Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis
Abstract
1. Introduction
2. Research Methodology
3. Results and Discussion
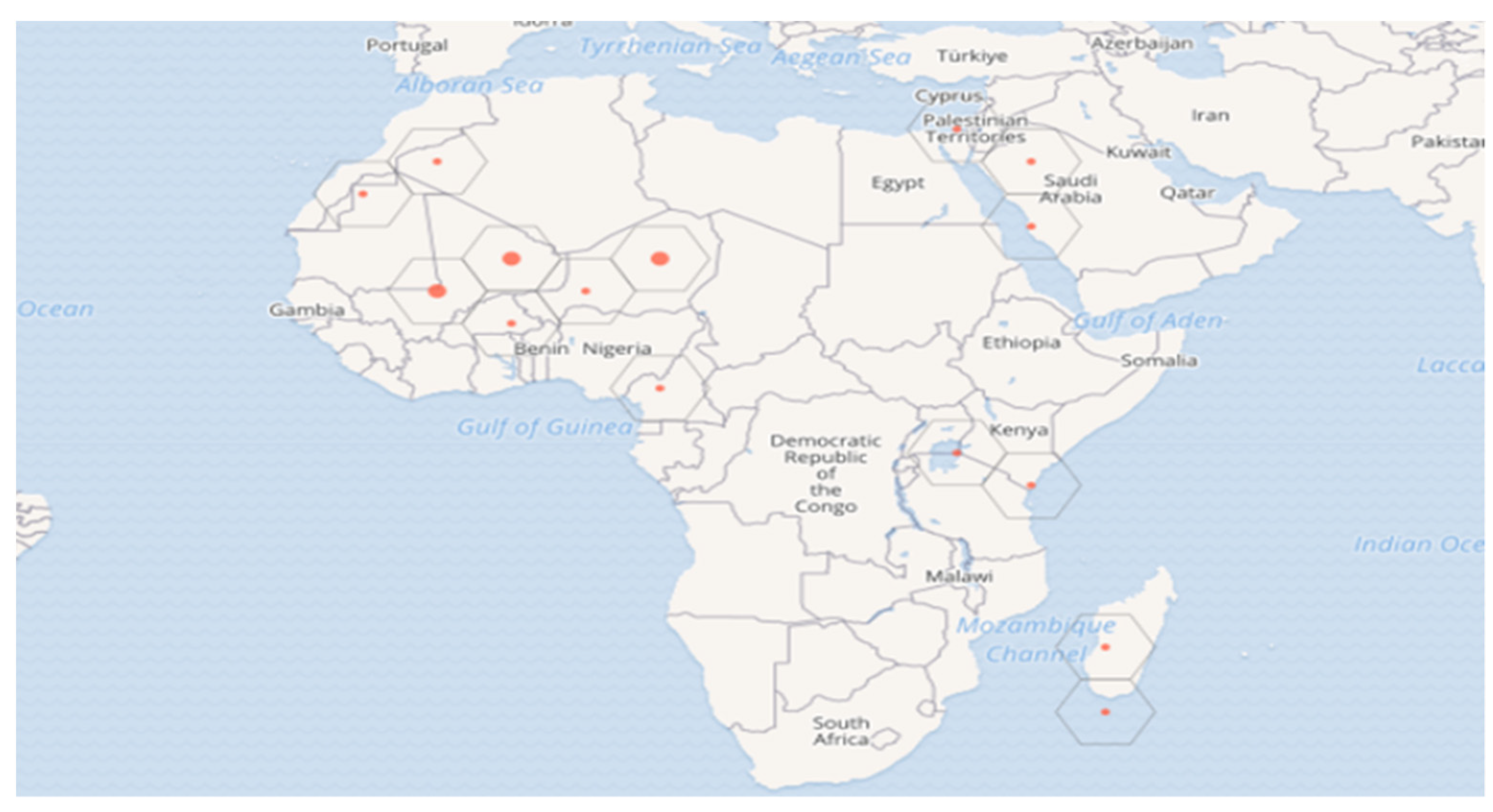
3.1. Taxonomy and Geographic Distribution
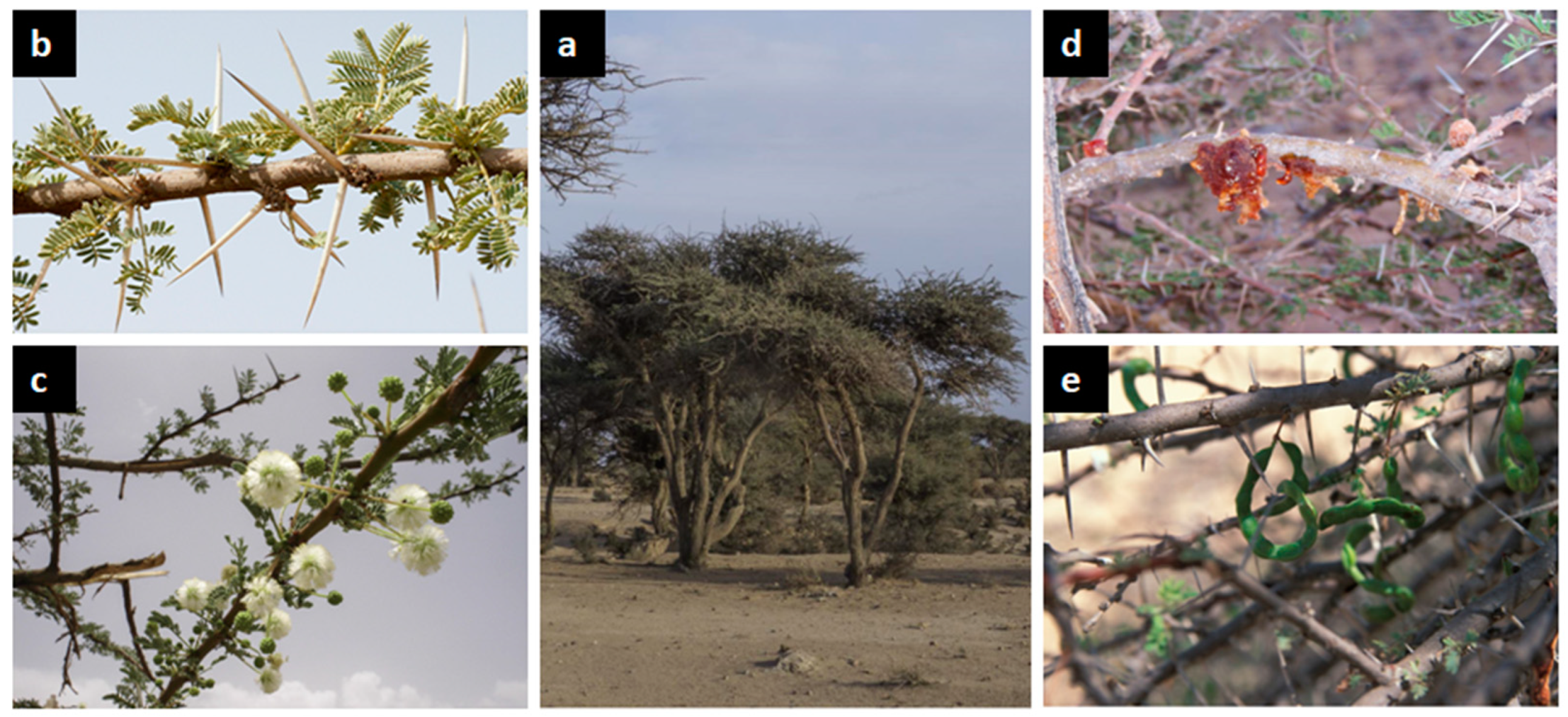
3.2. Botanical Description and Ecological Factors
3.3. Ethnomedicinal Use
3.4. Phytochemical Compounds
3.4.1. Fatty Acids
3.4.2. Monosaccharides
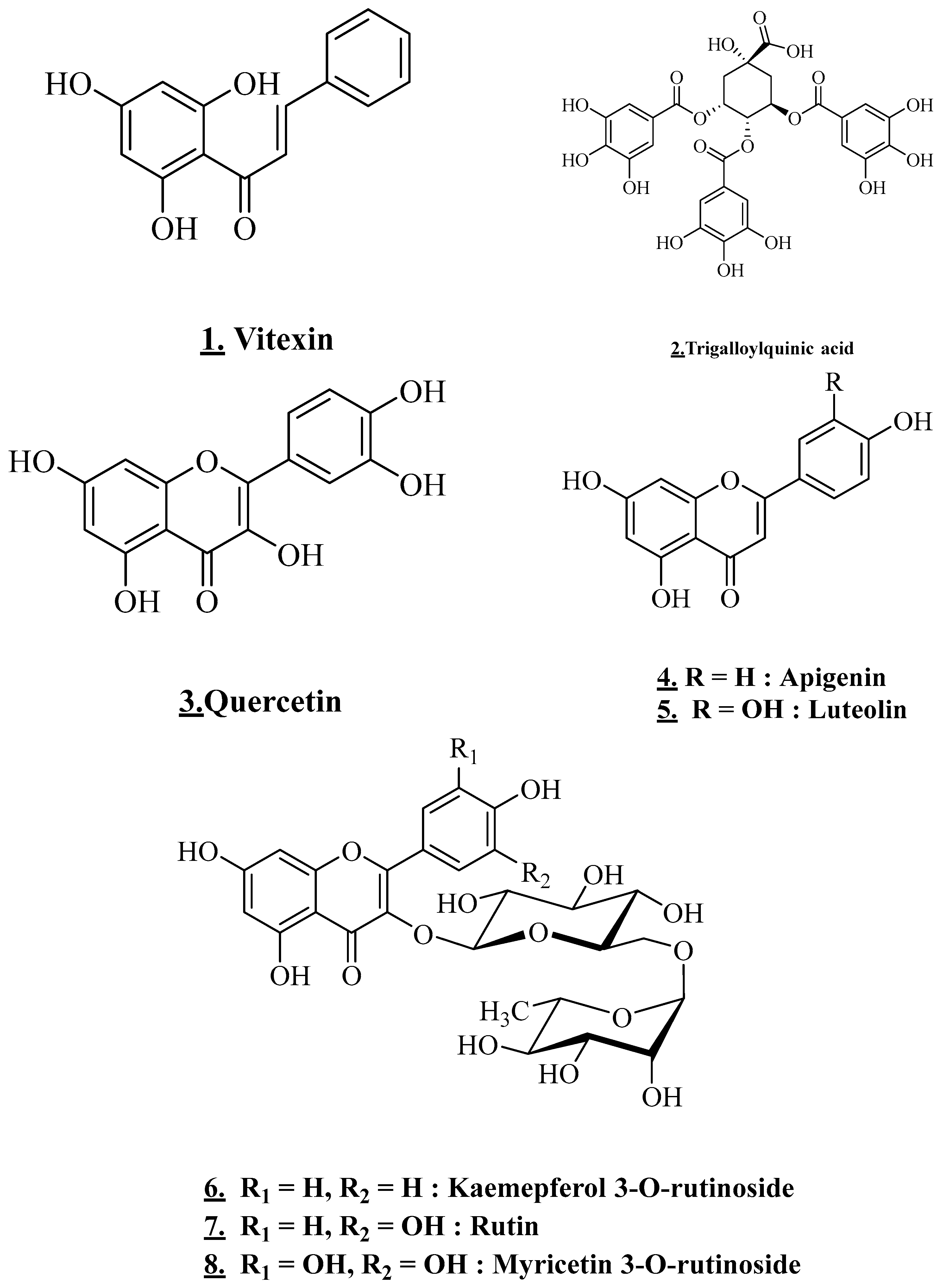
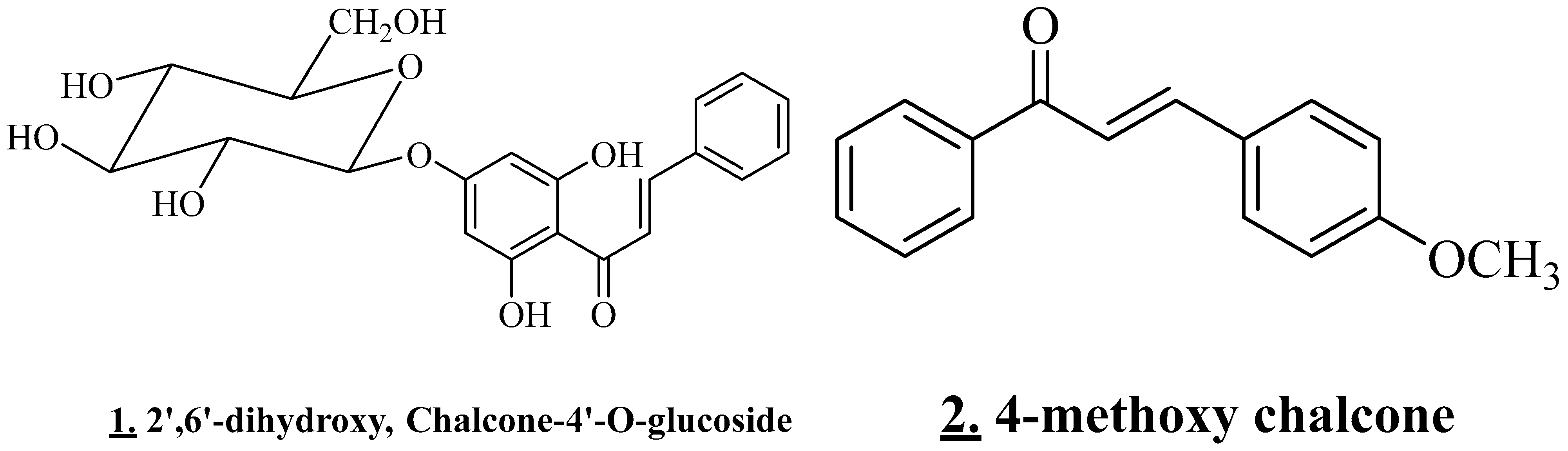
3.4.3. Flavonoids and Chalcone
3.4.4. Alcohols
3.5. Biological Properties
3.5.1. Antibacterial Activity
3.5.2. Antifungal Activity
3.5.3. Antiparasitic Effects
3.5.4. Antioxidant Activity
3.5.5. Antiproliferative Activity
3.5.6. Antidiabetic Effect
3.5.7. Anti-Inflammatory Effect
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABTS | 2,2’-Azino-bis (3-ethylBenzoThiazoline-6-Sulphonic)acid |
| ATCC | American Type Culture Collection |
| COX-2 | Cyclooxygenase-2 |
| DPPH | 2,2-Diphenyl-1-picryl hydrazyl radical |
| DW | Dry Weight |
| EC50 | Effective Concentration of 50% |
| FRAP | Ferric Reducing Antioxidant Power |
| GA | Gallic Acid |
| HDL | High-Density Lipoprotein |
| IC50 | Inhibition Concentration of 50% |
| iNOS | Inducible Nitric Oxide Synthase |
| IZD | Inhibition Zone Diameter |
| LD | Lethal Dose |
| LDL | Low-Density Lipoprotein |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
| SGOT | Glutamooxaloacetate Transferase Serum |
| SGPT | Glutamate Pyruvate Transaminase Serum |
| STZ | Streptozotocin-Induced Diabetic |
| TG | Triglyceride |
| TNF-α | Tumor Necrosis Factor |
| VLDL | Very Low-Density Lipoprotein |
References
- Abouri, M.; Mousadik, A.E.; Msanda, F.; Boubaker, H.; Saadi, B.; Cherifi, K. An ethnobotanical survey of medicinal plants used in the Tata Province, Morocco. Int. J. Med. Plants Res. 2012, 27, 99–123. [Google Scholar]
- Jaouadi, W.; Mechergui, K.; Ammari, Y.; Hamrouni, L.; Hanana, M.; Khouja, M.L. Étude ethnobotanique et ethnopharmacologique d’Acacia tortilis (Forssk) Hayne subsp. raddiana (Savi) de la steppe arborée du Nord de l’Afrique. Phytothérapie 2016, 14, 285–292. [Google Scholar] [CrossRef]
- Ali, M.R.; EL Said, R.M. Assessment of the potential of Arabic gum as an antimicrobial and antioxidant agent in developing vegan “egg-free” mayonnaise. J. Food Saf. 2020, 40, e12771. [Google Scholar] [CrossRef]
- Ziani, B.E.C.; Carocho, M.; Abreu, R.M.V.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Talhi, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profiling, biological activities and in silico studies of Acacia tortilis (Forssk.) Hayne ssp. raddiana extracts. Food Biosci. 2020, 36, 100616. [Google Scholar] [CrossRef]
- Abdllha, H.B.; Mohamed, A.I.; Almoniem, K.A.; Adam, N.I.; Alhaadi, W.; Elshikh, A.A.; Ali, A.J.; Makuar, I.G.; Elnazeer, A.M.; Elrofaei, N.A.; et al. Evolution of Antimicrobial, Antioxidant Potentials and Phytochemical Studies of Three Solvent Extracts of Five Species from Acacia Used in Sudanese Ethnomedicine. AiM 2016, 6, 691–698. [Google Scholar] [CrossRef]
- Sulayli, A.I.; Moustafa, M.F.; Eid, E.M. Genetic Variability, Antimicrobial Activity And Natural Water-Soluble Vitamins Contents Of Five Acacia Species Growing In Jazan Region, Saudi Arabia. Pak. J. Agric. Sci. 2019, 56, 289–300. [Google Scholar]
- Alam, P.; Alajmi, M.F.; Arbab, A.H.; Parvez, M.K.; Siddiqui, N.A.; Alqasoumi, S.I.; Al-Rehaily, A.J.; Al-Dosari, M.S.; Basudan, O.A. Comparative study of antioxidant activity and validated RP-HPTLC analysis of rutin in the leaves of different Acacia species grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 715–723. [Google Scholar] [CrossRef]
- Embaby, H.E.; Rayan, A.M. Chemical composition and nutritional evaluation of the seeds of Acacia tortilis (Forssk.) Hayne ssp. raddiana. Food Chem. 2016, 200, 62–68. [Google Scholar] [CrossRef]
- Verma, S. A Review Study on Acacia tortillis Sunita Verma. Int. J. Life Sci. Pharma Res. 2016, 6, 22–24. [Google Scholar]
- A Guide to Medicinal Plants in North Africa; IUCN: Grand, Switzerland, 2009; Volume 14.
- Muhaisen, H.M.H. A Review on Chemical Constituents of Acacia tortilis (Leguminosae). J. Pharm. 2021, 11, 10–21. [Google Scholar]
- Benkhnigue, O.; Akka, F.B.; Salhi, S.; Fadli, M.; Zidane, L. Catalogue des plantes médicinales utilisées dans le traitement du diabète dans la région d’Al Haouz-Rhamna (Maroc). J. Anim. Plant Sci. 2014, 30, 3539–3568. [Google Scholar]
- Bouyahya, A.; El Omari, N.; Elmenyiy, N.; Guaouguaou, F.E.; Balahbib, A.; Belmehdi, O.; Bakri, Y. Moroccan antidiabetic medicinal plants: Ethnobotanical studies, phytochemical bioactive compounds, preclinical investigations, toxicological validations and clinical evidences; challenges, guidance and perspectives for future management of diabetes worldwide. Trends Food Sci. Technol. 2021, 115, 147–254. [Google Scholar]
- Koch, A.; Tamez, P.; Pezzuto, J.; Soejarto, D. Evaluation of plants used for antimalarial treatment by the Maasai of Kenya. J. Ethnopharmacol. 2005, 101, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Al-Fatimi, M.; Wurster, M.; Schröder, G.; Lindequist, U. Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J. Ethnopharmacol. 2007, 111, 657–666. [Google Scholar] [CrossRef]
- Ghourri, M.; Zidane, L.; Douira, A. Usage des plantes médicinales dans le traitement du Diabète Au Sahara marocain (Tan-Tan). J. Anim. Plant Sci. 2013, 17, 2388–2411. [Google Scholar]
- El Hafian, M.; Benlandini, N.; Elyacoubi, H.; Zidane, L.; Rochdi, A. Étude floristique et ethnobotanique des plantes médicinales utilisées au niveau de la préfecture d’Agadir-Ida-Outanane (Maroc). J. App. Bioscience. 2014, 81, 7198. [Google Scholar] [CrossRef]
- Roué, M.; Molnar, Z. Knowing Our Lands and Resources: Indigenous and Local Knowledge of Biodiversity and Ecosystem Services in Europe and Central Asia; UNESCO Publishing: Paris, France, 2017; Volume 9. [Google Scholar]
- Hammiche, V.; Maiza, K. Traditional medicine in Central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Jada, E.K.M. Taxonomic Study on Woody Plants in Soba Area Khartoum State Sudan. Ph.D. Thesis, Sudan University of Science and Technology, Khartoum, Sudan, 2007. [Google Scholar]
- Mahmoud, T.; Gairola, S. Traditional knowledge and use of medicinal plants in the Eastern Desert of Egypt: A case study from Wadi El-Gemal National Park. J. Med. Plants 2013, 1, 10–17. [Google Scholar]
- Ramde-Tiendrebeogo, A.; Zerbo, R.; Ouattara, B.; Doulkom, A.; Guissou, I.P. Plantes sahéliennes adaptées dans la récupération des terres dégradées et leurs usages pour la santé: Cas de la province du Soum au Nord du Burkina Faso. J. Anim. Plant Sci. 2019, 41, 6767–6783. [Google Scholar]
- Alphonsine, R.-T.; Moumouni, K.; Nabere, O.; Marius, L.; Innocent, P.G. A comparative study of phytochemical profile and antioxidant activity of Sahelian plants used in the treatment of infectious diseases in northern part of Burkina Faso: Acacia seyal Delile and Acacia tortilis (Forssk.) Hayne subsp. raddiana (Savi). J. Pharmacogn. Phytother. 2019, 11, 74–79. [Google Scholar] [CrossRef]
- Gabr, S.; Nikles, S.; Pferschy Wenzig, E.M.; Ardjomand-Woelkart, K.; Hathout, R.M.; El-Ahmady, S.; Motaal, A.A.; Singab, A.; Bauer, R. Characterization and optimization of phenolics extracts from Acacia species in relevance to their anti-inflammatory activity. Biochem. Syst. Ecol. 2018, 78, 21–30. [Google Scholar] [CrossRef]
- Muhaisen, H.M.H.; Ilyas, M.; Mushfiq, M.; Parveen, M.; Basudan, O.A. Flavonoids from Acacia Tortilis. J. Chem. Res. 2002, 2002, 276–278. [Google Scholar] [CrossRef]
- Lakhera, A.K.; Kumar, V. Monosaccharide composition of acidic gum exudates from Indian Acacia tortilis ssp. raddiana (Savi) Brenan. Int. J. Biol. Macromol. 2017, 94, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Hagos, M.; Samuelsson, G.; Kenne, L.; Modawi, B.M. Isolation of smooth muscle relaxing 1, 3-diarylpropan-2-ol derivatives from Acacia tortilis. Planta Med. 1987, 53, 27–31. [Google Scholar] [CrossRef]
- Srivastava, M.; Kumar, S. Fatty Acid Compositional Studies of Lesser Known Acacia tortilis Seed Oil. FNS 2013, 4, 59–62. [Google Scholar] [CrossRef]
- Khan, R.; Srivastava, R.; Khan, M.A.; Alam, P.; Abdin, M.Z. Mahmooduzzafr Variation in oil content and fatty acid composition of the seed oil of Acacia species collected from the northwest zone of India. J. Sci. Food Agric. 2012, 92, 2310–2315. [Google Scholar] [CrossRef] [PubMed]
- Alajmi, M.F.; Alam, P.; Alqasoumi, S.I.; Ali Siddiqui, N.; Basudan, O.A.; Hussain, A.; Mabood Husain, F.; Ali Khan, A. Comparative anticancer and antimicrobial activity of aerial parts of Acacia salicina, Acacia laeta, Acacia hamulosa and Acacia tortilis grown in Saudi Arabia. Saudi Pharm. J. 2017, 25, 1248–1252. [Google Scholar] [CrossRef]
- Muhaisen, H.M. Flavonoid Glycosides from Acacia tortilis. Merit Res. J. Med. Med. Sci. 2020, 8, 575–580. [Google Scholar]
- Taveira, M.; Silva, L.R.; Vale-Silva, L.A.; Pinto, E.; Valentao, P.; Ferreres, F.; Guedes de Pinho, P.; Andrade, P.B. Lycopersicon esculentum seeds: An industrial byproduct as an antimicrobial agent. J. Agric. Food Chem. 2010, 58, 9529–9536. [Google Scholar] [CrossRef]
- Kigondu, E.V.M.; Rukunga, G.M.; Keriko, J.M.; Tonui, W.K.; Gathirwa, J.W.; Kirira, P.G.; Irungu, B.; Ingonga, J.M.; Ndiege, I.O. Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J. Ethnopharmacol. 2009, 123, 504–509. [Google Scholar] [CrossRef]
- Al-Busafi, S.; Al-Riyami, M.; Al-Ouwaisi, K.; Hisham, A. Screening of Antioxidant and Radical Scavenging Activities of Some Omani Medicinal Plants. SQU J. Sci. 2007, 12, 1. [Google Scholar] [CrossRef]
- Dudai, N.; Raz, A.; Hofesh, N.; Rozenzweig, N.; Aharon, R.; Fischer, R.; Chaimovitsh, D.; Segev, D. Antioxidant activity and phenol content of plant germplasm originating in the Dead Sea area. Isr. J. Plant Sci. 2008, 56, 227–232. [Google Scholar] [CrossRef]
- Habib, H.M.; Al Meqbali, F.T.; Kamal, H.; Souka, U.D.; Ibrahim, W.H. Bioactive components, antioxidant and DNA damage inhibitory activities of honeys from arid regions. Food Chemistry 2014, 153, 28–34. [Google Scholar] [CrossRef]
- Hédia Hannachi Chemicals profiling and antioxidants activities of Acacia seeds. J. Med. Plants Res. 2011, 5, 6869–6875. [CrossRef]
- Sakthive, K.M.; Kannan, N.; Angeline, A.; Guruvayoorappan, C. Anticancer activity of Acacia nilotica (L.) Wild. Ex. Delile subsp. indica against Dalton’s ascitic lymphoma induced solid and ascitic tumor model. Asian Pac. J. Cancer Prev. 2012, 13, 3989–3995. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; CFR Ferreira, I. The role of phenolic compounds in the fight against cancer–a review. Anti-Cancer Agents Med. Chem. 2013, 13, 1236–1258. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Quiet, L.; Rezk, A.; Jaiswal, R.; Rehders, M. Phenolic Profile and In Vitro Assessment of Cytotoxicity and Antibacterial Activity of Ziziphus spina-christi Leaf Extracts. Med. Chem. 2016, 6, 143–156. [Google Scholar] [CrossRef]
- Padmaharish, V.; Lakshmi, T. Anticancer activities of medicinal plants–an update. J. Pharm. Sci. Res. 2017, 9, 432. [Google Scholar]
- Guimarães, R.; Barros, L.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.J.R.; Ferreira, I.C. Bioactivity of different enriched phenolic extracts of wild fruits from Northeastern Portugal: A comparative study. Plant Foods Hum. Nutr. 2014, 69, 37–42. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Gupta, U.; Misra, P.; Singh, S.P.; Verma, R.C. Antidiabetic Effects of Acacia Tortilis Seed Extract in Normal and Alloxan-Induced Diabetic Rats. IJPSR 2013, 4, 1392–1397. [Google Scholar]
- Alharbi, W.D.M.; Azmat, A. Hypoglycemic and hypocholesterolemic effects of Acacia tortilis (fabaceae) growing in makkah. Pak. J. Pharmacol. 2011, 28, 1–8. [Google Scholar]
- Kumar Bhateja, P.; Singh, R. Antidiabetic Activity of Acacia tortilis (Forsk.) Hayne ssp. raddiana Polysaccharide on Streptozotocin-Nicotinamide Induced Diabetic Rats. BioMed Res. Int. 2014, 2014, e572013. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.J.; Loganathan, P. Hypoglycemic effect of Spinacia oleracea in alloxan induced diabetic rat. Glob. J. Biotechnol. Biochem. 2010, 5, 87–91. [Google Scholar]
- Hossain, M.S.; Ahmed, M.; Islam, A. Hypolipidemic and hepatoprotective effects of different fractions of ethanolic extract of immature leaves of Mangifera indica (Linn.) in alloxan induced diabetic rats. Int. J. Pharm. Sci. Res. 2010, 1, 132. [Google Scholar]
- Pi-Sunyer, F.X. Weight loss in type 2 diabetic patients. Diabetes Care 2005, 28, 1526–1527. [Google Scholar] [CrossRef]
- Niwa, A.; Tajiri, T.; Higashino, H. Ipomoea batatas and Agarics blazei ameliorate diabetic disorders with therapeutic antioxidant potential in streptozotocin-induced diabetic rats. J. Clin. Biochem. Nutr. 2011, 48, 1101050064. [Google Scholar] [CrossRef]
- Anjaneyulu, M.; Chopra, K. Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 244–248. [Google Scholar] [CrossRef]
- Xiao, M.; Jia, X.; Wang, N.; Kang, J.; Hu, X.; Goff, H.D.; Cui, S.W.; Ding, H.; Guo, Q. Therapeutic potential of non-starch polysaccharides on type 2 diabetes: From hypoglycemic mechanism to clinical trials. Crit. Rev. Food Sci. Nutr. 2022, 1–34. [Google Scholar] [CrossRef]
- Khosla, P.; Gupta, D.D.; Nagpal, R.K. Effect of Trigonella foenum graecum (Fenugreek) on serum lipids in normal and diabetic rats. Indian J. Pharmacol. 1995, 27, 89. [Google Scholar]
- Moritoh, Y.; Takeuchi, K.; Hazama, M. Chronic administration of voglibose, an α-glucosidase inhibitor, increases active glucagon-like peptide-1 levels by increasing its secretion and decreasing dipeptidyl peptidase-4 activity in ob/ob mice. J. Pharmacol. Exp. Ther. 2009, 329, 669–676. [Google Scholar] [CrossRef]
- Khalatbary, A.R.; Ahmadvand, H. Anti-inflammatory effect of the epigallocatechin gallate following spinal cord trauma in rat. Iran. Biomed. J. 2011, 15, 31. [Google Scholar] [PubMed]
- Wu, Y.R.; Choi, H.J.; Kang, Y.G.; Kim, J.K.; Shin, J.-W. In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano-and microscale particles. Int. J. Nanomed. 2017, 12, 7007. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, C.; Filippin-Monteiro, F.B.; Centa, A.; Creczinsky-Pasa, T.B. Antioxidant, Antitumoral and Anti-Inflammatory Activities of Gallic Acid. Handbook on Gallic Acid: Natural Occurrences, Antioxidant Properties and Health Implications, 4th ed.; Nova Publishers: New York, NY, USA, 2013; pp. 1–23. [Google Scholar]
- Chu, C.; Deng, J.; Man, Y.; Qu, Y. Green tea extracts epigallocatechin-3-gallate for different treatments. BioMed Res. Int. 2017, 2017, 5615647. [Google Scholar] [CrossRef] [PubMed]
- Sowndhararajan, K.; Santhanam, R.; Hong, S.; Jhoo, J.-W.; Kim, S. Suppressive effects of acetone extract from the stem bark of three Acacia species on nitric oxide production in lipopolysaccharide-stimulated RAW 264.7 macrophage cells. Asian Pac. J. Trop. Biomed. 2016, 6, 658–664. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
| Study of Area | Used Part | Mode of Preparation | Traditional Use | References |
|---|---|---|---|---|
| Morocco (Al Haouz Rhamna region) | Root, fruit and leaf | Decoction and powder | Diabetes | [12] |
| Moroccan Sahara (Tan Tan) | Plant pod | Powder | Diabetes | [16] |
| Morocco (Tata Province) | Gum | Infusion | Neuralgia, asthma, hepatitis, jaundice | [1] |
| Bark | Infusion | Astringent, demulcent, haemostatic, expectorant, angina | ||
| Fruit | Decoction | Kidney stones | ||
| Morocco (Agadir Ida Outanane) | Leaves | Powder | Diarrhea, stomach diseases, burns | [17] |
| Leaves | Poutine of powder mixed with olive oil | |||
| Northern Kenya (Samburu) | Roots | Not reported | Minimize bloating | [18] |
| Southern Algerian Sahara (Tassili N’ajjer) | Fruit | Powder | Stomach diseases, diarrhoeaaches | [19] |
| Central Sudan (Soba area, Khartoum State) | No reported | Not reported | Malaria, swollen joint problems, skin allergies | [20] |
| Eastern Desert of Egypt (Wadi El-Gemal National Park) | Gum | Not reported | Stomach acidity, ocular affections, jaundice | [21] |
| Yemen | Fruits | Not reported | Stomach aches, digestive disorders | [15] |
| Some regions of Africa | Leaves, trunk bark | Not reported | Jaundice, bilious fevers, skin allergies, diabetes, hypertension, diuretic properties | [13] |
| Northern Burkina Fasso | No reported | Not reported | Urogenital and pulmonary Infectious, schistosomiasis, ulcers, malaria, yellow fever, dysentery | [22] |
| Part Used | Origin | Type of Extract/ Seed Oil | Chemical Composition | Compounds Class | References |
|---|---|---|---|---|---|
| Leaves | Saudi Arabia | Methanolic Extract | 2′,6′-dihydroxy, Chalcone-4′-O-glucoside, 4-methoxy chalcone | Flavonoids (chalcone glycosides) | [11] |
| Vitexin | Flavonoids (flavones) | ||||
| Leaves | Algerian Sahara | Ethanolic extract | Epigallocatechin-3,7,3′,4′,5′-penta-Ogallate; | Flavonoids (flavanol) | [4] |
| Epigallocatechin-3,5,4′,5′-tetra-Ogallate; | |||||
| (Epi)gallocatechin-3,5′di-O-gallate; | |||||
| Epigallocatechin-3,5,3′-tri-O-gallate; | |||||
| Trigalloylquinic acid; | |||||
| (Epi)gallocatechin-5,7-di-O-gallate; | |||||
| Epigallocatechin-3,5,5′-tri-O-gallate; | |||||
| Epigallocatechin-5,7,4′-tri-O-gallate; | |||||
| Epigallocatechin-3,7,5′-tri-O-gallate; | |||||
| Epigallocatechin-3,5,4′-tri-O-gallate. | |||||
| Leaves | Egypt | Ethanol extract | Myricetin 3-O-rutinoside; Rutin (Quercetin 3-O-rutinoside); Kaemepferol 3-O-rutinoside | Flavonol glycoside | [24] |
| Leaves | Yemen | Methanol extract | 5,7-dihydroxy-4-p-methyl benzyl isoflavone | Flavonoids (isoflavone) | [25] |
| Apigenin Luteolin | Flavonoids (flavone) | ||||
| Quercetin | Flavonoids (flavanols) | ||||
| Gum exudates | India | Aqueous extract | L-arabinose, D-galactose, L-rhamnose, D-mannose, D-glucose, D-galacturonic acid, and D-glucuronic acid | Monosaccharides and derivatives | [26] |
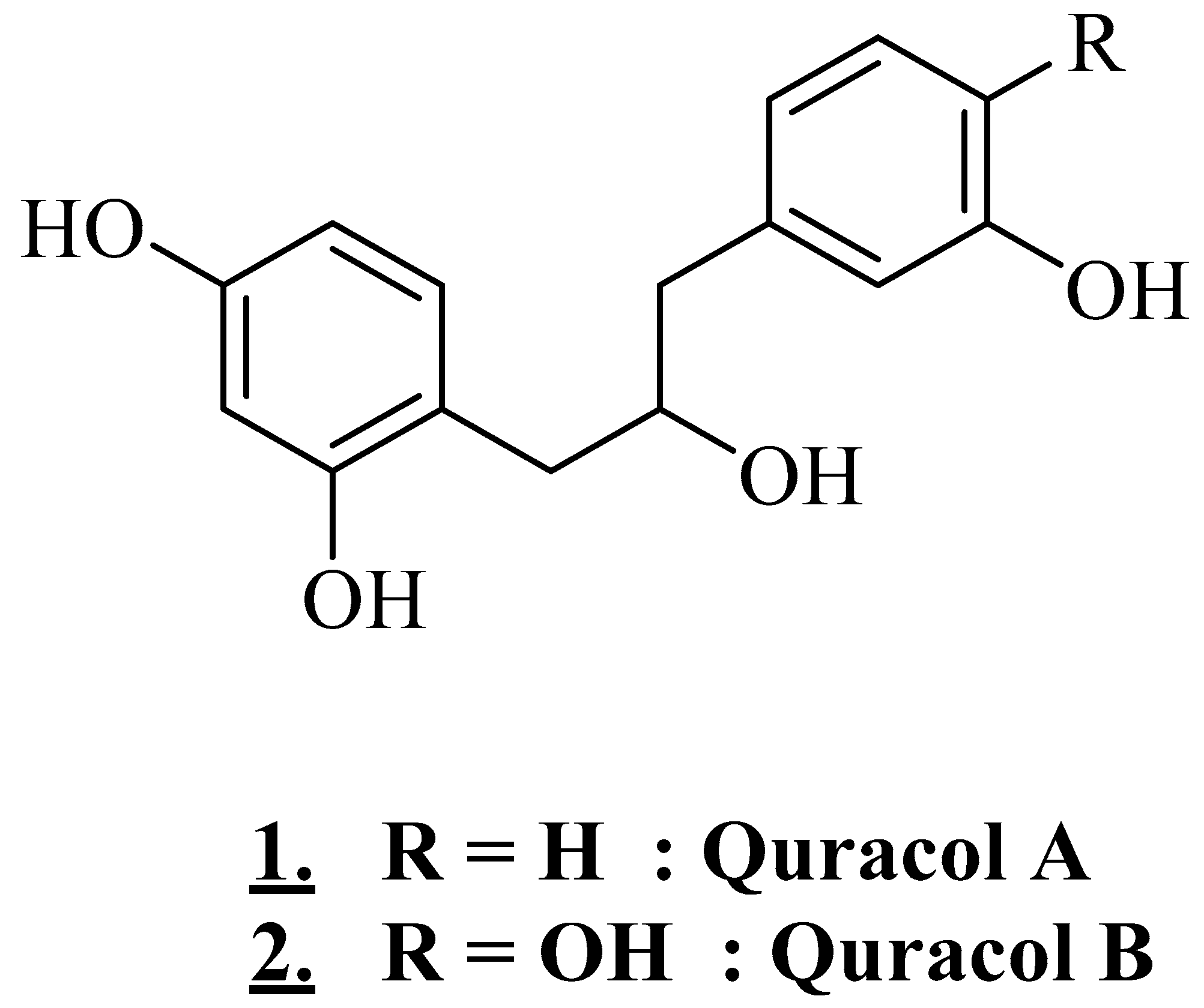
| Stem bark (with gum) | Somalia | Aqueous extract | Quracol A and Quracol B | Alcohols | [27] |
| Seeds | Israel | Seed oil | Linolenic acid, linoleic acid, palmitic acid, oleic acid, and stearic acid | Fatty acids | [28] |
| Egypt | Linoleic acid, palmitic acid, stearic acid, oleic acid, and arachidic acids | Fatty acids | [8] |
| Use Part | Extract | Bacterial Strain | Key Results | References |
|---|---|---|---|---|
| Aerial part | Ethanolic extract | Staphylococcus aureus (ATCC25923) | Ø = 20 mm Control not reported | [5] |
| Pseudomonas aeruginosa (ATCC27853) | Ø = 20 mm Control not reported | |||
| Chloroform extract | Staphylococcus aureus (ATCC25923) | nd | ||
| Pseudomonas aeruginosa (ATCC 27853) | nd | |||
| Acetonic extract | Staphylococcus aureus (ATCC 25923) | Ø = 23 mm Control not reported | ||
| Pseudomonas aeruginosa (ATCC 27853) | Ø = 18 mm Control not reported | |||
| Aerial part | Ethanolic extract | Staphylococcus aureus | Ø = 17 ± 0.9 mm MIC = 0.4 mg/mL Ampicillin Ø = 21 ± 1.9 mm | [30] |
| Escherichia coli | Ø = 19 ± 0.8 mm MIC = 0.8 mg/mL Doxycycline Ø = 25 ± 1.2 mm | |||
| Pseudomonas aeruginosa | Ø = 16 ± 1.5 mm MIC = 0.8 mg/mL Doxycycline Ø = 24 ± 1.7 mm | |||
| Fruit | Dichloromethanic extract | Staphylococcus aureus ATCC 29213 | Ø = 20 mm Ampicillin Ø = 26 mm | [15] |
| Bacillus subtilis ATCC 6059 | Ø = 20 mm Ampicillin Ø = 28 mm | |||
| Micrococcus flavus SBUG | Ø = 15 mm Ampicillin Ø = 31 mm | |||
| Methanolic extract | Staphylococcus aureus ATCC 29213 | Ø = 10 mm Ampicillin Ø = 26 mm | ||
| Bacillus subtilis ATCC 6059 | Ø = 8 mm Ampicillin Ø = 28 mm | |||
| Micrococcus flavus SBUG | Ø = 8 mm Ampicillin Ø = 31 mm | |||
| Pseudomonas aeruginosa ATCC 27853 | Ø = 10 mm Gentamicin Ø = 18 mm | |||
| Aqueous extract | Staphylococcusaureus ATCC 29213 | Ø = 10 mm Ampicillin Ø = 26 mm | ||
| Micrococcus flavus SBUG | Ø = 8 mm Ampicillin Ø = 31mm | |||
| Pseudomonas aeruginosa ATCC 27853 | Ø = 8 mm Gentamicin Ø = 18 mm | |||
| Dichloromethanic extract | Staphylococcus aureus ATCC 29213 | MIC = 500 µg/mL Ampicillin =0.05 µg/mL | ||
| Bacillus subtilis ATCC 6059 | MIC = 500 µg/mL Control not tested | |||
| Micrococcus flavus SBUG | MIC = 1000 µg/mL Ampicillin = 0.25 µg/mL | |||
| Methanolic extract | Staphylococcus aureus ATCC 29213 | MIC = 500 µg/mL Ampicillin = 0.05 µg/mL | ||
| Pseudomonas aeruginosa ATCC 27853 | MIC = 1000 µg/mL Control not tested | |||
| Aqueous extract | Staphylococcus aureus ATCC 29213 | MIC = 1000 µg/mL Ampicillin = 0.05 µg/mL | ||
| Gum | Aqueous extract | Salmonella typhi | Ø = 19 ± 0.5 mm Control not tested | [3] |
| Escherichia coli | Ø = 17 ± 0.4 mm Control not tested | |||
| Staphylococcus aureus | Ø = 24 ± 0.6 mm Control not tested | |||
| Bacillus subtilis | Ø = 23 ± 0.1 mm Control not tested | |||
| Fresh leaves | Chloroform extract | Klebsiella oxytoca | Ø = 10.0 ± 0.57 mm Control not reported | [6] |
| Staphylococcus aureus | Ø = 10.6 ± 0.66 mm Control not reported | |||
| Proteus mirabilis | Ø = 10.0 ± 0.57 mm Control not reported | |||
| Klebsiella pneumoniae | Ø = 14.3 ± 0.88 mm Control not reported | |||
| Pseudomonas aeruginosa | Ø = 9.3 ± 0.33 mm Control not reported | |||
| Alcoholic extract | Klebsiella oxytoca | Ø = 12.0 ± 1.15 mm Control not reported | ||
| Staphylococcus aureus | Ø = 9.3 ± 0.33 mm Control not reported | |||
| Proteus mirabilis | Ø = 8.6 ± 0.66 mm Control not reported | |||
| Klebsiella pneumoniae | Ø = 15.0 ± 0.57 mm Control not reported | |||
| Pseudomonas aeruginosa | Ø = 11.0 ± 0.00 mm Control not reported | |||
| Petroleum ether extract | Klebsiella oxytoca | Ø = 10.6 ± 0.66 mm | ||
| Staphylococcus aureus | Ø = 9.0 ± 1.00 mm | |||
| Proteus mirabilis | Ø = 8.3 ± 0.88 mm | |||
| Klebsiella pneumoniae | Ø = 14.3 ± 0.33 mm | |||
| Pseudomonas aeruginosa | Ø = 8.6 ± 0.33 mm | |||
| Control not reoprted | ||||
| Methanolic extract | Klebsiella oxytoca | Ø = 13.0 ± 0.58 mm | ||
| Staphylococcus aureus | Ø = 11.3 ± 0.33 mm | |||
| Proteus mirabilis | Ø = 9.6 ± 1.30 mm | |||
| Klebsiella pneumoniae | Ø = 15.3 ± 0.33 mm | |||
| Pseudomonas aeruginosa | Ø = 11.3 ± 0.33mm | |||
| Control not reoprted | ||||
| Petroleum Benzin extract | Klebsiella oxytoca | Ø = 9.3 ± 0.88 mm | ||
| Staphylococcus aureus | Ø = 8.6 ± 0.33 mm | |||
| Proteus mirabilis | Ø = 9.6 ± 1.45 mm | |||
| Control not reoprted | ||||
| Klebsiella pneumoniae | Ø = 16.0 ± 0.00 mm | |||
| Pseudomonas aeruginosa | Ø = 8.6 ± 0.88 mm | |||
| Control not reoprted | ||||
| Dry Bark | Chloroform extract | Klebsiella oxytoca | Ø = 7.3 ± 0.33 mm | [6] |
| Staphylococcus aureus | Ø = 7.6 ± 0.66 mm | |||
| Proteus mirabilis | Ø = 12.0 ± 0.00 mm | |||
| Klebsiella pneumoniae | Ø = 11.3 ± 0.33 mm | |||
| Pseudomonas aeruginosa | Ø = 7.0 ± 0.00 mm | |||
| Control not reported | ||||
| Alcoholic extract | Klebsiella oxytoca | Ø = 9.0 ± 0.57 mm | ||
| Staphylococcus aureus | Ø = 7.3 ± 0.33 mm | |||
| Proteus mirabilis | Ø = 13.3 ± 0.33 mm | |||
| Klebsiella pneumoniae | Ø = 13.6 ± 0.88 mm | |||
| Pseudomonas aeruginosa | Ø = 7.0 ± 0.00 mm | |||
| Control not reported | ||||
| Petroleum ether extract | Klebsiella oxytoca Staphylococcus aureus Proteus mirabilis Klebsiella pneumoniae Pseudomonas aeruginosa | Ø = 7.6 ± 0.33 mm Ø = 8.3 ± 0.88 mm Ø = 12.3 ± 0.33 mm Ø = 12.3 ± 0.88 mm Ø = 7.6 ± 0.33 mm | ||
| Methanolic extract | Klebsiella oxytoca Staphylococcus aureus Proteus mirabilis Klebsiella pneumoniae Pseudomonas aeruginosa | Ø = 7.6 ± 0.67 mm Ø = 7.0 ± 0.00 mm Ø = 11.3 ± 0.88 mm Ø = 13.0 ± 0.58 mm Ø = 7.0 ± 0.00 mm | ||
| Petroleum benzin extract | Klebsiella oxytoca Staphylococcus aureus Proteus mirabilis Klebsiella pneumoniae Pseudomonas aeruginosa | Ø = 7.0 ± 0.00 mm Ø = 8.6 ± 0.67 mm Ø = 12.0 ± 0.00 mm Ø = 13.3 ± 0.33 mm Ø = 7.3 ± 0.33 mm | ||
| Fresh leaves | Aqueous extract | Escherichia coli | MIC = 1.25 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | [4] |
| Klebsiella pneumoniae | MIC = 2.5 mg/mL Ampicillin (20 mg/mL) = 10 MBC = 20 mg/mL Ampicillin (20 mg/mL) = 20 | |||
| Morganella morganii | MIC = 1.25 mg/mL Ampicillin (20 mg/mL) = 20 MBC = 20 mg/mL Ampicillin (20 mg/mL) > 20 | |||
| Proteus mirabilis | MIC =2.5 mg/mL ; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |||
| Pseudomonas aeruginosa | MIC = 2.5 mg/mL ; MBC = 20 mg/mL Ampicillin (20 mg/mL) > 20 | |||
| Enterococcus faecalis | MIC = 5 mg/mL ; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |||
| Listeria monocytogenes | MIC = 1.25 mg/mL ; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |||
| Fresh leaves | Ethanolic extract | Escherichia coli | MIC = 1.25 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |
| Klebsiella pneumoniae | MIC = 1.25 mg/mL Ampicillin (20 mg/mL) = 10 MBC = 20 mg/mL Ampicillin (20 mg/mL) = 20 | |||
| Morganella morganii | MIC = 1.25 mg/mL Ampicillin (20 mg/mL) = 20 MBC = 20 mg/mL Ampicillin (20 mg/mL) > 20 | |||
| Proteus mirabilis | MIC =1.25 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |||
| Pseudomonas aeruginosa | MIC = 1.25 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) > 20 | |||
| Enterococcus faecalis | MIC = 2.5 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 | |||
| Listeria monocytogenes | MIC = 1.25 mg/mL; MBC = 20 mg/mL Ampicillin (20 mg/mL) < 0.15 |
| Use Part | Type of Extract | Tested Microorganisms | Key Results | References |
|---|---|---|---|---|
| Aerial part | Ethanolic extract | Candida albicans (ATCC90028) | Ø = 23 mm | [5] |
| Chloroform extract | nd | |||
| Acetonic extract | Ø = 25 mm | |||
| Aerial part | Ethanolic extract | Candida albicans | Ø = 15 ± 1.0 mm | [30] |
| MIC = 0.8 mg/mL | ||||
| Fruit | Methanolic extract | Candida maltose | Ø = 8 mm | [15] |
| Aerial part | Chloroform extract | Candida albicans | Ø = 16.0 ± 0.00 mm | [6] |
| Alcoholic extract | Ø = 16.6 ± 0.33 mm | |||
| Petroleum ether extract | Ø = 15.3 ± 0.33 mm | |||
| Methanolic extract | Ø = 16.6 ± 0.67 mm | |||
| Petroleum benzin extract | Ø = 15.6 ± 0.33 mm | |||
| Dry Bark | Chloroform extract | Candida albicans | Ø = 12.3 ± 1.20 mm | [6] |
| Alcoholic extract | Ø = 10.3 ± 1.76 mm | |||
| Petroleum ether extract | Ø = 11.0 ± 1.52 mm | |||
| Methanolic extract | Ø = 12.3 ± 0.33 mm | |||
| Petroleum benzin extract | Ø = 12.3 ± 0.33 mm |
| Use Part | Extracts/Method Extraction | Used Method | Key Results | References |
|---|---|---|---|---|
| Aerial parts | Ethanolic extract/maceration | DPPH | RSA = 83 ± 0.02% Control not reported | [5] |
| Chloroform extract/maceration | DPPH | RSA = 42 ± 0.7% Control not reported | ||
| Acetonic extract/maceration | DPPH | RSA = 82 ± 0.04% Control not reported | ||
| Leaves | Ethanolic extract/ultrasound | DPPH | IC50 = 250.13 µg/mL Rutin IC50 = 250.13 µg/mL | [7] |
| Leaves | n-Butanol extract/maceration | DPPH | RSA = 89.8% Control not tested | [34] |
| Leaves | Methanolic extract/infusion | DPPH | 84.3 ± 9.7 mg/g DW (Chlorogenic acid equivalent) Control not tested | [35] |
| Leaves | Methanolic extract/maceration | DPPH | IC50 = 0.03 ± 0.01 µg/mL Trolox = 0.01 ± 0.00 µg/mL | [23]. |
| Trunk bark | Methanolic extract/maceration | DPPH | IC50 = 0.01 ± 0.01 µg/mL Trolox = 0.01 ± 0.00 µg/mL | [23]. |
| Fruit | Methanolic extract/maceration | DPPH | RSA = 26.17% (at 100 µg/mL) Ascorbic acid RSA = 96.9% (at 100 µg/mL) | [15] |
| Fruit | Aqueous extract/maceration | ABTS | RSA = 80.62 ± 0.14% Control not tested | [36] |
| DPPH | RSA = 19.12 ± 1.34% Control not tested | |||
| Seeds | Methanolic extract/maceration | DPPH | RSA = 0.84 ± 0.03 (TEAC mM) Control not tested | [37] |
| ABTS | RSA = 2.22 ± 0.20 (TEAC mM) Control not tested | |||
| Gum | Aqueous extract/maceration | DPPH | RSA = 92.13 ± 0.13% (at 20 mg/mL) Control not tested | [3] |
| Activities | Use Part | Extracts | Experimental Approach | Key Results | References |
|---|---|---|---|---|---|
| Cytotoxic activity | Aerial part | Ethanolic extract | HepG2, HEK-293, MCF-7, and MDA-MB-231 cancer cells were tested in vitro for anticancer efficacy. | The estimated IC50 (μg·mL−1 ± SD): HepG2 (Liver) = 42.3 ± 1.78 5-Flurourasil = 3.1 ± 0.07 HEK-293 (Kidney) = 49.1 ± 1.92 5-Flurourasil = 2.5 ± 0.05 MCF-7 (Breast) = 65.7 ± 2.49 5-Flurourasil = 3.7 ± 0.07 MDA-MB-231 (Breast) = 52.2 ± 1. 995-Flurourasil = 3.9 ± 0.09 | [30] |
| Cytotoxic activity | Fruit | Methanolic extract | FL-cells, a human amniotic epithelial cell line | IC50% (µg/mL) against FL-cells > 1000 | [15] |
| Cytotoxic activity | Root bark | Chloroform extract | One human cancer cell line was used to test cytotoxic activity (KB, a human oral epidermoid cancer cell line) | Cytotoxicity assay; KB IC50 (µg/mL) > 20 Chloroquine = 17.4 µg/mL | [14] |
| Cytotoxic activity | Leaves | Ethanolic extract | Cytotoxicity, Growth inhibition values (GI50, μg/mL) | Cell lung cancer (NCI–H460) = 52 ± 1. Ellipticine = 1.0 ± 0.1 Cervical carcinoma (HeLa) = 48.2 ± 0.1. Ellipticine = 1.9 ± 0.1 Hepatocellular carcinoma (HepG2) = 33 ± 1 (p < 0.05) Ellipticine = 1.1 ± 0.2 Breast carcinoma (MCF-7) = 52 ± 1 (p < 0.05). Ellipticine = 0.91 ± 0.04 PLP2 = 259 ± 0.1 Ellipticine = 3.2 ± 0.7 | [4] |
| Part Used | Extract Tested | Dose | Model | Keys Results | References |
|---|---|---|---|---|---|
| Seed | Aqueous extract | 100 and 200 mg/kg body weight | Normoglycaemic and Alloxan-induced diabetic rats | Decreases blood glucose levels, fluid intake by 34.49%, and food intake | [43] |
| Leaves | Aqueous extract | 800 mg/kg | Normoglycaemic rats | Reduces blood glucose, serum total cholesterol and LDL level, and body weight Increase serum HDL-cholesterol | [44] |
| Stem and branches | Aqueous extract | 250–1000 mg/kg | Streptozotocin-Nicotinamide Induced diabetic rats | Minimizes fasting blood glucose level, glycated hemoglobin level, total cholesterol, triglyceride, LDL, VLDL, SGOT, and SGPT levels, and improved HDL level | [45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, D.; El Hajjaji, S.; Mourabit, Y.; Bouyahya, A.; Lee, L.-H.; El Menyiy, N.; Tarik, A.; Benali, T.; El Moudden, H.; Gallo, M.; et al. Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis. Plants 2022, 11, 3348. https://doi.org/10.3390/plants11233348
Taha D, El Hajjaji S, Mourabit Y, Bouyahya A, Lee L-H, El Menyiy N, Tarik A, Benali T, El Moudden H, Gallo M, et al. Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis. Plants. 2022; 11(23):3348. https://doi.org/10.3390/plants11233348
Chicago/Turabian StyleTaha, Douae, Souad El Hajjaji, Yassine Mourabit, Abdelhakim Bouyahya, Learn-Han Lee, Naoual El Menyiy, Aanniz Tarik, Taoufiq Benali, Hamza El Moudden, Monica Gallo, and et al. 2022. "Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis" Plants 11, no. 23: 3348. https://doi.org/10.3390/plants11233348
APA StyleTaha, D., El Hajjaji, S., Mourabit, Y., Bouyahya, A., Lee, L.-H., El Menyiy, N., Tarik, A., Benali, T., El Moudden, H., Gallo, M., Iba, N., & Bourais, I. (2022). Traditional Knowledge, Phytochemistry, and Biological Properties of Vachellia tortilis. Plants, 11(23), 3348. https://doi.org/10.3390/plants11233348








