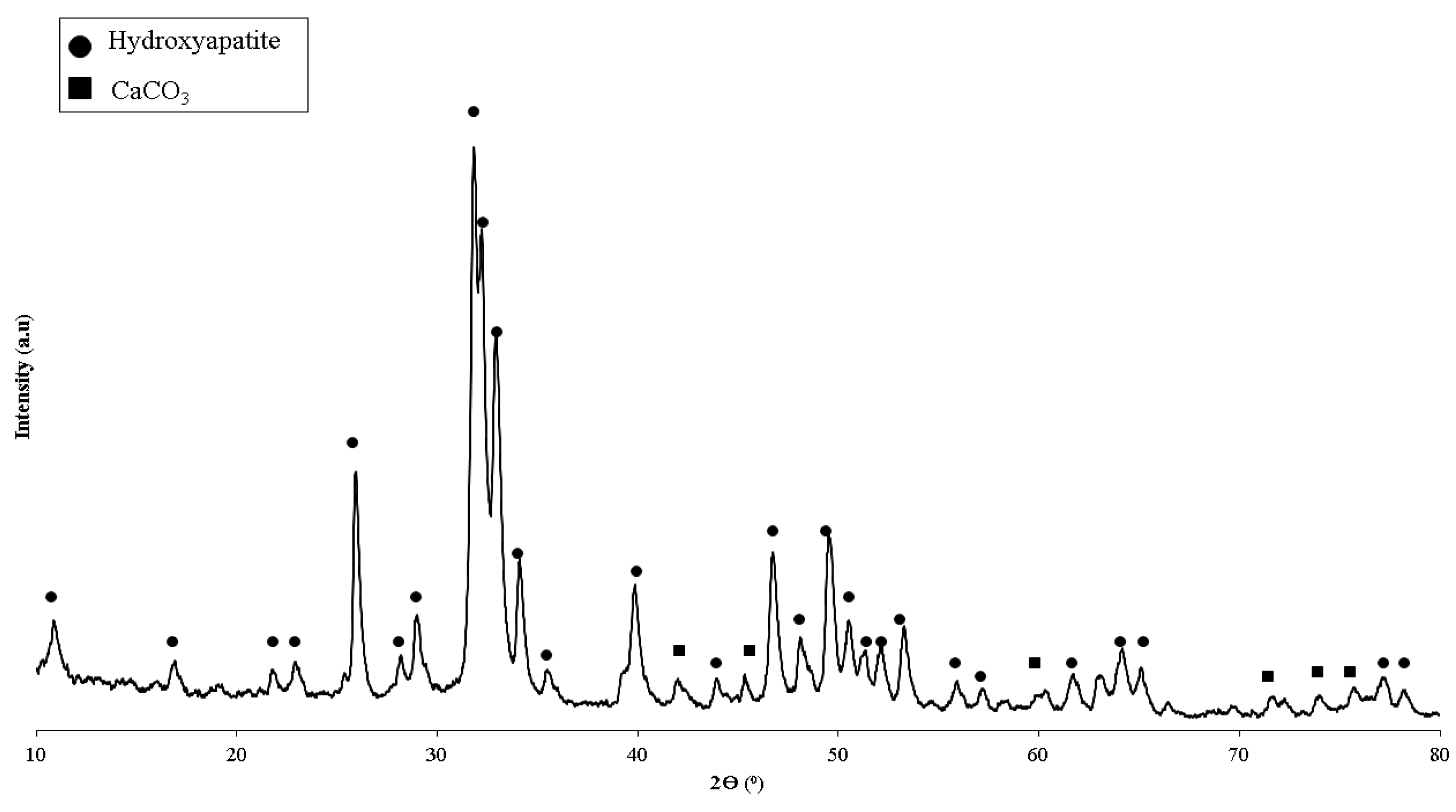
| Polymeric Blends | Ratio (w/w) | HA (wt%) | Sample Codes |
|---|---|---|---|
| PLA/PCL | 40/60 | 5 | PLA40/PCL60/HA5 |
| PLA/PCL | 40/60 | 10 | PLA40/PCL60/HA10 |
| PLA/PCL | 40/60 | 15 | PLA40/PCL60/HA15 |
| PLA/PCL | 40/60 | 20 | PLA40/PCL60/HA20 |
| PLA/PCL | 85/15 | 5 | PLA85/PCL15/HA5 |
| PLA/PCL | 85/15 | 10 | PLA85/PCL15/HA10 |
| PLA/PCL | 85/15 | 15 | PLA85/PCL15/HA15 |
| PLA/PCL | 85/15 | 20 | PLA85/PCL15/HA20 |
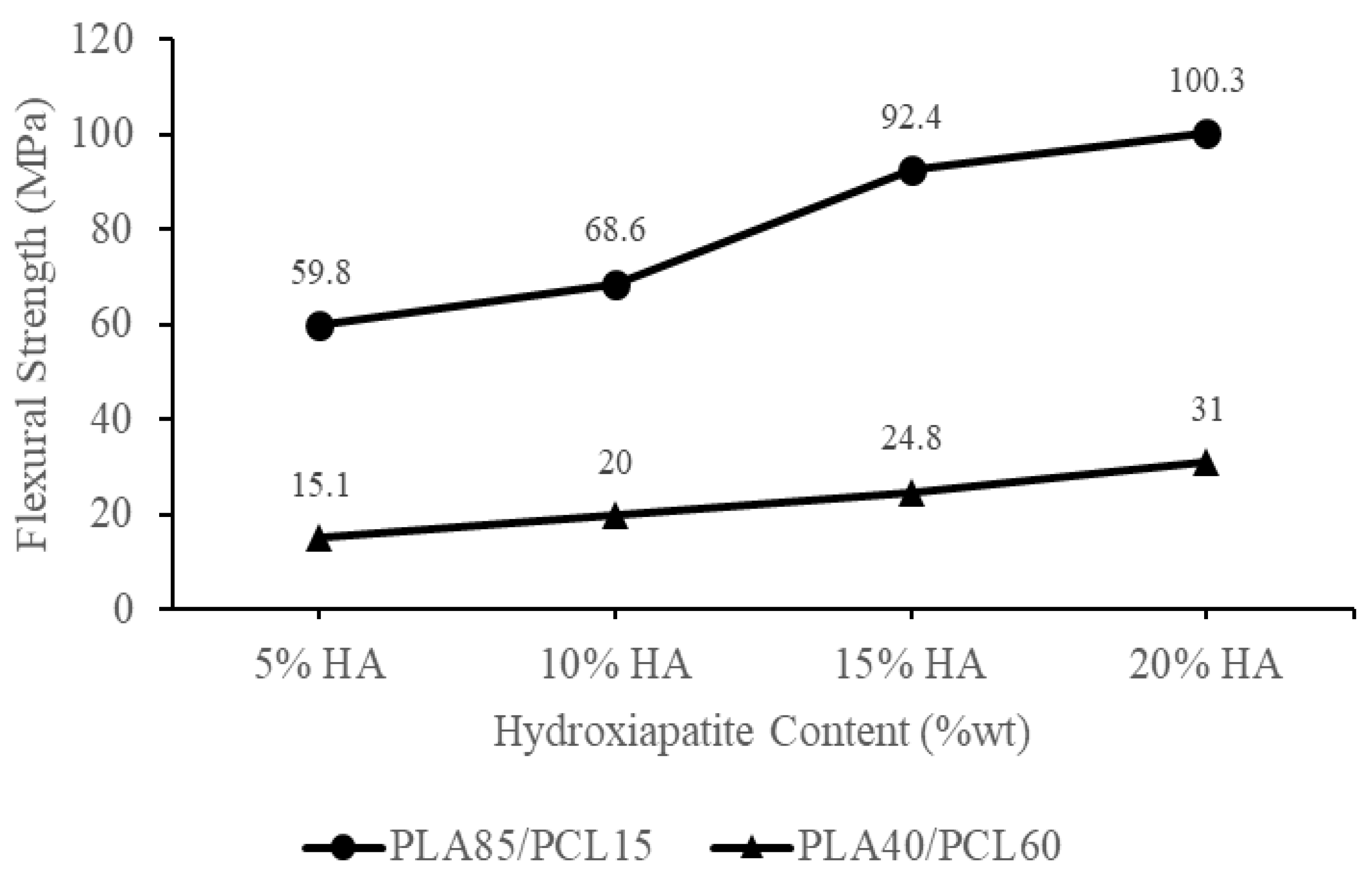
| Materials | Flexural Strength (MPa) | Ref |
|---|---|---|
| Polylactic acid: PLA | 72.3–108 | [14,57,58] |
| Polycaprolactone: PCL | 7.2–29 | [14] |
| PLA30/70PCL | 8 | [42] |
| PLA/30HA | 54 | [57] |
| PLA/5HA | 75.275 | [59] |
| PLA/5HA | 84 | [60] |
| PCL/20HA | 7.2 | [61] |
| PLA85/15PCL/20HA | 97.7 | [28] |
| PLA85/15PCL/15HA | 82.8 | |
| PLA85/15PCL/10HA | 61.3 | |
| PLA85/15PCL/5HA | 56.6 | |
| PLA/PCL/15HA 3D-Printed | 101.10 | [14] |
| PLA/PCL/5HA 3D-Printed | 101.21 | |
| PLA40/PCL60/HA5 | 15.1 | Current study |
| PLA40/PCL60/HA10 | 20 | |
| PLA40/PCL60/HA15 | 24.8 | |
| PLA40/PCL60/HA20 | 31 | |
| PLA85/PCL15/HA5 | 59.8 | |
| PLA85/PCL15/HA10 | 68.6 | |
| PLA85/PCL15/HA15 | 92.4 | |
| PLA85/PCL15/HA20 | 100.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, R.; Cionita, T.; Lai, Y.L.; Fitriyana, D.F.; Siregar, J.P.; Bayuseno, A.P.; Nugraha, F.W.; Muhamadin, R.C.; Irawan, A.P.; Hadi, A.E. Characterization of PLA/PCL/Green Mussel Shells Hydroxyapatite (HA) Biocomposites Prepared by Chemical Blending Methods. Materials 2022, 15, 8641. https://doi.org/10.3390/ma15238641
Ismail R, Cionita T, Lai YL, Fitriyana DF, Siregar JP, Bayuseno AP, Nugraha FW, Muhamadin RC, Irawan AP, Hadi AE. Characterization of PLA/PCL/Green Mussel Shells Hydroxyapatite (HA) Biocomposites Prepared by Chemical Blending Methods. Materials. 2022; 15(23):8641. https://doi.org/10.3390/ma15238641
Chicago/Turabian StyleIsmail, Rifky, Tezara Cionita, Yin Ling Lai, Deni Fajar Fitriyana, Januar Parlaungan Siregar, Athanasius Priharyoto Bayuseno, Fariz Wisda Nugraha, Rilo Chandra Muhamadin, Agustinus Purna Irawan, and Agung Efriyo Hadi. 2022. "Characterization of PLA/PCL/Green Mussel Shells Hydroxyapatite (HA) Biocomposites Prepared by Chemical Blending Methods" Materials 15, no. 23: 8641. https://doi.org/10.3390/ma15238641







