Xylitol Production from Pineapple Cores (Ananas comosus (L.) Merr) by Enzymatic and Acid Hydrolysis Using Microorganisms Debaryomyces hansenii and Candida tropicalis
Abstract
1. Introduction
2. Materials and Methods
2.1. Tools and Materials
2.2. Enzymatic Hydrolysis
2.3. Acid Hydrolysis
2.4. Fermentation
2.5. Purification of Fermentation Product
2.6. Analysis Method
2.7. Data Interpretation
2.8. Statistical Analysis
3. Results and Discussion
3.1. Lignocellulose Composition of Pineapple Core
3.2. Impact of Hydrolysis Types on Hydrolysate Composition
3.3. Influence of Different Hydrolysate Types on Substrate Utilization by D. hansenii and C. tropcalis
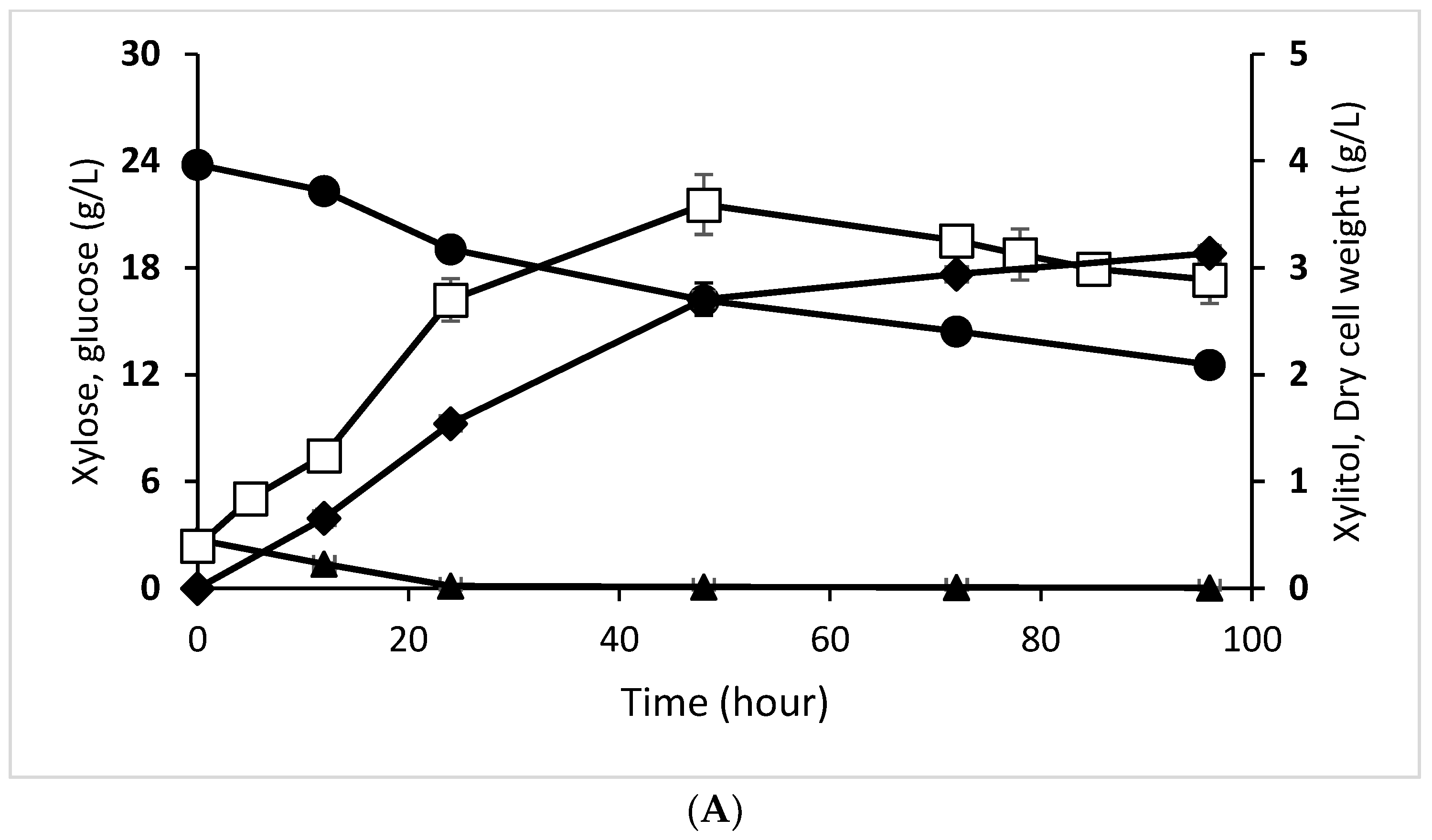
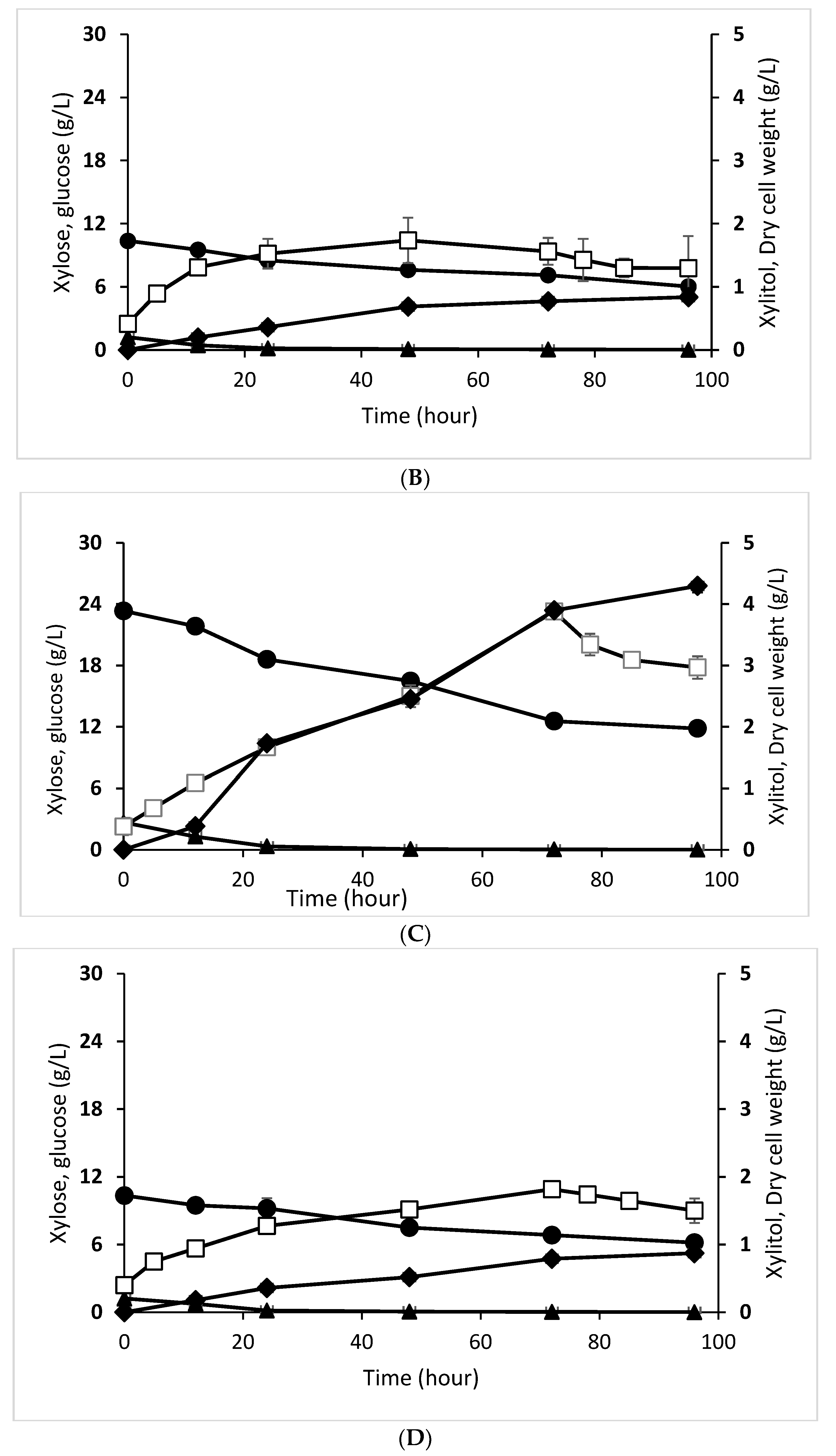
3.4. Effect of Enzymatic and Acid Hydrolysate on Xylitol Production Profile by D. hansenii and C. tropicalis
3.5. Morphology Changes of Fresh and Hydrolyzed Pineapple Core
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hikal, W.M.; Mahmoud, A.A.; Said-Al Ahl, H.A.H.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), Waste Streams, Characterisation and Valorisation: An Overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, X. Physicochemical Characteristics of Pineapple (Ananas Mill.) Peel Cellulose Prepared by Different Methods. Adv. Mater. Res. 2012, 554–556, 1038–1041. [Google Scholar] [CrossRef]
- Pardo, M.E.S.; Cassellis, M.E.R.; Escobedo, R.M.; García, E.J. Chemical Characterisation of the Industrial Residues of the Pineapple (Ananas comosus). J. Agric. Chem. Environ. 2014, 3, 53–56. [Google Scholar] [CrossRef]
- Dasgupta, D.; Bandhu, S.; Adhikari, D.K.; Ghosh, D. Challenges and Prospects of Xylitol Production with Whole Cell Bio-Catalysis: A Review. Microbiol. Res. 2017, 197, 9–21. [Google Scholar] [CrossRef]
- Winkelhausen, E.; Kuzmanova, S. Microbial Conversion of D-Xylose to Xylitol. J. Ferment. Bioeng. 1998, 86, 1–14. [Google Scholar] [CrossRef]
- Venkateswar Rao, L.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of Lignocellulosic Biomass to Xylitol: An Overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Suhartini, S.; Rohma, N.A.; Mardawati, E.; Kasbawati; Hidayat, N.; Melville, L. Biorefining of Oil Palm Empty Fruit Bunches for Bioethanol and Xylitol Production in Indonesia: A Review. Renew. Sustain. Energy Rev. 2022, 154, 111817. [Google Scholar] [CrossRef]
- Kresnowati, M.; Mardawati, E.; Setiadi, T. Production of Xylitol from Oil Palm Empty Friuts Bunch: A Case Study on Bioefinery Concept. Mod. Appl. Sci. 2015, 9, 206. [Google Scholar] [CrossRef]
- Mardawati, E.; Werner, A.; Bley, T.; Mtap, K.; Setiadi, T. The Enzymatic Hydrolysis of Oil Palm Empty Fruit Bunches to Xylose. J. Japan Inst. Energy 2014, 93, 973–978. [Google Scholar] [CrossRef]
- Mardawati, E.; Purwadi, R.; Setiadi, T. Evaluation of the Enzymatic Hydrolysis Process of Oil Palm Empty Fruit Bunch Using Crude Fungal Xylanase. ARPN J. Eng. Appl. Sci. 2017, 12, 5286–5292. [Google Scholar]
- Parajó, J.C.; Domínguez, H.; Domínguez, J.M. Biotechnological Production of Xylitol. Part 2: Operation in Culture Media Made with Commercial Sugars. Bioresour. Technol. 1998, 65, 203–212. [Google Scholar] [CrossRef]
- Azizah, N. Biotransformation of Xylitol Production from Xylose of Lignocellulose Biomass Using Xylose Reductase Enzyme: Review. J. Food Life Sci. 2019, 3, 103–112. [Google Scholar] [CrossRef]
- Ravella, S.R.; Gallagher, J.; Fish, S.; Prakasham, R.S. D-Xylitol; da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642318863. [Google Scholar]
- Mardawati, E.; Febrianti, E.A.; Fitriana, H.N.; Yuliana, T.; Putriana, N.A.; Suhartini, S.; Kasbawati. An Integrated Process for the Xylitol and Ethanol Production from Oil Palm Empty Fruit Bunch (OPEFB) Using Debaryomyces hansenii and Saccharomyces cerevisiae. Microorganisms 2022, 10, 2036. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, E.; Costa, S.; Marchetti, M.G.; Pedrini, P. Optimized Production of Xylitol from Xylose Using a Hyper-Acidophilic Candida tropicalis. Biomolecules 2015, 5, 1979–1989. [Google Scholar] [CrossRef]
- Harahap, B.M. Degradation Techniques of Hemicellulose Fraction from Biomass Feedstock for Optimum Xylose Production: A Review. J. Keteknikan Pertan. Trop. dan Biosist. 2020, 8, 107–124. [Google Scholar] [CrossRef]
- Mardawati, E.; Wira, D.W.; Kresnowati, M.; Purwadi, R.; Setiadi, T. Microbial Production of Xylitol from Oil Palm Empty Fruit Bunches Hydrolysate: The Effect of Glucose Concentration. J. Japan Inst. Energy 2015, 94, 769–774. [Google Scholar] [CrossRef]
- Sugiharto, Y.E.C.; Harimawan, A.; Kresnowati, M.T.A.P.; Purwadi, R.; Mariyana, R.; Andry; Fitriana, H.N.; Hosen, H.F. Enzyme Feeding Strategies for Better Fed-Batch Enzymatic Hydrolysis of Empty Fruit Bunch. Bioresour. Technol. 2016, 207, 175–179. [Google Scholar] [CrossRef]
- Qi, B.; Chen, X.; Su, Y.; Wan, Y. Enzyme Adsorption and Recycling during Hydrolysis of Wheat Straw Lignocellulose. Bioresour. Technol. 2011, 102, 2881–2889. [Google Scholar] [CrossRef]
- Vázquez, M.J.; Alonso, J.L.; Domínguez, H.; Parajó, J.C. Production of Xylose-Containing Fermentation Media by Enzymatic Post-Hydrolysis of Oligomers Produced by Corn Cob Autohydrolysis. World J. Microbiol. Biotechnol. 2001, 17, 817–822. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H. Enzymatic Hydrolysis of Lignocellulosic Biomass from Low to High Solids Loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Detoxification of Hemicellulosic Hydrolyzate from Olive Tree Pruning Residue. Ind. Crops Prod. 2013, 49, 196–203. [Google Scholar] [CrossRef]
- Purwadi, R.; Niklasson, C.; Taherzadeh, M.J. Kinetic Study of Detoxification of Dilute-Acid Hydrolyzates by Ca(OH)2. J. Biotechnol. 2004, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Roberto, I.C. Alternatives for Detoxification of Diluted-Acid Lignocellulosic Hydrolyzates for Use in Fermentative Processes: A Review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Ma, H.; Tian, Z.; Lyu, G.; Fang, G.; Chen, J.; Saeed, H.A.M. Production of Xylose from Diluted Sulfuric Acid Hydrolysis of Wheat Straw. BioResources 2017, 12, 7084–7095. [Google Scholar] [CrossRef]
- Mardawati, E.; Mandra Harahap, B.; Ayu Febrianti, E.; Try Hartono, A.; Putri Siahaan, N.; Wulandari, A.; Yudiastuti, S.; Suhartini, S.; Kasbawati, K. Integrated and Partial Process of Xylitol and Bioethanol Production from Oil Palm Empty Fruit Bunches. Adv. Food Sci. Sustain. Agric. Agroind. Eng. 2022, 5, 49–67. [Google Scholar] [CrossRef]
- Mohamad, N.L.; Kamal, S.M.M.; Abdullah, N.; Ismail, I. Evaluation of Fermentation Conditions by Candida tropicalis for Xylitol Production from Sago Trunk Cortex. BioResources 2013, 8, 2499–2509. [Google Scholar] [CrossRef][Green Version]
- Rahman, S.H.A.; Choudhury, J.P.; Ahmad, A.L. Production of Xylose from Oil Palm Empty Fruit Bunch Fiber Using Sulfuric Acid. Biochem. Eng. J. 2006, 30, 97–103. [Google Scholar] [CrossRef]
- Cheng, K.K.; Zhang, J.A.; Ling, H.Z.; Ping, W.X.; Huang, W.; Ge, J.P.; Xu, J.M. Optimization of PH and Acetic Acid Concentration for Bioconversion of Hemicellulose from Corncobs to Xylitol by Candida Tropicalis. Biochem. Eng. J. 2009, 43, 203–207. [Google Scholar] [CrossRef]
- Parajó, J.C.; Domínguez, H.; Domínguez, J.M. Biotechnological Production of Xylitol. Part 1: Interest of Xylitol and Fundamentals of Its Biosynthesis. Bioresour. Technol. 1998, 65, 191–201. [Google Scholar] [CrossRef]
- Tran, L.H.; Yogo, M.; Ojima, H.; Idota, O.; Kawai, K.; Suzuki, T.; Takamizawa, K. The Production of Xylitol by Enzymatic Hydrolysis of Agricultural Wastes. Biotechnol. Bioprocess Eng. 2004, 9, 223–228. [Google Scholar] [CrossRef]
- Soontornchaiboon, W.; Chunhachart, O.; Pawongrat, R. Ethanol Production from Pineapple Waste by Co-Culture of Saccharomycs cerevisiae TISTR 5339 and Candida shehatae KCCM 11422. Asia-Pacific J. Sci. Technol. 2016, 21, 347–355. [Google Scholar] [CrossRef]

| Lignocellulose Biomass | Result (%) |
|---|---|
| NDF 1 | 61.57 ± 0.65 |
| Hemicellulose | 36.06 ± 0.22 |
| ADF 2 | 25.51 ± 0.72 |
| Cellulose | 14.20 ± 0.31 |
| Lignin | 10.05 ± 0.93 |
| Silica | 1.26 ± 0.82 |
| Components | Type of Hydrolysis | |
|---|---|---|
| Enzymatic Hydrolysis | Acid Hydrolysis | |
| Solid Loading (%) | 20.000 ± 0.800 | 4.00 ± 0.800 |
| Xylose (g/L) | 23.792 ± 0.163 | 9.844 ± 0.159 |
| Glucose (g/L) | 2.73 ± 0.080 | 1.22 ± 0.010 |
| Hemicellulose hydrolysis efficiency (%) | 37.550 ± 0.900 | 31.074 ± 0.700 |
| Products | Type of Hydrolysis | |
|---|---|---|
| Enzymatic Hydrolysis | Acid Hydrolysis | |
| Initial Xylose Concentration (g/L) | 23.790 ± 0.163 | 10.380 ± 0.050 |
| Final xylose concentration (g/L) | 12.560 ± 0.217 | 6.030 ± 0.030 |
| Xylose Utilization (%) | 47.200 ± 0.570 | 41.860 ± 0.070 |
| Initial Glucose Concentration (g/L) | 2.730 ± 0.080 | 1.220 ± 0.010 |
| Final Glucose Concentration (g/L) | 0.030 ± 0.010 | 0.030 ± 0.010 |
| Glucose Utilization (%) | 98.890 ± 0.400 | 97.540 ± 0.810 |
| Initial Xylitol Concentration (g/L) | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Final Xylitol Concentration (g/L) | 3.140 ± 0.080 | 0.840 ± 0.040 |
| Xylitol Yield from xylose (g/g) (YP/s) | 0.279 ± 0.000 | 0.193± 0.000 |
| Initial Cells Concentration (g cell/L) | 0.400 ± 0.040 | 0.410 ± 0.080 |
| Final Cells Concentration (g cell/L) | 2.890 ± 0.022 | 1.290± 0.051 |
| Biomass Yield of Substrate (g/g) (YX/S) | 0.221 ± 0.000 | 0.203 ± 0.000 |
| Xylitol Yield of Concentration cells (g/g) (YP/X) | 0.279 ± 0.000 | 0.193 ± 0.000 |
| Specific Growth Rate (h−1) (μ) | 0.108 ± 0.000 | 0.102 ± 0.000 |
| Products | Type of Hydrolysis | |
|---|---|---|
| Enzymatic Hydrolysis | Acid Hydrolysis | |
| Initial Xylose Concentration (g/L) | 23.340 ± 0.075 | 10.350 ± 0.037 |
| Final xylose concentration (g/L) | 11.480 ± 0.379 | 6.300 ± 0.216 |
| Xylose Utilization (%) | 49.600 ± 0.370 | 40.490 ± 0.410 |
| Initial Glucose Concentration (g/L) | 2.680 ± 0.050 | 1.220 ± 0.010 |
| Final Glucose Concentration (g/L) | 0.020 ± 0.000 | 0.020 ± 0.100 |
| Glucose Utilization (%) | 98.370 ± 0.280 | 99.400 ± 0.810 |
| Initial Xylitol Concentration (g/L) | 0.000 ± 0.000 | 0.000 ± 0.000 |
| Final Xylitol Concentration (g/L) | 4.290 ± 0.110 | 0.873 ± 0.004 |
| Xylitol Yield from xylose (g/g) (YP/s) | 0.371 ± 0.000 | 0.210 ± 0.000 |
| Initial Cells Concentration (g cell/L) | 0.380 ± 0.070 | 0.400 ± 0.080 |
| Final Cells Concentration (g cell/L) | 2.970 ± 0.018 | 1.500 ± 0.018 |
| Biomass Yield of Substrate (g/g) (YX/S) | 0.225 ± 0.000 | 0.211 ± 0.000 |
| Xylitol Yield of Concentration cells (g/g) (YP/X) | 0.373 ± 0.000 | 0.209 ± 0.000 |
| Specific Growth Rate (h−1) (μ) | 0.088 ± 0.000 | 0.082 ± 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardawati, E.; Hartono, A.T.; Nurhadi, B.; Fitriana, H.N.; Hermiati, E.; Ermawar, R.A. Xylitol Production from Pineapple Cores (Ananas comosus (L.) Merr) by Enzymatic and Acid Hydrolysis Using Microorganisms Debaryomyces hansenii and Candida tropicalis. Fermentation 2022, 8, 694. https://doi.org/10.3390/fermentation8120694
Mardawati E, Hartono AT, Nurhadi B, Fitriana HN, Hermiati E, Ermawar RA. Xylitol Production from Pineapple Cores (Ananas comosus (L.) Merr) by Enzymatic and Acid Hydrolysis Using Microorganisms Debaryomyces hansenii and Candida tropicalis. Fermentation. 2022; 8(12):694. https://doi.org/10.3390/fermentation8120694
Chicago/Turabian StyleMardawati, Efri, Agus T. Hartono, Bambang Nurhadi, Hana Nur Fitriana, Euis Hermiati, and Riksfardini Annisa Ermawar. 2022. "Xylitol Production from Pineapple Cores (Ananas comosus (L.) Merr) by Enzymatic and Acid Hydrolysis Using Microorganisms Debaryomyces hansenii and Candida tropicalis" Fermentation 8, no. 12: 694. https://doi.org/10.3390/fermentation8120694
APA StyleMardawati, E., Hartono, A. T., Nurhadi, B., Fitriana, H. N., Hermiati, E., & Ermawar, R. A. (2022). Xylitol Production from Pineapple Cores (Ananas comosus (L.) Merr) by Enzymatic and Acid Hydrolysis Using Microorganisms Debaryomyces hansenii and Candida tropicalis. Fermentation, 8(12), 694. https://doi.org/10.3390/fermentation8120694





