Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole
Abstract
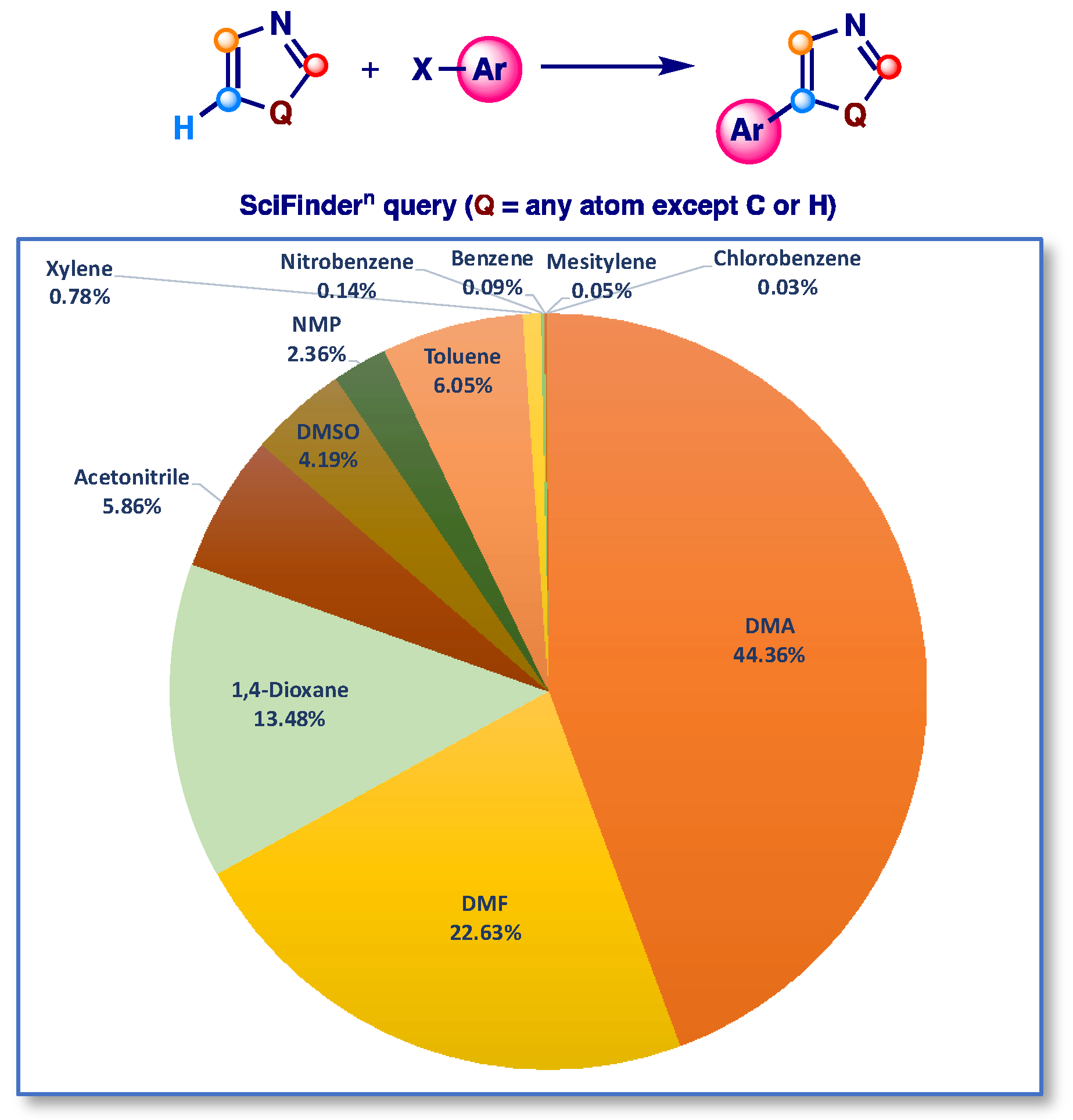
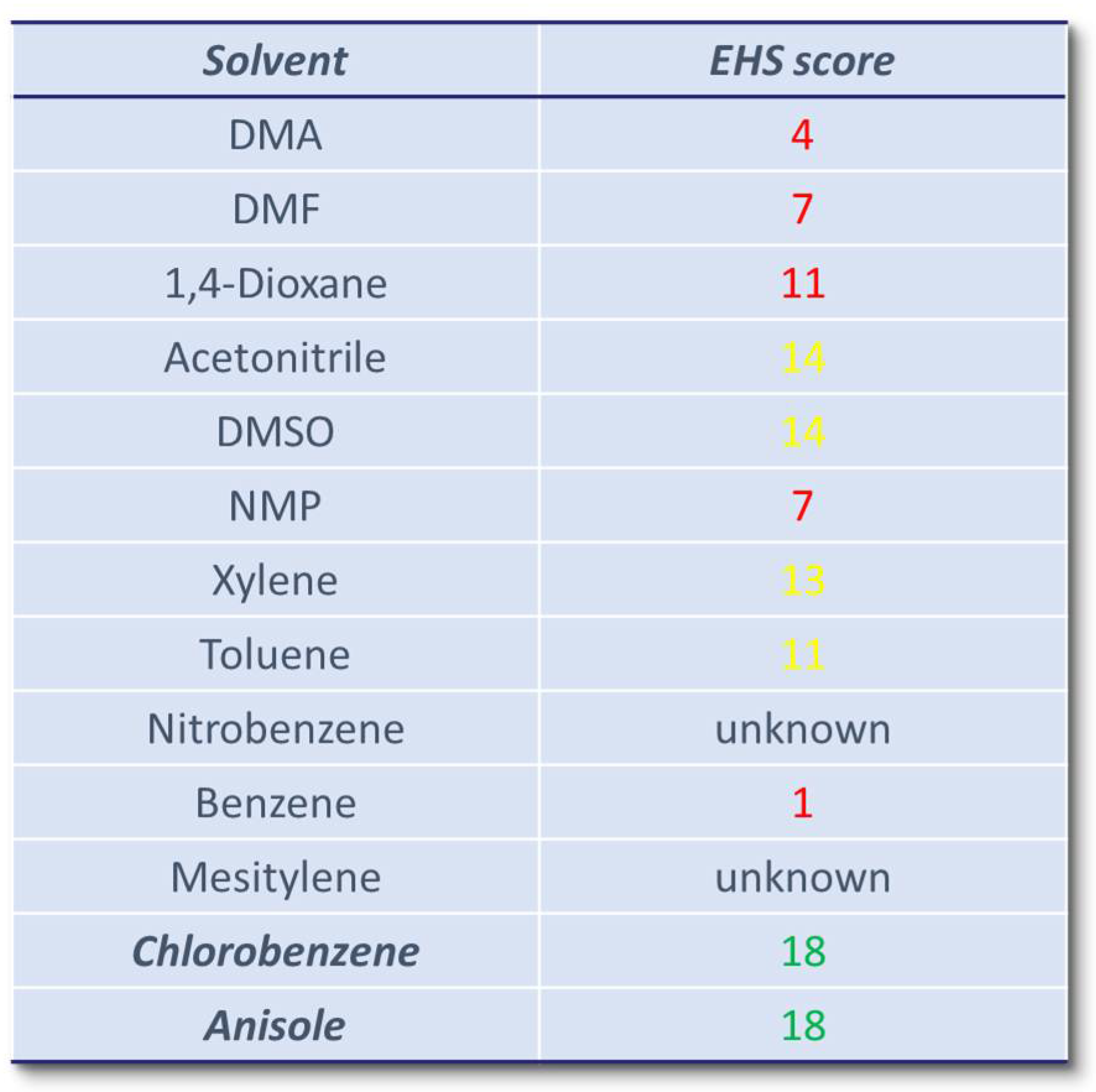
1. Introduction
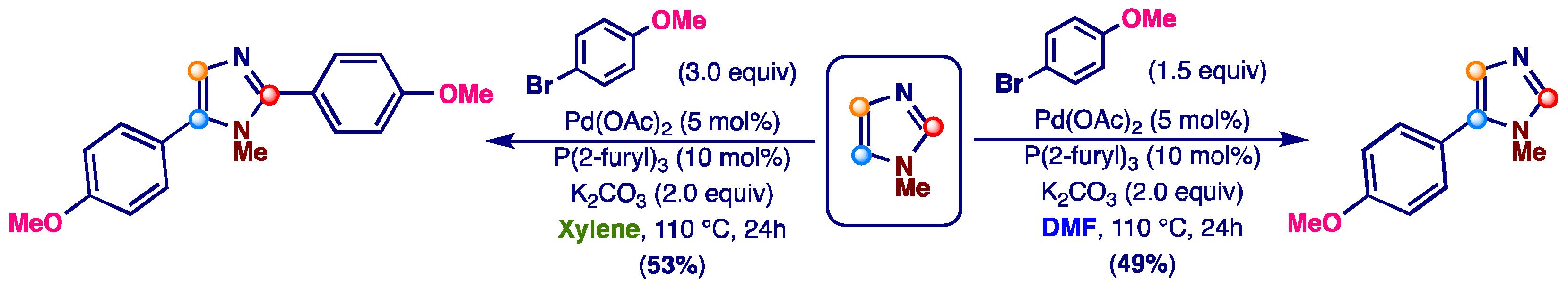
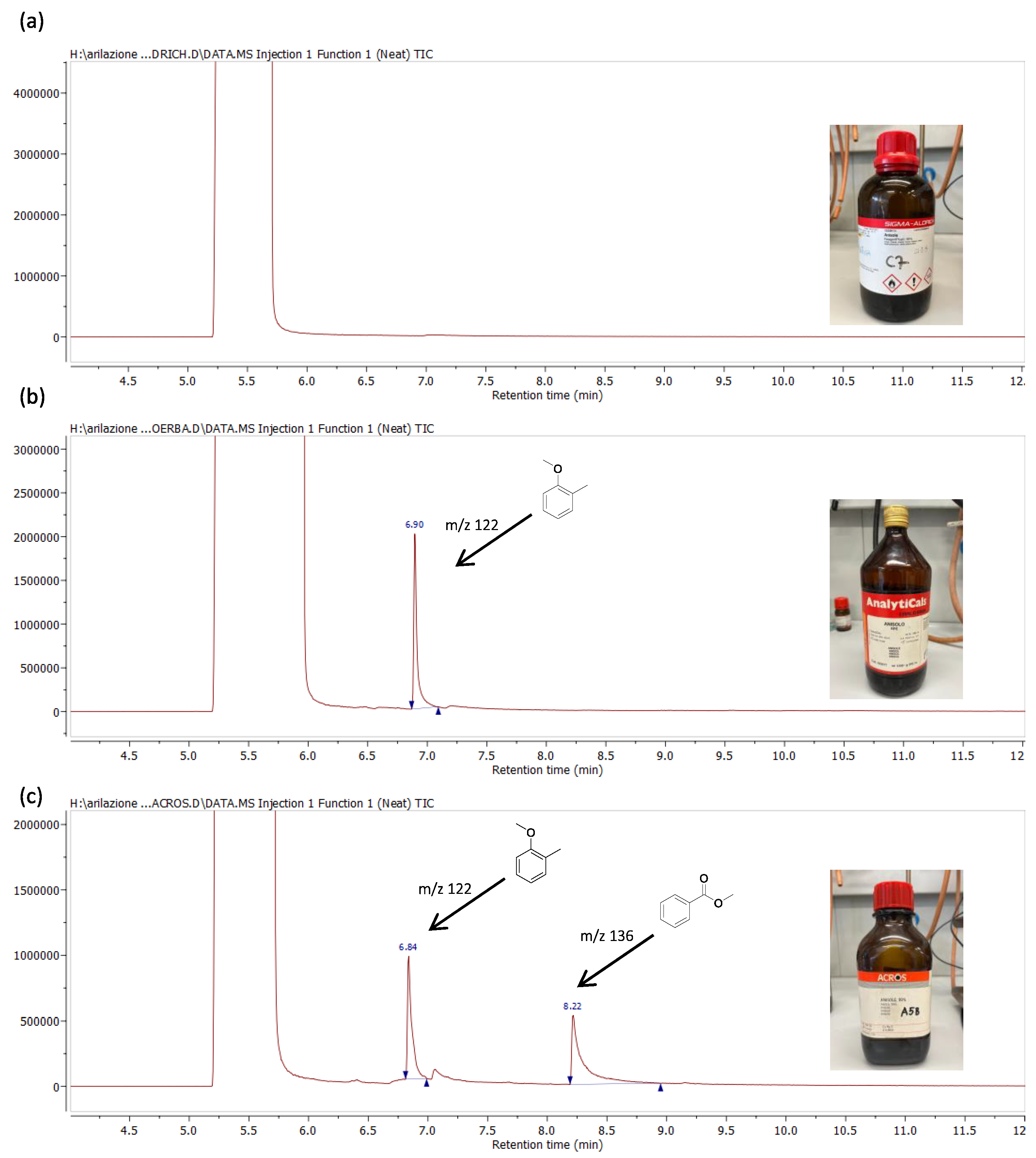
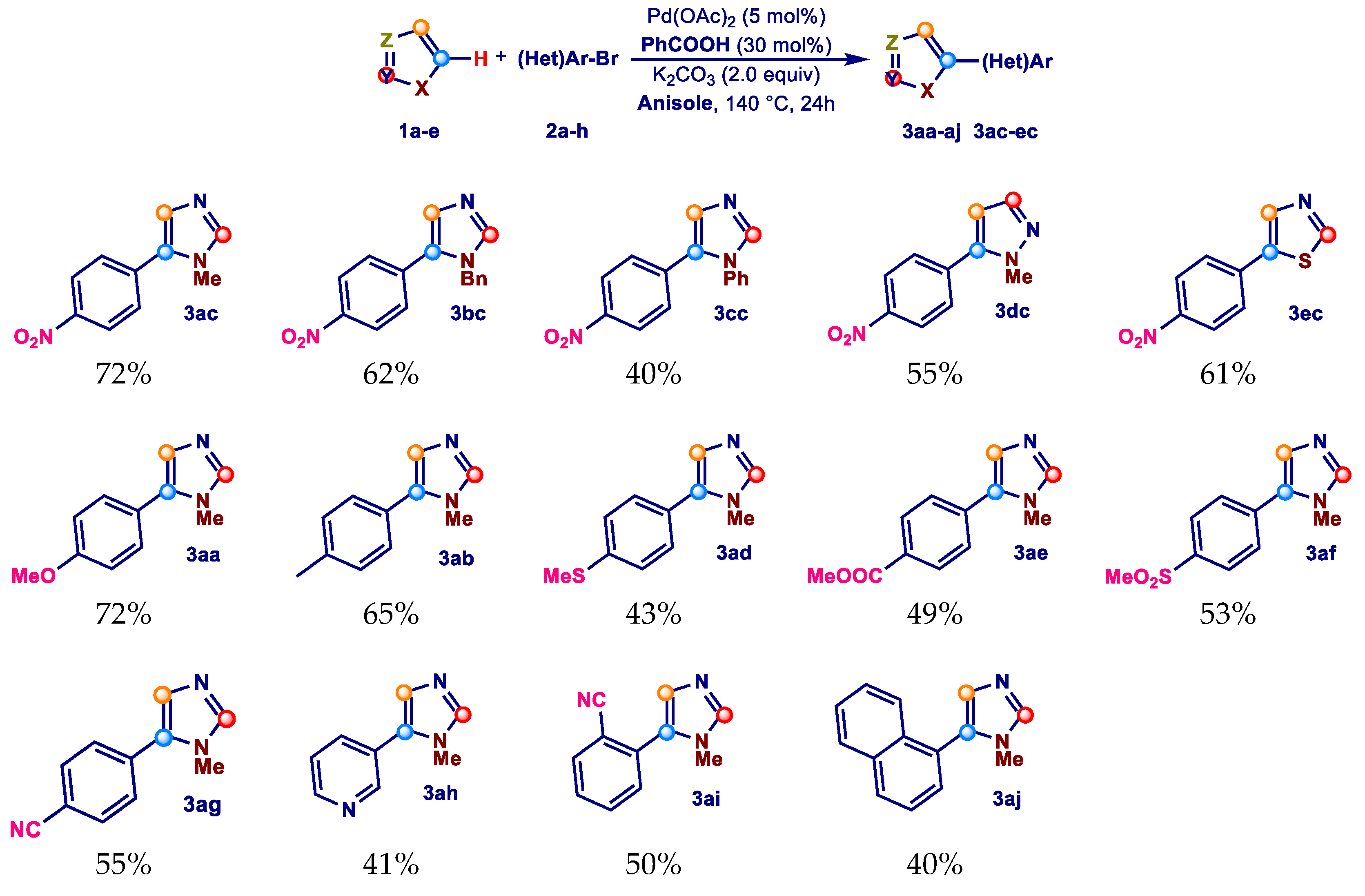
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Procedure for the Screening of the aromatic solvents for the Pd-Catalyzed 5-Arylation of 1-Methyl-1H-imidazole (1a) with Aryl Bromides 2a–c
3.3. Procedure for the Screening of the Reaction Conditions for the Pd-Catalyzed 5-Arylation of 1-Methyl-1H-imidazole (1a) with 1-Bromo-4-Nitrobenzene (2c) in Anisole (SA)
3.4. Procedure for the Screening of the Reaction Scope for the Pd-Catalyzed 5-Arylation of Azoles (1a–e) with Aryl Bromides (2a–j) in Anisole (SA)
3.4.1. 1-methyl-5-(4-nitrophenyl)-1H-imidazole (3ac)
3.4.2. 1-benzyl-5-(4-nitrophenyl)-1H-imidazole (3bc)
3.4.3. 5-(4-nitrophenyl)-1-phenyl-1H-imidazole (3cc)
3.4.4. 1-methyl-5-(4-nitrophenyl)-1H-pyrazole (3dc)
3.4.5. 5-(4-nitrophenyl)thiazole (3ec)
3.4.6. 5-(4-methoxyphenyl)-1-methyl-1H-imidazole (3aa)
3.4.7. 1-methyl-5-(p-tolyl)-1H-imidazole (3ab)
3.4.8. 1-methyl-5-(4-(methylthio)phenyl)-1H-imidazole (3ad)
3.4.9. methyl 4-(1-methyl-1H-imidazol-5-yl)benzoate (3ae)
3.4.10. 1-methyl-5-(4-(methylsulfonyl)phenyl)-1H-imidazole (3af)
3.4.11. 4-(1-methyl-1H-imidazol-5-yl)benzonitrile (3ag)
3.4.12. 3-(1-methyl-1H-imidazol-5-yl)pyridine (3ah)
3.4.13. 2-(1-methyl-1H-imidazol-5-yl)benzonitrile (3ai)
3.4.14. 1-methyl-5-(naphthalen-1-yl)-1H-imidazole (3aj)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R.; Lessi, M.; Manzini, C.; Marianetti, G. Direct (Hetero)arylation Reactions of (Hetero)arenes as Tools for the Step- and Atom-Economical Synthesis of Biologically Active Unnatural Compounds Including Pharmaceutical Targets. Synthesis 2016, 48, 3821–3862. [Google Scholar] [CrossRef]
- Bellina, F.; Guazzelli, N.; Lessi, M.; Manzini, C. Imidazole analogues of resveratrol: Synthesis and cancer cell growth evaluation. Tetrahedron 2015, 71, 2298–2305. [Google Scholar] [CrossRef]
- Bellina, F.; Rossi, R.; Lessi, M.; Manzini, C.; Perego, L. Synthesis of Multiply Arylated Heteroarenes, Including Bioactive Derivatives, via Palladium-Catalyzed Direct C–H Arylation of Heteroarenes with (Pseudo)Aryl Halides or Aryliodonium Salts. Synthesis 2014, 46, 2833–2883. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.M.; Damu, G.L.; Geng, R.X.; Zhou, C.H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Shalini, K.; Sharma, P.K.; Kumar, N. Imidazole and its biological activities: A review. Der Chem. Sin. 2010, 1, 36–47. [Google Scholar]
- Bonezzi, K.; Taraboletti, G.; Borsotti, P.; Bellina, F.; Rossi, R.; Giavazzi, R. Vascular disrupting activity of tubulin-binding 1,5-diaryl-1H-imidazoles. J. Med. Chem. 2009, 52, 7906–7910. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Synthesis and biological activity of vicinal diaryl-substituted 1H-imidazoles. Tetrahedron 2007, 63, 4571–4624. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Monti, S.; Rossi, R. Novel imidazole-based combretastatin A-4 analogues: Evaluation of their in vitro antitumor activity and molecular modeling study of their binding to the colchicine site of tubulin. Bioorganic Med. Chem. Lett. 2006, 16, 5757–5762. [Google Scholar] [CrossRef]
- Mori, A.; Sekiguchi, A.; Masui, K.; Shimada, T.; Horie, M.; Osakada, K.; Kawamoto, M.; Ikeda, T. Facile synthesis of 2,5-diarylthiazoles via palladium-catalyzed tandem C-H substitutions. Design of tunable light emission and liquid crystalline characteristics. J. Am. Chem. Soc. 2003, 125, 1700–1701. [Google Scholar] [CrossRef]
- Marianetti, G.; Lessi, M.; Barone, V.; Bellina, F.; Pucci, A.; Minei, P. Solar collectors based on luminescent 2,5-diarylimidazoles. Dye. Pigment. 2018, 157, 334–341. [Google Scholar] [CrossRef]
- Lessi, M.; Manzini, C.; Minei, P.; Perego, L.A.; Bloino, J.; Egidi, F.; Barone, V.; Pucci, A.; Bellina, F. Synthesis and Optical Properties of Imidazole-Based Fluorophores having High Quantum Yields. ChemPlusChem 2014, 79, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Chen, Y. Synthesis and photophysical properties of thiolactone derivatives. Tetrahedron 2013, 69, 1872–1876. [Google Scholar] [CrossRef]
- Kulhanek, J.; Bures, F. Imidazole as a parent pi-conjugated backbone in charge-transfer chromophores. Beilstein J. Org. Chem. 2012, 8, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Fridman, N.; Speiser, S.; Kaftory, M. Structures and Chromogenic Properties of Bisimidazole Derivatives. Cryst. Growth Des. 2006, 6, 1653–1662. [Google Scholar] [CrossRef]
- Basak, S.; Dutta, S.; Maiti, D. Accessing C2-Functionalized 1,3-(Benz)azoles through Transition Metal-Catalyzed C-H Activation. Chem. Eur. J. 2021, 27, 10533–10557. [Google Scholar] [CrossRef]
- Dhawa, U.; Kaplaneris, N.; Ackermann, L. Green strategies for transition metal-catalyzed C–H activation in molecular syntheses. Org. Chem. Front. 2021, 8, 4886–4913. [Google Scholar] [CrossRef]
- Bohra, H.; Wang, M. Direct C–H arylation: A “Greener” approach towards facile synthesis of organic semiconducting molecules and polymers. J. Mater. Chem. A 2017, 5, 11550–11571. [Google Scholar] [CrossRef]
- Bheeter, C.B.; Chen, L.; Soulé, J.-F.; Doucet, H. Regioselectivity in palladium-catalysed direct arylation of 5-membered ring heteroaromatics. Catal. Sci. Technol. 2016, 6, 2005–2049. [Google Scholar] [CrossRef]
- Bellina, F. Recent Developments in Pd-Catalyzed Direct Arylations of Heteroarenes with Aryl Halides. In C-H Bond Activation and Catalytic Functionalization I; Springer: Berlin, Germany, 2015; pp. 77–102. [Google Scholar] [CrossRef]
- Roger, J.; Gottumukkala, A.L.; Doucet, H. Palladium-Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds with Aryl Halides by CH Bond Activation. ChemCatChem 2010, 2, 20–40. [Google Scholar] [CrossRef]
- Ackermann, L.; Vicente, R.; Kapdi, A.R. Transition-metal-catalyzed direct arylation of (hetero)arenes by C-H bond cleavage. Angew. Chem. Int. Ed. 2009, 48, 9792–9826. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Recent advances in the synthesis of (hetero)aryl-substituted heteroarenes via transition metal-catalysed direct (hetero)arylation of heteroarene C–H bonds with aryl halides or pseudohalides, diaryliodonium salts, and potassium aryltrifluoroborates. Tetrahedron 2009, 65, 10269–10310. [Google Scholar] [CrossRef]
- Liegault, B.; Lapointe, D.; Caron, L.; Vlassova, A.; Fagnou, K. Establishment of broadly applicable reaction conditions for the palladium-catalyzed direct arylation of heteroatom-containing aromatic compounds. J. Org. Chem. 2009, 74, 1826–1834. [Google Scholar] [CrossRef] [PubMed]
- McGlacken, G.P.; Fairlamb, I.J.S. Palladium-Catalysed Cross-Coupling and Related Processes: Some Interesting Observations That Have Been Exploited in Synthetic Chemistry. Eur. J. Org. Chem. 2009, 2009, 4011–4029. [Google Scholar] [CrossRef]
- Alberico, D.; Scott, M.E.; Lautens, M. Aryl-aryl bond formation by transition-metal-catalyzed direct arylation. Chem. Rev. 2007, 107, 174–238. [Google Scholar] [CrossRef]
- Satoh, T.; Miura, M. Catalytic Direct Arylation of Heteroaromatic Compounds. Chem. Lett. 2007, 36, 200–205. [Google Scholar] [CrossRef]
- Miura, M.; Nomura, M. Direct Arylation via Cleavage of Activated and Unactivated C-H Bonds. In Cross-Coupling Reactions; Springer: Berlin, Germany, 2002; pp. 211–241. [Google Scholar] [CrossRef]
- Negishi, E.-I. Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar] [CrossRef]
- D’Alterio, M.C.; Casals-Cruanas, E.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef]
- Pagett, A.B.; Lloyd-Jones, G.C. Suzuki–Miyaura Cross-Coupling. In Organic Reactions; Wiley: Hoboken, NJ, USA, 2019; pp. 547–620. [Google Scholar] [CrossRef]
- Rossi, R.; Bellina, F.; Carpita, A. Palladium Catalysts for the Suzuki Cross-Coupling Reaction: An Overview of Recent Advances. Synthesis 2004, 2004, 2419–2440. [Google Scholar] [CrossRef]
- Kotha, S.; Lahiri, K.; Kashinath, D. Recent applications of the Suzuki–Miyaura cross-coupling reaction in organic synthesis. Tetrahedron 2002, 58, 9633–9695. [Google Scholar] [CrossRef]
- Suzuki, A. Overview of the Suzuki Protocol with B. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 249–262. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Cordovilla, C.; Bartolomé, C.; Martínez-Ilarduya, J.M.; Espinet, P. The Stille Reaction, 38 Years Later. ACS Catal. 2015, 5, 3040–3053. [Google Scholar] [CrossRef]
- Kosugi, M.; Fugami, K. Overview of the Stille Protocol with Sn. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 263–283. [Google Scholar] [CrossRef]
- Farina, V.; Krishnamurthy, V.; Scott, W.J. The Stille Reaction; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Mitchell, T.N. Palladium-Catalysed Reactions of Organotin Compounds. Synthesis 1992, 1992, 803–815. [Google Scholar] [CrossRef]
- Stille, J.K. The Palladium-Catalyzed Cross-Coupling Reactions of Organotin Reagents with Organic Electrophiles [New Synthetic Methods(58)]. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Diner, C.; Organ, M.G. The Negishi Cross-Coupling Reaction. In Organic Reactions; Wiley: Hoboken, NJ, USA, 2019; pp. 1–62. [Google Scholar] [CrossRef]
- Haas, D.; Hammann, J.M.; Greiner, R.; Knochel, P. Recent Developments in Negishi Cross-Coupling Reactions. ACS Catal. 2016, 6, 1540–1552. [Google Scholar] [CrossRef]
- Negishi, E.-I. Overview of the Negishi Protocol with Zn, Al, Zr, and Related Metals. In Handbook of Organopalladium Chemistry for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2002; pp. 229–247. [Google Scholar] [CrossRef]
- Ohta, A.; Aoyagi, Y.; Inoue, A.; Koizumi, I.; Hashimoto, R.; Tokunaga, K.; Gohma, K.; Komatsu, J.; Sekine, K.; Miyafuji, A.; et al. Palladium-catalyzed Cross-coupling Ractions of Chloropyrazines with Aromatic Heterocycles. Heterocycles 1992, 33, 257–272. [Google Scholar] [CrossRef]
- Akita, Y.; Itagaki, Y.; Takizawa, S.; Ohta, A. Cross-coupling reactions of chloropyrazines with 1-substituted indoles. Chem. Pharm. Bull. 1989, 37, 1477–1480. [Google Scholar] [CrossRef]
- Pivsa-Art, S.; Satoh, T.; Kawamura, Y.; Miura, M.; Nomura, M. Palladium-Catalyzed Arylation of Azole Compounds with Aryl Halides in the Presence of Alkali Metal Carbonates and the Use of Copper Iodide in the Reaction. Bull. Chem. Soc. Jpn. 1998, 71, 467–473. [Google Scholar] [CrossRef]
- Bellina, F.; Lessi, M.; Panzetta, G.; Marianetti, G. Improved Synthesis of Symmetrical 2,5-Diarylimidazoles by One-Pot Palladium-Catalyzed Direct Arylation Tailored on the Electronic Features of the Aryl Halide. Synthesis 2017, 49, 4676–4686. [Google Scholar] [CrossRef]
- Takfaoui, A.; Zhao, L.; Touzani, R.; Soulé, J.-F.; Dixneuf, P.H.; Doucet, H. One pot Pd(OAc)2-catalysed 2,5-diarylation of imidazoles derivatives. Tetrahedron 2014, 70, 8316–8323. [Google Scholar] [CrossRef]
- Bellina, F.; Lessi, M.; Manzini, C. Mild Palladium-Catalyzed Regioselective Direct Arylation of Azoles Promoted by Tetrabutylammonium Acetate. Eur. J. Org. Chem. 2013, 2013, 5621–5630. [Google Scholar] [CrossRef]
- Roger, J.; Doucet, H. Phosphine-free palladium-catalysed direct 5-arylation of imidazole derivatives at low catalyst loading. Tetrahedron 2009, 65, 9772–9781. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Marchetti, C.; Rossi, R. Highly selective synthesis of 4(5)-aryl-, 2,4(5)-diaryl-, and 4,5-diaryl-1H-imidazoles via Pd-catalyzed direct C-5 arylation of 1-benzyl-1H-imidazole. Tetrahedron 2008, 64, 6060–6072. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Efficient and practical synthesis of 4(5)-aryl-1H-imidazoles and 2,4(5)-diaryl-1H-imidazoles via highly selective palladium-catalyzed arylation reactions. J. Org. Chem. 2007, 72, 8543–8546. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Mannina, L.; Rossi, R.; Viel, S. Regiocontrolled Synthesis of 1,2-Diaryl-1H-imidazoles by Palladium- and Copper-Mediated Direct Coupling of 1-Aryl-1H-imidazoles with Aryl Halides under Ligandless Conditions. Eur. J. Org. Chem. 2006, 2006, 693–703. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Rossi, R. Palladium- and Copper-Mediated Direct C-2 Arylation of Azoles—Including Free (NH)-Imidazole, -Benzimidazole and -Indole—Under Base-Free and Ligandless Conditions. Eur. J. Org. Chem. 2006, 2006, 1379–1382. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Mannina, L.; Rossi, R.; Viel, S. Regioselective synthesis of 1,5-diaryl-1H-imidazoles by palladium-catalyzed direct arylation of 1-aryl-1H-imidazoles. J. Org. Chem. 2005, 70, 3997–4005. [Google Scholar] [CrossRef]
- Shi, X.; Soulé, J.F.; Doucet, H. Reaction Conditions for the Regiodivergent Direct Arylations at C2- or C5-Positions of Oxazoles using Phosphine-Free Palladium Catalysts. Adv. Synth. Catal. 2019, 361, 4748–4760. [Google Scholar] [CrossRef]
- Liu, X.W.; Shi, J.L.; Yan, J.X.; Wei, J.B.; Peng, K.; Dai, L.; Li, C.G.; Wang, B.Q.; Shi, Z.J. Reigoselective arylation of thiazole derivatives at 5-position via Pd catalysis under ligand-free conditions. Org. Lett. 2013, 15, 5774–5777. [Google Scholar] [CrossRef]
- Strotman, N.A.; Chobanian, H.R.; Guo, Y.; He, J.; Wilson, J.E. Highly regioselective palladium-catalyzed direct arylation of oxazole at C-2 or C-5 with aryl bromides, chlorides, and triflates. Org. Lett. 2010, 12, 3578–3581. [Google Scholar] [CrossRef]
- Derridj, F.; Djebbar, S.; Benali-Baitich, O.; Doucet, H. Direct arylation of oxazole and benzoxazole with aryl or heteroaryl halides using a palladium–diphosphine catalyst. J. Organomet. Chem. 2008, 693, 135–144. [Google Scholar] [CrossRef]
- Ohnmacht, S.A.; Mamone, P.; Culshaw, A.J.; Greaney, M.F. Direct arylations on water: Synthesis of 2,5-disubstituted oxazoles balsoxin and texaline. Chem. Commun. 2008, 10, 1241–1243. [Google Scholar] [CrossRef]
- Verrier, C.; Martin, T.; Hoarau, C.; Marsais, F. Palladium-catalyzed direct (hetero)arylation of ethyl oxazole-4-carboxylate: An efficient access to (hetero)aryloxazoles. J. Org. Chem. 2008, 73, 7383–7386. [Google Scholar] [CrossRef]
- Tani, S.; Uehara, T.N.; Yamaguchi, J.; Itami, K. Programmed synthesis of arylthiazoles through sequential C–H couplings. Chem. Sci. 2014, 5, 123–135. [Google Scholar] [CrossRef]
- Gottumukkala, A.L.; Doucet, H. Activated Aryl Chlorides: Useful Partners for the Coupling with 2-Substituted Thiazoles in the Palladium-Catalysed C-H Activation/Functionalisation Reaction. Eur. J. Inorg. Chem. 2007, 2007, 3629–3632. [Google Scholar] [CrossRef]
- Kumpulainen, E.T.T.; Pohjakallio, A. Selective Palladium-Catalyzed Direct C—H Arylation of Unsubstituted. N-Protected Pyrazoles. Adv. Synth. Catal. 2014, 356, 1555–1561. [Google Scholar] [CrossRef]
- Derridj, F.; Roger, J.; Djebbar, S.; Doucet, H. Catalytic System for Inhibition of Amination-Type Reaction and Palladium-Catalysed Direct Arylation using Non-Protected Pyrazole Derivatives. Adv. Synth. Catal. 2012, 354, 747–750. [Google Scholar] [CrossRef]
- Yan, T.; Chen, L.; Bruneau, C.; Dixneuf, P.H.; Doucet, H. Palladium-catalyzed direct arylation of 5-chloropyrazoles: A selective access to 4-aryl pyrazoles. J. Org. Chem. 2012, 77, 7659–7664. [Google Scholar] [CrossRef]
- Doucet, H.; Ammar, H.; Beladhria, A.; Beydoun, K.; Salem, R. Pd-Catalysed Direct 5-Arylation of 1-Methylpyrazole with Aryl Bromides. Synthesis 2011, 2011, 2553–2560. [Google Scholar] [CrossRef]
- Mateos, C.; Mendiola, J.; Carpintero, M.; Minguez, J.M. Regioselective palladium-catalyzed arylation of 4-chloropyrazoles. Org. Lett. 2010, 12, 4924–4927. [Google Scholar] [CrossRef]
- Goikhman, R.; Jacques, T.L.; Sames, D. C-H bonds as ubiquitous functionality: A general approach to complex arylated pyrazoles via sequential regioselective C-arylation and N-alkylation enabled by SEM-group transposition. J. Am. Chem. Soc. 2009, 131, 3042–3048. [Google Scholar] [CrossRef]
- Santelli, M.; Doucet, H.; Fall, Y. Palladium-Catalyzed Direct Arylation of Pyrazole Derivatives: A Green Access to 4-Arylpyrazoles. Synthesis 2009, 2010, 127–135. [Google Scholar] [CrossRef]
- Cernak, T.; Dykstra, K.D.; Tyagarajan, S.; Vachal, P.; Krska, S.W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016, 45, 546–576. [Google Scholar] [CrossRef]
- Wencel-Delord, J.; Glorius, F. C-H bond activation enables the rapid construction and late-stage diversification of functional molecules. Nat. Chem. 2013, 5, 369–375. [Google Scholar] [CrossRef]
- Breslow, R.; Baldwin, S.; Flechtner, T.; Kalicky, P.; Liu, S.; Washburn, W. Remote oxidation of steroids by photolysis of attached benzophenone groups. J. Am. Chem. Soc. 1973, 95, 3251–3262. [Google Scholar] [CrossRef]
- Gorelsky, S.I. Origins of regioselectivity of the palladium-catalyzed (aromatic)CH bond metalation–deprotonation. Coord. Chem. Rev. 2013, 257, 153–164. [Google Scholar] [CrossRef]
- Lapointe, D.; Markiewicz, T.; Whipp, C.J.; Toderian, A.; Fagnou, K. Predictable and site-selective functionalization of poly(hetero)arene compounds by palladium catalysis. J. Org. Chem. 2011, 76, 749–759. [Google Scholar] [CrossRef]
- Korenaga, T.; Suzuki, N.; Sueda, M.; Shimada, K. Ligand effect on direct arylation by CMD process. J. Organomet. Chem. 2015, 780, 63–69. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the palladium-catalyzed (aromatic)C-H bond metalation-deprotonation mechanism spanning the entire spectrum of arenes. J. Org. Chem. 2012, 77, 658–668. [Google Scholar] [CrossRef]
- Lapointe, D.; Fagnou, K. Overview of the Mechanistic Work on the Concerted Metallation–Deprotonation Pathway. Chem. Lett. 2010, 39, 1118–1126. [Google Scholar] [CrossRef]
- Gorelsky, S.I.; Lapointe, D.; Fagnou, K. Analysis of the concerted metalation-deprotonation mechanism in palladium-catalyzed direct arylation across a broad range of aromatic substrates. J. Am. Chem. Soc. 2008, 130, 10848–10849. [Google Scholar] [CrossRef]
- Gandon, V.; Hoarau, C. Concerted vs. Nonconcerted Metalation-Deprotonation in Orthogonal Direct C-H Arylation of Heterocycles with Halides: A Computational Study. J. Org. Chem. 2021, 86, 1769–1778. [Google Scholar] [CrossRef]
- Théveau, L.; Querolle, O.; Dupas, G.; Hoarau, C. Ligand controlled orthogonal base-assisted direct C–H bond arylation in oxa(thia)zole-4-carboxylate series. New insights in nCMD mechanism. Tetrahedron 2013, 69, 4375–4380. [Google Scholar] [CrossRef]
- Wang, L.; Carrow, B.P. Oligothiophene Synthesis by a General C-H Activation Mechanism: Electrophilic Concerted Metalation-Deprotonation (eCMD). ACS Catal. 2019, 9, 6821–6836. [Google Scholar] [CrossRef]
- El Kazzouli, S.; Boujdi, K.; El Brahmi, N.; Collet, S.; Dubreuil, D.; Mathé-Allainmat, M.; Akssira, M.; Guillaumet, G.; Lebreton, J. Solvent/Ligand-Controlled Switchable C3 or C7 C–H Arylations of 1-Methyl-4-nitro-1H-indazole. Synthesis 2022, 54, 4834–4842. [Google Scholar] [CrossRef]
- Kanai, Y.; Müller-Borges, D.; Plenio, H. The Regioselective Arylation of 1,3-Benzodioxoles. Adv. Synth. Catal. 2021, 364, 679–688. [Google Scholar] [CrossRef]
- Matsidik, R.; Komber, H.; Sommer, M. Rational Use of Aromatic Solvents for Direct Arylation Polycondensation: C-H Reactivity versus Solvent Quality. ACS Macro Lett. 2015, 4, 1346–1350. [Google Scholar] [CrossRef]
- Wang, X.; Wang, M. Synthesis of donor–acceptor conjugated polymers based on benzo[1,2-b:4,5-b′]dithiophene and 2,1,3-benzothiadiazole via direct arylation polycondensation: Towards efficient C–H activation in nonpolar solvents. Polym. Chem. 2014, 5, 5784–5792. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Prat, D.; Hayler, J.; Wells, A. A survey of solvent selection guides. Green Chem. 2014, 16, 4546–4551. [Google Scholar] [CrossRef]
- Bellina, F.; Cauteruccio, S.; Di Fiore, A.; Rossi, R. Regioselective Synthesis of 4,5-Diaryl-1-methyl-1H-imidazoles Including Highly Cytotoxic Derivatives by Pd-Catalyzed Direct C-5 Arylation of 1-Methyl-1H-imidazole with Aryl Bromides. Eur. J. Org. Chem. 2008, 2008, 5436–5445. [Google Scholar] [CrossRef]
- Bellina, F.; Calandri, C.; Cauteruccio, S.; Rossi, R. Efficient and highly regioselective direct C-2 arylation of azoles, including free (NH)-imidazole, -benzimidazole and -indole, with aryl halides. Tetrahedron 2007, 63, 1970–1980. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 2002, 91, 165–195. [Google Scholar] [CrossRef]
- Ackermann, L. Carboxylate-assisted transition-metal-catalyzed C-H bond functionalizations: Mechanism and scope. Chem. Rev. 2011, 111, 1315–1345. [Google Scholar] [CrossRef]
- Fagnou, K. Mechanistic considerations in the development and use of azine, diazine and azole N-oxides in palladium-catalyzed direct arylation. Top. Curr. Chem. 2010, 292, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Hartwig, J.F. Assessment of the intermediacy of arylpalladium carboxylate complexes in the direct arylation of benzene: Evidence for C-H bond cleavage by “ligandless” species. J. Am. Chem. Soc. 2011, 133, 3308–3311. [Google Scholar] [CrossRef]
- Perego, L.A.; Grimaud, L.; Bellina, F. Mechanistic Studies on the Palladium-Catalyzed Direct C-5 Arylation of Imidazoles: The Fundamental Role of the Azole as a Ligand for Palladium. Adv. Synth. Catal. 2016, 358, 597–609. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Lagowski, J.M. Reactivity of Five-membered Rings with Two or More Heteroatoms. In Comprehensive Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 1984; pp. 39–110. [Google Scholar] [CrossRef]
- Lessi, M.; Lucci, A.; Cuzzola, A.; Bellina, F. Imidazo-Fused Isoindoles by Pd(II)/Ag(I)-Promoted Intramolecular Dehydrogenative Coupling. Eur. J. Org. Chem. 2020, 2020, 796–802. [Google Scholar] [CrossRef]
- Van Leusen, A.M.; Wildeman, J.; Oldenziel, O.H. Chemistry of sulfonylmethyl isocyanides. 12. Base-induced cycloaddition of sulfonylmethyl isocyanides to carbon,nitrogen double bonds. Synthesis of 1,5-disubstituted and 1,4,5-trisubstituted imidazoles from aldimines and imidoyl chlorides. J. Org. Chem. 1977, 42, 1153–1159. [Google Scholar] [CrossRef]
- Hu, L.-Q.; Deng, R.-L.; Li, Y.-F.; Zeng, C.-J.; Shen, D.-S.; Liu, F.-S. Developing Bis(imino)acenaphthene-Supported N-Heterocyclic Carbene Palladium Precatalysts for Direct Arylation of Azoles. Organometallics 2018, 37, 214–226. [Google Scholar] [CrossRef]
- He, X.-X.; Li, Y.; Ma, B.-B.; Ke, Z.; Liu, F.-S. Sterically Encumbered Tetraarylimidazolium Carbene Pd-PEPPSI Complexes: Highly Efficient Direct Arylation of Imidazoles with Aryl Bromides under Aerobic Conditions. Organometallics 2016, 35, 2655–2663. [Google Scholar] [CrossRef]
- Mao, S.X.; Shi, X.Z.; Soule, J.F.; Doucet, H. Exploring Green Solvents Associated to Pd/C as Heterogeneous Catalyst for Direct Arylation of Heteroaromatics with Aryl Bromides. Adv. Synth. Catal. 2018, 360, 3306–3317. [Google Scholar] [CrossRef]
- Ouyang, J.-S.; Li, Y.-F.; Shen, D.-S.; Ke, Z.; Liu, F.-S. Bulky α-diimine palladium complexes: Highly efficient for direct C–H bond arylation of heteroarenes under aerobic conditions. Dalton Trans. 2016, 45, 14919–14927. [Google Scholar] [CrossRef] [PubMed]




| Entry | Solvent | 2 | R | 3 Yield (%) 2 | 4 Yield (%) 2 | Selectivity 3 |
|---|---|---|---|---|---|---|
| 1 | Nitrobenzene | 2a | OMe | 30 | 33 | 0.9 |
| 2 | Nitrobenzene | 2b | Me | 67 | 20 | 3.4 |
| 3 | Nitrobenzene | 2c | NO2 | 65 | 18 | 3.6 |
| 4 | Chlorobenzene | 2a | OMe | 41 | 35 | 1.2 |
| 5 | Chlorobenzene | 2b | Me | 27 | 46 | 0.6 |
| 6 | Chlorobenzene | 2c | NO2 | 60 | 8 | 7.5 |
| 7 | Xylenes | 2a | OMe | 20 | 38 | 0.5 |
| 8 | Xylenes | 2b | Me | 53 | 36 | 1.5 |
| 9 | Xylenes | 2c | NO2 | 64 | 8 | 8.0 |
| 10 | Anisole | 2a | OMe | 40 | 6 | 6.7 |
| 11 | Anisole | 2b | Me | 60 | 12 | 5.0 |
| 12 | Anisole | 2c | NO2 | 56 (43) | 24 | 2.3 |
| Entry | Supplier | Cat. N. | 3ac Yield (%) 2 | 4ac Yield (%) 2 | Selectivity 3 |
|---|---|---|---|---|---|
| 1 | Acros (AC) | 15,392 | 56 | 24 | 2.3 |
| 2 | Carlo Erba (CE) | 422,677 | 49 | 40 | 1.2 |
| 3 | Sigma-Aldrich (SA) | 123,266 | 47 | 30 | 1.6 |
| Entry | Acidic Additive | 1a Conv (%) 2 | 3ac Yield (%) 3 | 4ac Yield (%) 3 |
|---|---|---|---|---|
| 1 | Benzoic acid | 93 | 75(72) | 14 |
| 2 4 | Benzoic acid | 87 | 71 | 10 |
| 3 5 | Benzoic acid | 56 | 35 | 1 |
| 4 6 | Benzoic acid | 90 | 63 | 22 |
| 5 | Pivalic acid | 75 | 35 | 12 |
| 6 | Phenol | 79 | 47 | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosadoni, E.; Banchini, F.; Bellini, S.; Lessi, M.; Pasquinelli, L.; Bellina, F. Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole. Molecules 2022, 27, 8454. https://doi.org/10.3390/molecules27238454
Rosadoni E, Banchini F, Bellini S, Lessi M, Pasquinelli L, Bellina F. Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole. Molecules. 2022; 27(23):8454. https://doi.org/10.3390/molecules27238454
Chicago/Turabian StyleRosadoni, Elisabetta, Federico Banchini, Sara Bellini, Marco Lessi, Luca Pasquinelli, and Fabio Bellina. 2022. "Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole" Molecules 27, no. 23: 8454. https://doi.org/10.3390/molecules27238454
APA StyleRosadoni, E., Banchini, F., Bellini, S., Lessi, M., Pasquinelli, L., & Bellina, F. (2022). Ligandless Palladium-Catalyzed Direct C-5 Arylation of Azoles Promoted by Benzoic Acid in Anisole. Molecules, 27(23), 8454. https://doi.org/10.3390/molecules27238454






