Growth and Influence of White-Rot Fungi on the Chemical Composition of Wheat Straw Inoculated under Varying Pre-Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Inoculum
Grain Spawn
2.2. Solid State Fermentation of Wheat Straw
2.2.1. Trial 1—Soaked and Drained Straw
2.2.2. Trial 2 and 3—Remoistened Straw
2.3. Microbial Analysis
2.4. Chemical Analysis
2.5. Statistical Analysis
3. Results
3.1. Temperature Development
3.2. Fungal Growth and pH Development
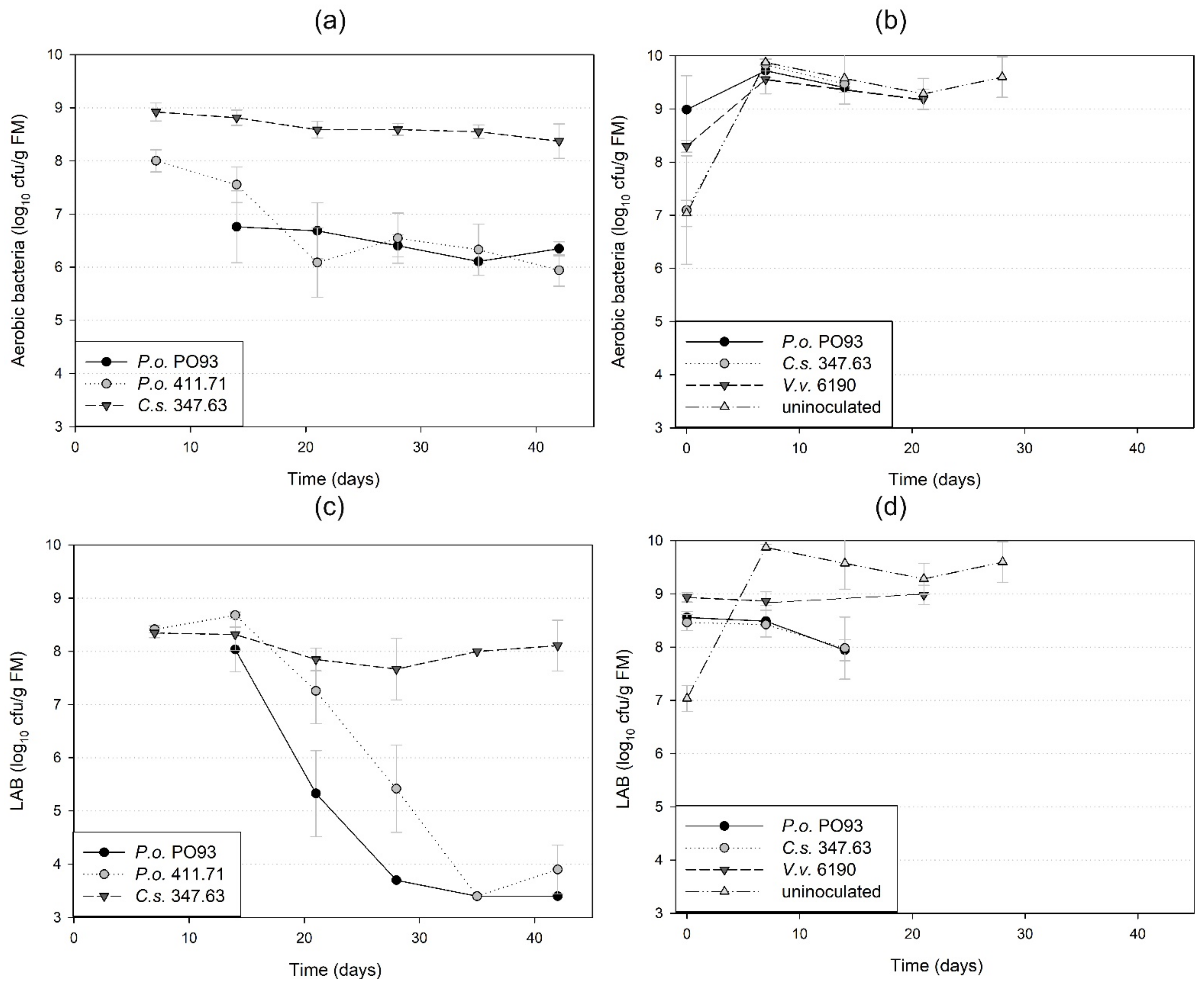
3.3. Accompanying Microflora
3.4. Changes in the Chemical Composition
3.4.1. Fiber Fractions
3.4.2. Non-Fiber Carbohydrates
3.4.3. Non-Starch Polysaccharides
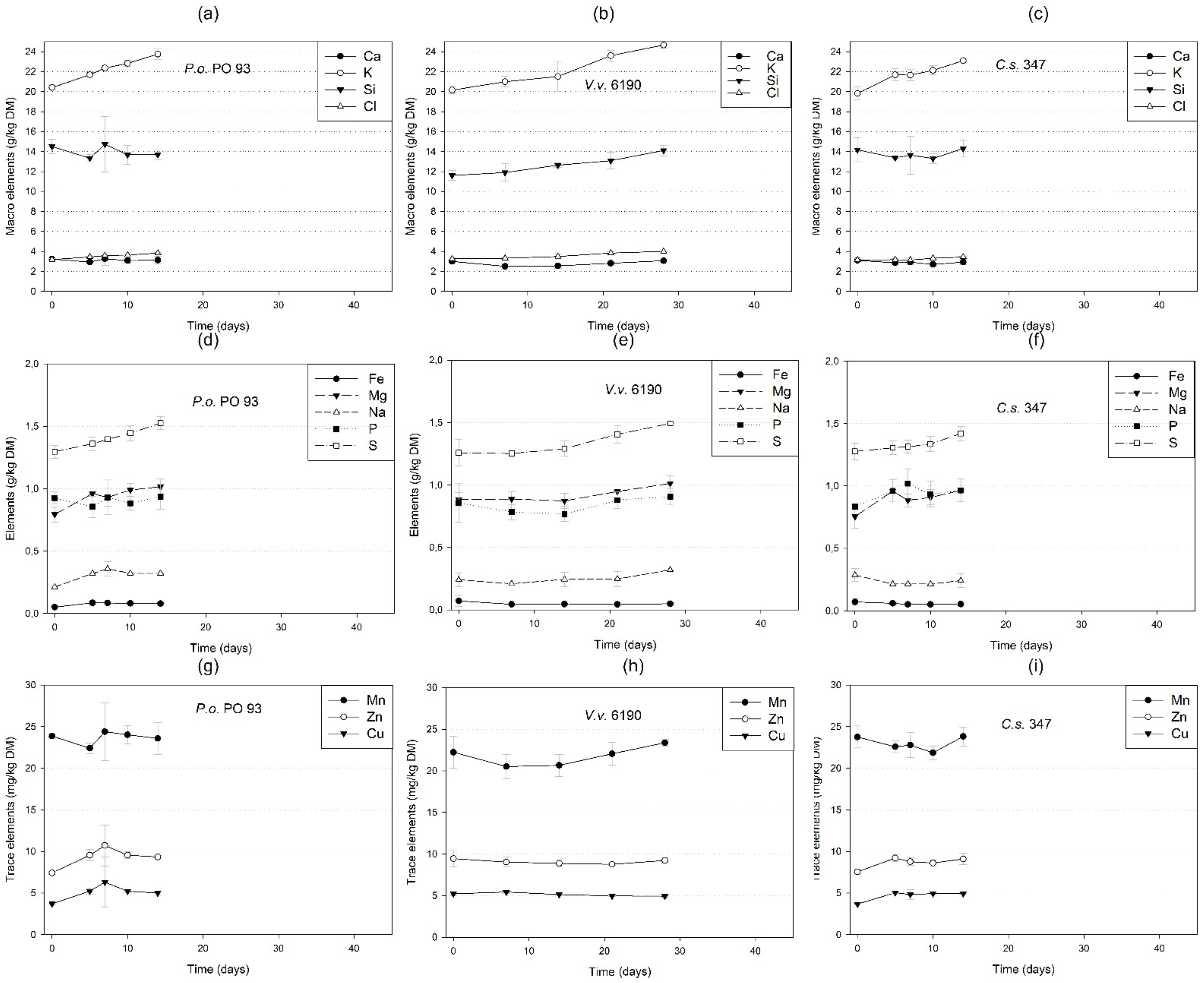
3.4.4. Minerals and Trace Elements
4. Discussion
4.1. Fungal Growth
4.2. Fibre and Minerals
4.3. Sterilization of Substrate
4.4. Accompanying Microflora
4.5. Lactic Acid Bacteria
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Crops Primary 2020. In FAOSTAT. 2022. Available online: http://www.fao.org/faostat/en/#data/QCL] (accessed on 19 October 2022).
- Chivenge, P.; Rubianes, F.; Chin, D.V.; Thach, T.V.; Khang, V.T.; Romasanta, R.R.; Hung, N.V.; Trinh, M.V. Rice straw incorporation influences nutrient cycling and soil organic matter. In Sustainable Rice Straw Management; Springer: Cham, Switzerland, 2020; pp. 131–144. [Google Scholar]
- Logeswaran, J.; Shamsuddin, A.H.; Silitonga, A.S.; Mahlia, T.M.I. Prospect of using rice straw for power generation: A review. Environ. Sci. Pollut. Res. 2020, 27, 25956–25969. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, X.; Fadel, J.G. Oyster mushroom cultivation with rice and wheat straw. Bioresour. Technol. 2002, 82, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Norring, M.; Manninen, E.; de Passille, A.M.; Rushen, J.; Munksgaard, L.; Saloniemi, H. Effects of Sand and Straw Bedding on the Lying Behavior, Cleanliness, and Hoof and Hock Injuries of Dairy Cows. J. Dairy Sci. 2008, 91, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Lahrmann, H.P.; Oxholm, L.C.; Steinmetz, H.; Nielsen, M.B.F.; D’Eath, R.B. The effect of long or chopped straw on pig behaviour. Animal 2015, 9, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Akins, M.S.; Esser, N.M.; Ogden, R.; Coblentz, W.K.; Kalscheur, K.F.; Hatfield, R. Effects of feeding alfalfa stemlage or wheat straw for dietary energy dilution on nutrient intake and digestibility, growth performance, and feeding behavior of Holstein dairy heifers. J. Dairy Sci. 2017, 100, 7106–7115. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 2014, 631013. [Google Scholar] [CrossRef]
- Zadrazil, F.; Kamra, D.N.; Isikhuemhen, O.S.; Schuchardt, F.; Flachowsky, G. Bioconversion of Lignocellulose into Ruminant Feed with White Rot Fungi—Review of Work Done at the FAL, Braunschweig. J. Appl. Anim. Res. 1996, 10, 105–124. [Google Scholar] [CrossRef]
- Mahesh, M.; Mohini, M. Biological treatment of crop residues for ruminant feeding: A review. Afr. J. Biotechnol. 2013, 12, 4221–4231. [Google Scholar]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; Cone, J.W. Fungal treated lignocellulosic biomass as ruminant feed ingredient: A review. Biotechnol. Adv. 2015, 33, 191–202. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Screening of white-rot fungi for bioprocessing of wheat straw into ruminant feed. J. Appl. Microbiol. 2018, 125, 468–479. [Google Scholar] [CrossRef]
- Srivastava, D.; Kumar, R.; Singh, V. Wood Decaying Fungi; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2013. [Google Scholar]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef]
- Amirta, R.; Tanabe, T.; Watanabe, T.; Honda, Y.; Kuwahara, M.; Watanabe, T. Methane fermentation of Japanese cedar wood pretreated with a white rot fungus, Ceriporiopsis subvermispora. J. Biotechnol. 2006, 123, 71–77. [Google Scholar] [CrossRef]
- Aguiar, A.; Ferraz, A. Relevance of extractives and wood transformation products on the biodegradation of Pinus taeda by Ceriporiopsis subvermispora. Int. Biodeterior. Biodegrad. 2008, 61, 182–188. [Google Scholar] [CrossRef]
- Buswell, J.A.; Cai, Y.J.; Chang, S.T.; Peberdy, J.F.; Fu, S.Y.; Yu, H.-S. Lignocellulolytic enzyme profiles of edible mushroom fungi. World J. Microbiol. Biotechnol. 1996, 12, 537–542. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; John Wiley & Sons Ltd.: Chichester, UK, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Streeter, C.L.; Conway, K.E.; Horn, G.W.; Mader, T.L. Nutritional evaluation of wheat straw incubated with the edible mushroom, Pleurotus ostreatus. J. Anim. Sci. 1982, 54, 183–188. [Google Scholar] [CrossRef]
- Valmaseda, M.; Martinez, M.J.; Martinez, A.T. Kinetics of wheat straw solid-state fermentation with Trametes versicolor and Pleurotus ostreatus: Lignin and polysaccharide alteration and production of related enzymatic activities. Appl. Microbiol. Biotechnol. 1991, 35, 817–823. [Google Scholar] [CrossRef]
- Salvachua, D.; Prieto, A.; Lopez-Abelairas, M.; Lu-Chau, T.; Martinez, A.T.; Martinez, M.J. Fungal pretreatment: An alternative in second-generation ethanol from wheat straw. Bioresour. Technol. 2011, 102, 7500–7506. [Google Scholar] [CrossRef]
- Van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; del Río, J.C.; Rencoret, J.; Gutiérrez, A.; de Ruijter, N.C.A.; Cone, J.W. Characterization of wheat straw and oak wood chips treated with the white rot fungi Ceriporiopsis subvermispora and Lentinula edodes. In Fungal Treatment of Lignocellulosic Biomass; Wageningen University: Wageningen, The Netherlands, 2016; pp. 88–110. [Google Scholar]
- Belewu, M.A.; Belewu, K.Y. Cultivation of mushroom (Volvariella volvacea) on banana leaves. Afr. J. Biotechnol. 2005, 4, 1401–1403. [Google Scholar]
- Bao, D.; Gong, M.; Zheng, H.; Chen, M.; Zhang, L.; Wang, H.; Jiang, J.; Wu, L.; Zhu, Y.; Zhu, G.; et al. Sequencing and Comparative Analysis of the Straw Mushroom (Volvariella volvacea) Genome. PLoS ONE 2013, 8, e58294. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Jiang, D.; Tian, P.; Zheng, M.; Xu, C. Treatment using white rot fungi changed the chemical composition of wheat straw and enhanced digestion by rumen microbiota in vitro. Anim. Feed Sci. Technol. 2018, 237, 46–54. [Google Scholar] [CrossRef]
- Breck, H. Erzeugung von Körner Pilzbrut [Production of Grain Spawn]. 2021. Available online: www.pilzmaennchen.de (accessed on 5 April 2019).
- Breck, A.; Breck, H. Pilzzuchtanleitung auf Stroh [Manual for Mushroom Cultivation on Straw]. 2021. Available online: www.pilzmaennchen.de (accessed on 5 April 2019).
- VDLUFA. Mikrobiologische Verfahren [Microbiological Procedures]. In VDLUFA Book of Methods, Volume III—The Chemical Analysis of Feedstuff (1976). Supplement 8 as of 2012, 3rd ed.; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- VDLUFA. VDLUFA Book of Methods, Volume III—The Chemical Analysis of Feedstuff, 3rd ed.; VDLUFA-Verlag: Darmstadt, Germany, 1976. [Google Scholar]
- AOAC. AOAC Official Method 994.13: Total Dietary Fiber. In Official Methods of Analysis, 17th ed.; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2000; pp. 84–88. [Google Scholar]
- Thomsen, S.T.; Londono, J.E.G.; Ambye-Jensen, M.; Heiske, S.; Kadar, Z.; Meyer, A.S. Combination of ensiling and fungal delignification as effective wheat straw pretreatment. Biotechnol. Biofuels 2016, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Umor, N.A.; Abdullah, S.; Mohamad, A.; Ismail, S.; Ismail, S.I.; Misran, A. Challenges and Current State-of-Art of the Volvariella volvacea Cultivation Using Agriculture Waste: A Brief Review. In Advances in Waste Processing Technology; Yaser, A.Z., Ed.; Springer: Singapore, 2020; pp. 145–156. [Google Scholar]
- Zervakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium growth kinetics and optimal temperature conditions for the cultivation of edible mushroom species on lignocellulosic substrates. Folia Microbiolog. 2001, 46, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Flachowsky, G. Stroh und andere faserreiche Futtermittel [Straw and other feed stuff rich in fiber]. In Futtermittelkunde; Jeroch, H., Flachowsky, G., Weissbach, F., Eds.; Jena: Stuttgart, Germany, 1993; pp. 155–191. [Google Scholar]
- Fernandez-Fueyo, E.; Castanera, R.; Ruiz-Duenas, F.J.; Lopez-Lucendo, M.F.; Ramirez, L.; Pisabarro, A.G.; Martinez, A.T. Ligninolytic peroxidase gene expression by Pleurotus ostreatus: Differential regulation in lignocellulose medium and effect of temperature and pH. Fungal Genet. Biol. 2014, 72, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Zhang, H.T.; Meng, Q.H.; Wang, Y.R. Studies on Production Conditions and Properties of Ligninolytic Enzymes from White Rot Fungi. J. Jilin Agric. Sci. 2008, 2. Available online: https://en.cnki.com.cn/Article_en/CJFDTotal-JLNK200802015.htm (accessed on 19 October 2022).
- Tapia, J.; Vicuna, R. Synthetic Lignin Mineralization by Ceriporiopsis subvermispora Is Inhibited by an Increase in the pH of the Cultures Resulting from Fungal Growth. Appl. Environ. Microbiol. 1995, 61, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.R.; Pinnamaneni, R.; Koona, S. Occurrences, Physical and Biochemical Properties of Laccase. Univers. J. Environ. Res. Technol. 2012, 2, 1–13. [Google Scholar]
- Soccol, C.R.; da Costa, E.S.F.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; de Souza Vandenberghe, L.P. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Belal, E.B.; Khalafalla, M.M.E. Biodegradation of Panicum repens residues by Pleurotus ostreatus for its use as a non conventional feedstuff in diets of Oreochromis niloticus. ID. Afr. J. Microb. Res. 2011, 5, 3038–3050. [Google Scholar]
- Mukherjee, R.; Nandi, B. Improvement of in vitro digestibility through biological treatment of water hyacinth biomass by two Pleurotus species. Intern. Biodeter. Biodegrad. 2004, 53, 7–12. [Google Scholar] [CrossRef]
- Nayan, N.; van Erven, G.; Kabel, M.A.; Sonnenberg, A.S.; Hendriks, W.H.; Cone, J.W. Improving ruminal digestibility of various wheat straw types by white-rot fungi. J. Sci. Food Agric. 2019, 99, 957–965. [Google Scholar] [CrossRef]
- Tuyen, V.D.; Cone, J.W.; Baars, J.J.P.; Sonnenberg, A.S.M.; Hendriks, W.H. Fungal strain and incubation period affect chemical composition and nutrient availability of wheat straw for rumen fermentation. Bioresour. Technol. 2012, 111, 336–342. [Google Scholar] [CrossRef]
- Van Aken, B.; Agathos, S.N. Biodegradation of nitro-substituted explosives by white-rot fungi: A mechanistic approach. In Advances in Applied Microbiology, 48th ed.; Academic Press: Cambridge, MA, USA, 2001; pp. 1–77. [Google Scholar]
- Otjen, L.; Blanchette, R.; Effland, M.; Leatham, G. Assessment of 30 White Rot Basidiomycetes for Selective Lignin Degradation. Holzforschung 1987, 41, 343. [Google Scholar] [CrossRef]
- Otjen, L.; Blanchette, R.A. Selective Delignification of Birch Wood (Betula papyrifera) by Hirschioporus pargamenus in the Field and Laboratory. Holzforschung 1986, 40, 183–190. [Google Scholar] [CrossRef]
- Otjen, L.; Blanchette, R.A. A discussion of microstructural changes in wood during decomposition by white rot basidiomycetes. Can. J. Bot. 1986, 64, 905–911. [Google Scholar] [CrossRef]
- Otjen, L.; Blanchette, R.A. Xylobolus frustulatus Decay of Oak: Patterns of Selective Delignification and Subsequent Cellulose Removal. Appl. Environ. Microbiol. 1984, 47, 670–676. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, S.; Cai, L.; Liu, X.; Wu, H.; Xin, F.; Zhang, M.; Jiang, M. Improved Treatment and Utilization of Rice Straw by Coprinopsis cinerea. Appl. Biochem. Biotechnol. 2018, 184, 616–629. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Chapter One—Insights into Lignin Degradation and its Potential Industrial Applications. In Advances in Applied Microbiology, 82nd ed.; Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1–28. [Google Scholar] [CrossRef]
- Kerem, Z.; Hadar, Y. Effect of manganese on preferential degradation of lignin by Pleurotus ostreatus during solid-state fermentation. Appl. Environ. Microbiol. 1995, 61, 3057–3062. [Google Scholar] [CrossRef]
- Tyagi, S.; Lee, K.J.; Mulla, S.I.; Garg, N.; Chae, J.C. Chapter 2—Production of Bioethanol From Sugarcane Bagasse: Current Approaches and Perspectives. In Applied Microbiology and Bioengineering; Shukla, P., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–42. [Google Scholar] [CrossRef]
- Flachowsky, G.; Grün, M. Influence of type of diet and incubation time on major elements release in sacco from Italian ryegrass, untreated and ammonia-treated wheat straw. Anim. Feed Sci. Technol. 1992, 36, 239–254. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Sniffen, C.J. Nitrogen Fractions in NDF and ADF. In Proceedings of the Distillers Feed Conference, Cincinnati, OH, USA, 1984; pp. 73–81. [Google Scholar]
- Okano, K.; Ohkoshi, N.; Nishiyama, A.; Usagawa, T.; Kitagawa, M. Improving the nutritive value of madake bamboo, Phyllostachys bambusoides, for ruminants by culturing with the white-rot fungus Ceriporiopsis subvermispora. Anim. Feed Sci. Technol. 2009, 152, 278–285. [Google Scholar] [CrossRef]
- Baker, P.W.; Charlton, A.; Hale, M.D.C. Fibre degradation of wheat straw by Pleurotus erygnii under low moisture conditions during solid-state fermentation. Lett. Appl. Microbiol. 2019, 68, 182–187. [Google Scholar] [CrossRef]
- Milenkovic, I.; Mllosvljevic, I. (Eds.) Mushroom Cultivation Manual for the Small Mushroom Entrepreneur; Ekofungi: Belgrade, Serbia, 2017. [Google Scholar]
- Wan, C.; Li, Y. Fungal pretreatment of lignocellulosic biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Blanchette, R.A.; Myers, G.; Kirk, T. An overview of biomechanical pulping research. In Environmentally Friendly Technologies for the Pulp and Paper Industry; Young, R.A., Akhtar, M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1998; pp. 309–340. [Google Scholar]
- Blanchette, R.A.; Shaw, C.G.; Cohen, A.L. SEM study of the effects of bacteria and yeasts on wood decay by brown and white-rot fungi. [Enterobacter, Cryptococcus Pichia, and Saccharomyces]. Scanning Electron. Microsc. 1978, 2, 61–68. [Google Scholar]
- Frey-Klett, P.; Burlinson, P.; Deveau, A.; Barret, M.; Tarkka, M.; Sarniguet, A. Bacterial-Fungal Interactions: Hyphens between Agricultural, Clinical, Environmental, and Food Microbiologists. Microbiol. Mol. Biol. Rev. 2011, 75, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Ricciardi, A.; Ianniello, R.G.; Storti, L.V.; Glibota, N.A.; Parente, E. Aerobic and respirative growth of heterofermentative lactic acid bacteria: A screening study. Food Microbiol. 2018, 76, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar Tiwari, S. Optimization of culture conditions for bacteriocin production by soil isolates Pediococcus pentosaceus LB44 and Weissella confusa LM85. Int. J. Infect. 2017, 4, e15842. [Google Scholar] [CrossRef]
- Dobrogosz, W.J.; Stone, R.W. Oxidative metabolism in Pediococcus pentosaceus. II. Factors controlling the formation of oxidative activities. J. Bacteriol. 1962, 84, 724–729. [Google Scholar] [CrossRef]
- Wahyudi, A.; Cahyanto, M.N.; Soejono, M.; Bachruddin, Z. Potency of lignocellulose degrading bacteria isolated from buffalo and horse gastrointestinal tract and elephant dung for feed fiber degradation. J. Indones. Tropic. Anim. Agric. 2010, 35, 34–41. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, R.; Rathi, M.; Bhatia, D.; Malik, D.K. Lignocellulose biodegradation: An advance technology for sustainable environment. Biosci. Biotechnol. Res. Commun. 2018, 11, 634–637. [Google Scholar] [CrossRef]
- Quan, L.H.; Piao, J.Y.; Min, J.W.; Yang, D.U.; Lee, H.N.; Yang, D.C. Bioconversion of ginsenoside Rb1 into compound K by Leuconostoc citreum LH1 isolated from kimchi. Braz. J. Microbiol. 2011, 42, 1227–1237. [Google Scholar] [CrossRef]





| Trial 1—Soak and Drain | Trial 2—Remoisten and V.v. | Trial 3—Remoisten and C.s./P.o. | ||
|---|---|---|---|---|
| Inoculant | Strains | C. subvermispora CBS 347.63, P. ostreatus CBS 411.71 and PO93 | V. volvacea DSM 6190, uninoculated control | C. subvermispora CBS 347.63, P. ostreatus CBS 411.71 |
| Growth medium | Wheat grains | Wheat grains | Wheat grains | |
| Straw | Soaking | Abundant H2O | 2.7 L H2O/kg straw (25% target DM) | 2.7 L H2O/kg straw (25% target DM) |
| Draining | Yes | No | No | |
| Storage | Ambient °C | 21 °C | 24 °C | 23 °C |
| Sampling | After d | 0, 7, 14, 21, 28, 35, 42 | 0, 7, 14, 21, 28 | 0, 5, 7, 10, 14 |
| Storage Duration (d) | Treatment | Isolates (n) | Genus (n) | Species (n) |
|---|---|---|---|---|
| 0 | Remoistened for 24 h | 49 | Enterococcus (2) | durans |
| mundtii | ||||
| Lactobacillus (12) | curvatus (6) | |||
| pentosus/paraplantarum/plantarum (6) | ||||
| Pediococcus (8) | pentosaceus | |||
| Weissella (24) | cibaria/confusa | |||
| unidentified (3) | (3) | |||
| 7 | C. subvermispora | 8 | Enterococcus (1) | casseliflavus |
| Pediococcus (1) | pentosaceus | |||
| Weissella (5) | cibaria/confusa | |||
| unidentified (1) | ||||
| P. ostreatus PO93 | 11 | Lactobacillus (1) | pentosus/paraplantarum/plantarum | |
| Lactococcus (3) | lactis | |||
| Leuconostoc (1) | sp. | |||
| Pediococcus (4) | pentosaceus | |||
| Weissella (1) | cibaria/confusa | |||
| unidentified (1) | ||||
| epiphytic microflora | 14 | Enterococcus (5) | casseliflavus (1) | |
| (uninoculated) | faecium (1) | |||
| mundtii (3) | ||||
| Pediococcus (1) | pentosaceus | |||
| Weissella (6) | cibaria/confusa | |||
| unidentified (2) | (2) | |||
| 14 | C. subvermispora | 5 | Pediococcus (2) | pentosaceus |
| Weissella (3) | cibaria/confusa | |||
| P. ostreatus PO93 | 16 | Enterococcus (4) | casseliflavus(2) | |
| gallinarum (2) | ||||
| Lactobacillus (1) | pentosus/paraplantarum/plantarum | |||
| Lactococcus (1) | lactis | |||
| Leuconostoc (2) | citreum | |||
| Pediococcus (1) | pentosaceus | |||
| Weissella (1) | cibaria/confusa | |||
| unidentified (6) | (4), (1), (1) |
| Strain | P.o. PO 93 | P.o. PO 93 | P.o. 411 | C.s. 347 | C.s. 347 | V.v. |
|---|---|---|---|---|---|---|
| drained | remoistened | drained | drained | remoistened | remoistened | |
| Trial | 1 | 3 | 1 | 1 | 3 | 2 |
| 0 d | 54.9 | 127 | 54.9 | 54.9 | 106 | 106 |
| 5 d | 109 | 82.2 | ||||
| 7 d | 53.3 | 113 | 52.7 | 41.3 | 78.3 | 123 |
| 10 d | 112 | 79.6 | ||||
| 14 d | 43.7 | 128 | 65.3 | 65.7 | 81.8 | 127 |
| 21 d | 89.3 | 92.0 | 77.7 | 81.4 | ||
| 28 d | 95.3 | 101 | 51.3 | 79.0 | ||
| 35 d | 99.0 | 80.0 | 71.3 | |||
| 42 d | 78.7 | 98.0 | 75.0 | |||
| mean | 76.6 | 118 | 81.6 | 64.3 | 85.6 | 103 |
| SEM | 1.90 | 3.09 | 1.90 | 1.90 | 3.09 | 3.09 |
| Monomers | g/kg DM | % of NSP |
|---|---|---|
| Arabinose | 28.22 | 5.17 |
| Xylose | 220.35 | 40.4 |
| Mannose | 1.98 | 0.36 |
| Galactose | 6.90 | 1.27 |
| Glucose | 287.96 | 52.8 |
| Xylose/Arabinose | Ratio 7.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martens, S.D.; Wildner, V.; Greef, J.M.; Zeyner, A.; Steinhöfel, O. Growth and Influence of White-Rot Fungi on the Chemical Composition of Wheat Straw Inoculated under Varying Pre-Conditions. Fermentation 2022, 8, 695. https://doi.org/10.3390/fermentation8120695
Martens SD, Wildner V, Greef JM, Zeyner A, Steinhöfel O. Growth and Influence of White-Rot Fungi on the Chemical Composition of Wheat Straw Inoculated under Varying Pre-Conditions. Fermentation. 2022; 8(12):695. https://doi.org/10.3390/fermentation8120695
Chicago/Turabian StyleMartens, Siriwan D., Vicki Wildner, Jörg M. Greef, Annette Zeyner, and Olaf Steinhöfel. 2022. "Growth and Influence of White-Rot Fungi on the Chemical Composition of Wheat Straw Inoculated under Varying Pre-Conditions" Fermentation 8, no. 12: 695. https://doi.org/10.3390/fermentation8120695
APA StyleMartens, S. D., Wildner, V., Greef, J. M., Zeyner, A., & Steinhöfel, O. (2022). Growth and Influence of White-Rot Fungi on the Chemical Composition of Wheat Straw Inoculated under Varying Pre-Conditions. Fermentation, 8(12), 695. https://doi.org/10.3390/fermentation8120695






