Abstract
Strokes are the second most common cause of death worldwide and a leading cause of disability. Regular consumption of polyphenols has been shown to reduce the risk of suffering a cardiovascular event. For this reason, we have investigated the protective effect of Salicornia ramosissima, a seasonal halophyte that synthetizes high amounts of bioactive compounds, including polyphenols, in response to environmental stress. Aqueous, hydroalcoholic, and ethanolic extracts were prepared to investigate if dietary supplementation prior to ischemic challenge can prevent subsequent damage using two animal models. First, we screened the protective effect against hypoxia–reoxygenation in Drosophila melanogaster and observed that both ethanolic and hydroalcoholic extracts protected flies from the deleterious effects of hypoxia. Second, we confirmed the protective effect of S. ramosissima ethanolic extract against brain ischemia using the transient middle cerebral artery occlusion mice model. Four weeks of oral supplementation with the ethanolic extract before artery occlusion reduced infarct volume and lowered the plasma levels of the DNA peroxidant product 8-hydroxydeoxyguanosine. Phytochemical profiling of S. ramosissima ethanolic extract revealed 50 compounds. Thus, it represents a valuable source of bioactive compounds that show promising disease-modifying activities and could be further developed as an effective food supplement for the prevention or treatment of neurovascular disorders.
1. Introduction
Stroke is the most common neurovascular disease, representing the second most common cause of death and the third most common cause of disability worldwide, according to the World Health Organization. Ischemic stroke encompasses different mechanisms of vascular occlusion and represent around 80% of all stroke cases [1]. Changes in brain-regional blood flow deprive this highly demanding tissue of oxygen and nutrients, leading to a cascade of metabolic and molecular processes resulting in significant neuronal death shortly after ischemia [2]. More than a hundred drug candidates for stroke treatment have been tested in clinical trials [3], but mechanical thrombectomy and intravenous thrombolysis using tissue plasminogen activator (t-PA) or tenecteplase (TNK) are still the only approved medical therapies for ischemic stroke and, unfortunately, no neuroprotectant drug has ever been shown to be useful in stroke patients [4].
Epidemiologic data show that the incidence of stroke has fallen in high-income countries with the improvement in the control of risk factors, such as hypertension [5], hyperlipidaemia, diabetes [6], or atrial fibrillation [7]. Most risk factors are very prevalent and, potentially, treatable. Therefore, a suggested strategy to prevent or reduce ischemic brain injury is identifying populations at high vascular risk, such as patients having coexistence of several vascular risk factors or silent brain infarcts, or those undergoing carotid artery or heart surgery, to whom neuroprotective therapies could be administered before a stroke might occur [8]. In this sense, nutraceuticals have been reported to reduce stroke risk and improve recovery [9] and, if necessary, can be administered for years as prophylactic treatments depending on their safety profile.
Salicornia ramosissima, commonly known as glasswort, is a seasonal Mediterranean saltmarsh plant that, in response to saline stress and UV radiation, synthetizes bioactive compounds, such as polyphenols [10], which are of great interest for their therapeutic effects in cardiovascular diseases [11]. Green aerial parts of different Salicornia species have been used for a long time as food sources in salads, pickles, beverages and, more recently, as green salt. Historically, Salicornia has also been used for non-edible purposes in traditional medicine as a remedy for constipation, infections, or diabetes, among other conditions [12]. More recently, a S. fragilis extract rich in polyphenols has been attributed with antioxidant activities [13]. Moreover, S. europaea extracts have been reported to ameliorate hypertension and vascular dysfunction induced by high salt consumption in vivo [14], as well as reduce hyperplasia during vascular remodeling [15]. In addition, oral administration of a desalted S. europaea extract prior to scopolamine-induced amnesia protected mice from cognitive impairment and promoted neurogenesis [16].
Among phytochemicals previously found in S. ramosissima, phenolic compounds, such as flavonoids derivatives (luteolin or quercetin glucosides), have been reported to be relevant compounds in the prevention of several disorders, such as cancer, hypertension, or neurodegeneration, taking action in the regulation of several metabolic pathways or decreasing cancer cell growth [17,18]. Moreover, phenolic acids, such as tungtungmadic acid, have been reported to be hepatoprotective by reducing necrosis and inflammation of the liver in a mice model of hepatic fibrosis induced by carbon tetrachloride [19].
Although accumulating evidence supports the therapeutic potential of Salicornia’s phytochemical profile, there are no reports investigating its role against hypoxia or ischemia. In the present study, we have evaluated whether a S. ramosissima ethanolic extract, a powerful source of polyphenols, protects from the deleterious effects induced by ischemia.
2. Materials and Methods
2.1. Extracts Preparation Procedure
Freshly collected S. ramosissima aerial parts were freeze-dried until complete loss of moisture and subsequently ground to facilitate the extraction process. Plant materials were subjected to solid–liquid extraction (plant to solvent ratio 1:10, m:v (g:mL)) by using ethanol (Salicornia ethanolic extract (S-EE)), hydroalcoholic mixture (50:50; ethanol: water; v:v) (Salicornia hydroalcoholic extract (S-HE)), or water (Salicornia aqueous extract (S-AE)) for 2 h at 45 °C and at a stirring speed of 170 rpm. Then, supernatant was collected, centrifuged, and filtered (0.45 μm). Extracts were evaporated under vacuum and dry extracts were kept light-protected at −20 °C until later use.
2.2. Determination of Total Phenolic Content
The total content of phenolic compounds was assessed using the Folin–Ciocalteu method. Briefly, Salicornia extract stocks (20 mg/mL in 20:80 v/v ethanol:water) were diluted to 1 mg/mL in water. Then, 30 μL of each sample (1 mg/mL) or standard (Gallic acid) were mixed in triplicates with 30 μL of Folin–Ciocalteu reagent (1:10 dilution in water) in a 96-well plate and incubated at room temperature for 5 min. Finally, 5% sodium carbonate (w/v, 240 μL) was added and the plate was incubated for 1.5 h at 30 °C. Absorbance was measured at 760 nm using a CLARIOstar spectrophotometer (BMG Labtech, Ortenberg, Germany). Total phenolic content was calculated using a Gallic acid calibration curve within a range of 0–100 μg/mL. Results were expressed as mg Gallic acid equivalents (GAE)/g of dry extract.
2.3. Phytochemical Profile of S-EE by HPLC-ESI-QTOF-MS Methodology
The sample was reconstituted from the dry extract at a concentration of 5 mg/mL and filtered before high-performance liquid chromatography coupled to electrospray ionization and quadrupole time-of-flight mass spectrometry (HPLC-ESI-QTOF-MS) analysis. The analysis was carried out in a high-pressure liquid chromatography platform (Agilent 1290 HPLC, Agilent Technologies, Palo Alto, CA, USA) coupled to mass spectrometry using a quadrupole time-of-flight analyzer (Agilent 6545 QTOF Ultra High Definition, Agilent Technologies, Palo Alto, CA, USA).
The chromatographic method was carried out in reverse phase with a C18 column (ACQUITY UPLC BEH, 1.7 µm, 2.1 mm, 150 mm, 130 Å, Waters Corporation). The column temperature was fixed at 60 °C. As mobile phases, water with 0.1% of formic acid (A) and acetonitrile (B) were used. The following mobile phase gradient was used for the separation: 0 min [A:B 95/5], 5 min [A:B 90/10], 18 min [A:B 15/85], 24 min [A:B 0/100], 25.50 min [A:B 0/100], 26.50 min [A:B 95/5], and 32.50 min [A:B 95/5]. The mobile phase flow rate was 0.4 mL/min and the injection volume of the sample was 5 µL.
MS acquisition was performed in negative electrospray ionization mode (ESI-) with a mass/charge ratio range between 50 and 1200 m/z. Two reference masses—purine (m/z 121.0509) and HP-921 (m/z 922.0098)—were used for the calibration of all m/z values by means of a continuous infusion. S-EE was analyzed in full scan acquisition mode with a scan rate of 3 spectra/sec. The MS data were acquired in centroid mode. Other parameters were as follows: gas temperature 200 °C; gas flow 10 L/min; nebulizer 20 psig; sheath gas temperature 350 °C; sheath gas flow 12 L/min, VCap 4000 V; and Nozzle Voltage 500 V. The acquired MS data were converted to an mzML format using MSConverter (Proteowizard) and then processed using the mzMine 2.53 software. The compounds were annotated according to the comparison of the experimental data (Retention time, m/z, molecular formula) with those found in the literature and databases.
2.4. Drosophila Treatments and Exposure to Hypoxia
D. melanogaster (Oregon R strain) flies were kindly provided by Dr. Luisma Escudero. Flies were reared on a standard medium in plastic vials at a constant temperature and humidity (25 °C; 40% humidity) under a 12 h/12 h light/dark cycle. Flies were sexed under CO2 anesthesia 1–3 days after emergence and males were selected and placed in plastic vials containing Nutrifly instant formulation media (Genesee Scientific, San Diego, CA, USA). Each experiment was performed in triplicates (3 drosophila vials per condition containing 10–15 male flies each). The treated groups received instant formulation prepared in water containing Salicornia extracts at the indicated concentrations for 5 days. The control and hypoxia groups were kept on instant formulation media prepared in vehicle (0.5% ethanol in water). Treatment media were refreshed once during the experimental procedure.
To study any possible toxic effect of Salicornia treatments, male survival was assessed daily during the treatment length in normoxic conditions. After 5 days of treatment, vials containing 10–15 male flies were subjected to 1% O2 for 2.5 h using a Coy O2 Control In Vitro Glove Box (Coy, Grass Lake, MI, USA). N2 was used to displace the atmospheric gas, and temperature (25 °C) and humidity (30–40%) were controlled during the hypoxia challenge. Then, flies were allowed to recover in normoxia maintained in standard medium. Mortality rate in each tube was assessed.
2.5. Drosophila Locomotor Activity Assay
To study the impact of hypoxia on locomotor activity, each group of flies was transferred into a 25 mm empty polycarbonate vial and placed in the Drosophila Activity Monitoring (DAM) system (LAM25H-3, Trikinetics Inc., Waltham, MA, USA). Locomotor activity was assessed following the hypoxia exposure by registering the infrared light beam crossing at 3 different heights vertically on each tube. The DAMSystem3 Data Collection Software was used for data acquisition every 5 s. Raw data were then processed using the FileScan Software to ensure that all data records are complete and converted to 30 min intervals for analysis. Live flies were counted at the beginning and the end of the assessment period and mean beam crosses every 30 min was calculated.
2.6. Animals and Transient Focal Cerebral Ischemia Model
All animal care and experimental procedures were performed in accordance with the European Union guideline and approved by the Animal Research Ethic Committee of the Vall d’Hebrón Institute (protocol number 03/19) and the Regional Committee for Animal Experimentation. Six-week-old C57BL/6J male mice (Janvier Laboratories, Le Genest-Saint-Isle, France) were housed under a 12 h light–dark cycle at a temperature of 22 °C and allowed to consume food and tap water ad libitum throughout the study period. Thirty mice were randomly assigned into two groups, and per os (p.o.) administered with 100 mg/kg of S-EE or vehicle (20% ethanol solution in water) for 28 days before surgery. All animals were weighed daily during the experimental procedure.
A 90 min transient proximal middle cerebral artery occlusion (t-pMCAo) model was performed blindly by introducing an intraluminal filament (Doccol 602256PK10Re) as described previously [20]. Only animals presenting a reduction in cerebral blood flow of 80% after filament introduction and a recovery of 75% after filament removal were included in the study. Overall, 10 mice were excluded because of treatment or surgery failure. Finally, 10 mice per group were included in this study.
Then, 24 h after the surgery, animals received deep anesthesia and blood samples were collected in EDTA anticoagulated tubes, centrifuged at 1000× g for 15 min and plasma was collected and stored in −80 °C until use. Animals were then transcardially perfused with cold saline and brains were quickly collected for analysis.
2.7. Infarct Volume Assessment
Immediately after collection, the mice brains were sectioned into 1 mm thick slices and stained using 2.5% 2,3,5-triphenyl-2H-tetrazolium chloride (TTC; Sigma-Aldrich, Saint Louis, MO, USA). TTC images were captured using a CanoScan 4200F scanner (Canon, Tokyo, Japan) and quantified with Image J software. The white ischemic lesion was calculated blindly as a percentage of infarct volume of the hemisphere ipsilateral to the lesion.
2.8. Functional and Neurological Assessment
Grip strength test was performed to assess the peak forelimb force using a computerized grip strength meter (Harvard Apparatus, Cambridge, MA, USA) one day before the operation, and then repeated at 24 h post-surgery, as described previously [21]. At 24 h after occlusion, as well as the grip test, a latency to full body movement test and a 39-point neurological score were also performed blindly on each mouse [22,23,24].
For the latency–movement test, more than 3 min was recorded as 180 s. Both general deficits (total score 13) and focal deficits (total score 26) were evaluated using a 39-point neurological score method, in which a higher score represents a worse outcome.
2.9. Preparation of Brain Homogenates
Brain slices were split in two hemispheres: the ipsilateral and contralateral hemisphere. After 1 mL of PBS was added to each sample, brain samples were homogenized with a sonicator and centrifuged at 8000× g for 10 min. The supernatants were collected and stored at −80 °C until analysis. Bicinchoninic acid (BCA) assay was performed to detect the total protein amount in brain homogenate. The molecule amount in the brains was calibrated using the total protein amount of each sample.
2.10. Quantification of Brain and Plasma Antioxidation Markers
Antioxidation-related molecules, including antioxidants, lipid peroxidation and DNA oxidation products, were analyzed in both plasma and brain homogenate samples. Sample size was reduced to n = 6–8 (2 out of 10 animals in vehicle were not included in the ELISA measurements due to the maximal measure capability in one 96-well plate and one plasma sample of the S-EE-treated mice did not reach detection limits for the measured parameters). Experiments were performed following the instructions provided by the commercially available ELISA kits: Thioredoxine (TRX, Abx254796, Abbexa, Cambridge, UK), 4-Hydroxynonenal (4-HNE, MOFI01251, AssayGenie, Dublin, Ireland), and 8-hydroxydeoxyguanosine (8-OHdG, CSB-E10527m, Cusabio, Hertfordshire, UK). The detection sensitivity of TRX, 4-HNE, and 8-OHdG is 37.5 pg/mL, 18.75 pg/mL, and 0.195 pg/mL, respectively. The intra- and inter-assay coefficients of variation (CVs) of TRX, 4-HNE and 8-OHdG were all < 10%. All standards and samples (except TRX measurement) were tested in duplicate wells. Values showing CV value of OD > 20% were excluded for the analysis. Data reflecting molecule amount in brain homogenates were then calibrated with the total protein amount in each sample and represented as molecule amount per nanogram (ng) total protein. To compare the difference in molecule expression in two groups, the percentage in the ipsilateral hemisphere versus the contralateral hemisphere was introduced.
2.11. Statistics
Data are expressed as mean ± standard deviation (s.d.). Data were checked for normal distribution using the Kolmogorov–Smirnov test. For Drosophila assays, groups were compared using one-way ANOVA analysis followed by Dunnett´s multiple comparisons test. For mice experiments, data were compared using unpaired t-tests. Differences in mortality between groups in mice experiments were assessed using the chi-square test. All statistical analysis was performed using GraphPad Prism v.8 and significance was set as p < 0.05.
3. Results
3.1. Content of Phenolic Compounds in S. ramosissima Extracts
It is known that halophytes are a great source of biological compounds [25]. The total phenolic content in Salicornia extracts was determined using the Folin–Ciocalteu assay. The content of polyphenols varied considerably among the three extracted fractions, ranging from 7 to 46.3 mg GAE/g (Table 1). The S-EE showed the highest phenolic content (46.3 mg GAE/g) followed by the S-HE extract (23.5 mg GAE/g). In contrast, the S-AE was less rich in phenolic compounds (7 mg GAE/g).

Table 1.
Total phenolic content in S. ramosissima extracts. Results are expressed as mean ± standard deviation of five independent experiments.
3.2. Tentative Characterization of S-EE by HPLC-ESI-QTOF-MS
Following the non-targeted method for the annotation of the phytochemical profile of S-EE, fifty compounds have been detected. Table 2 shows the analytical information of each compound (retention time, m/z, molecular formula, and proposed compound) and the previous data found in the literature. The major abundance of bioactive compounds in this extract is associated with caffeoylquinic acid derivatives, flavonoids, and fatty acids.

Table 2.
Annotated compounds in S-EE by HPLC-ESI-QTOF-MS.
3.3. Effect of Supplementation with S. ramosissima Extracts on Drosophila Melanogaster Performance after Severe Hypoxia
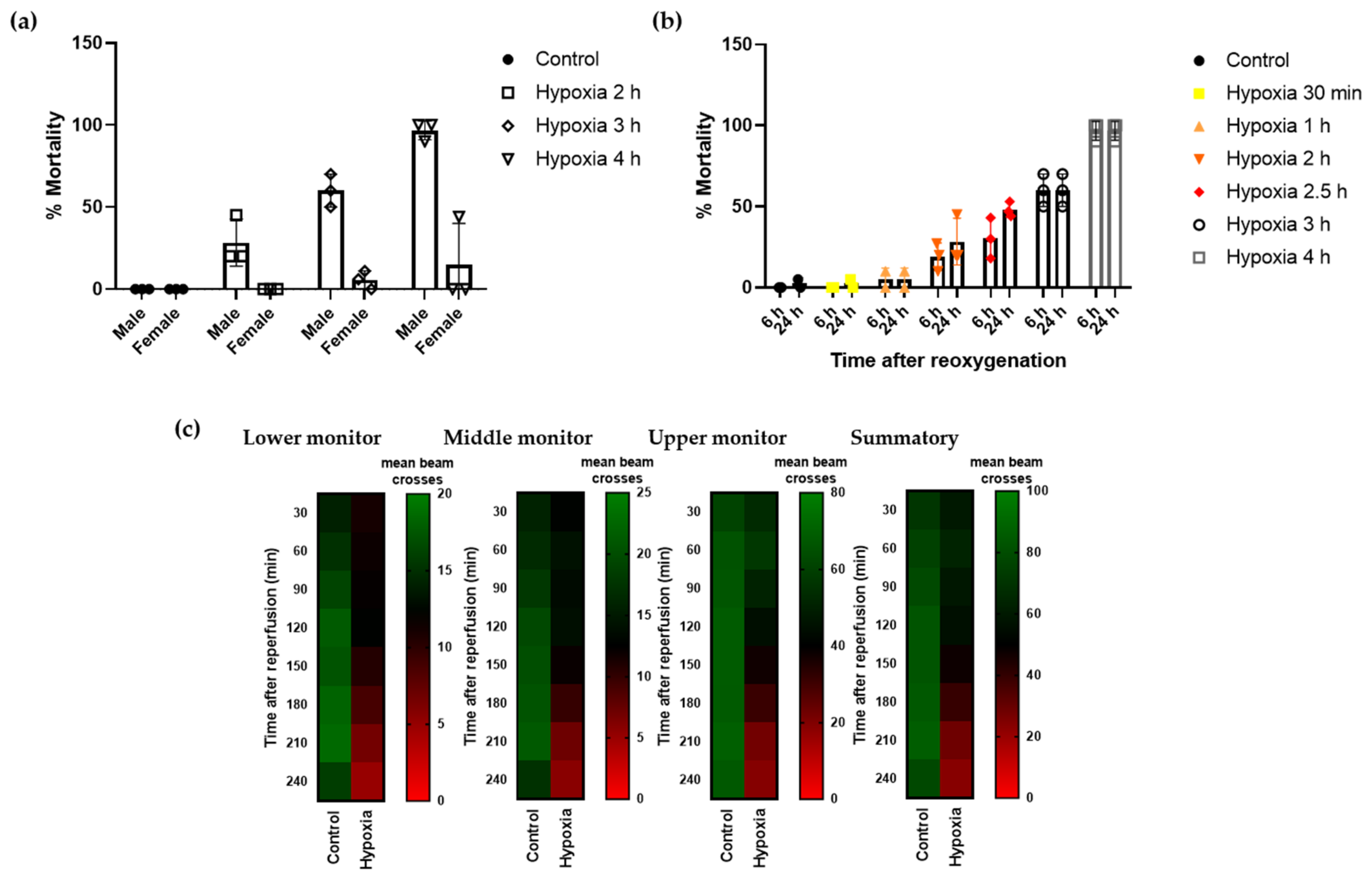
Drosophila melanogaster has been widely used as a model system in neurodegeneration for its limited need for resources and the high degree of conservation, including the entire hypoxia cascade [37]. In initial assays, we observed dimorphic response to the detrimental effects of hypoxia, with males being more vulnerable to hypoxia/reperfusion injury than females (Figure 1a). Therefore, to standardize our experiments, we conducted hypoxia using male flies only. To investigate the influence of severe hypoxia on Drosophila males, flies were subjected to hypoxic stress under controlled conditions (1% O2; 25 °C; 30–40% relative humidity) at increasing time durations. As shown, increasing the time of severe hypoxia exposure resulted in higher mortality rates, with 4 h hypoxia resulting in almost 100% mortality (Figure 1b). Therefore, we selected the half-lethal hypoxic stimulus of 2.5 h to define a reliable protocol to study the effect of the treatments on mortality rates. Next, we confirmed that flies exposed to 2.5 h hypoxia exhibited a locomotor activity deficit compared to control flies. We monitored the number of beam crosses of each experimental group using the DAM system and observed that control flies moved uniformly over time and the number of beam crosses were higher at the upper monitor, as expected given the innate locomotor behavior in Drosophila. Flies undergoing hypoxia showed decreased locomotor activity, indicated by fewer beam crosses in all the monitors of the unit (lower, middle, and upper monitor). Moreover, locomotor activity after hypoxia was further reduced over time, especially after 120–150 min of reoxygenation, revealing a delayed effect of hypoxic injury on flies’ behavior (Figure 1c).
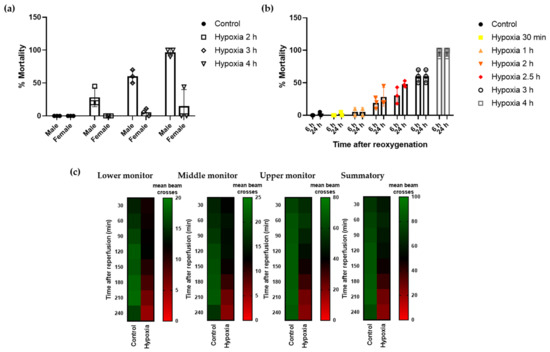
Figure 1.
Mortality rate and locomotor activity of Drosophila after hypoxia. Flies were submitted to 1% O2 for different time periods followed by reoxygenation (n = 3 experiments, 90 individuals per condition). (a) Mortality rate of male and female flies 24 h after exposure to different durations of hypoxia. (b) Mortality rate of male flies 6 and 24 h after hypoxia exposure. (c) Heatmap displaying the locomotor activity of male flies following 2.5 h of hypoxia or normoxia. Flies were transferred into the DAM system immediately after hypoxia and activity was recorded for 240 min after reperfusion. Each cell shows the mean beam crosses per fly.
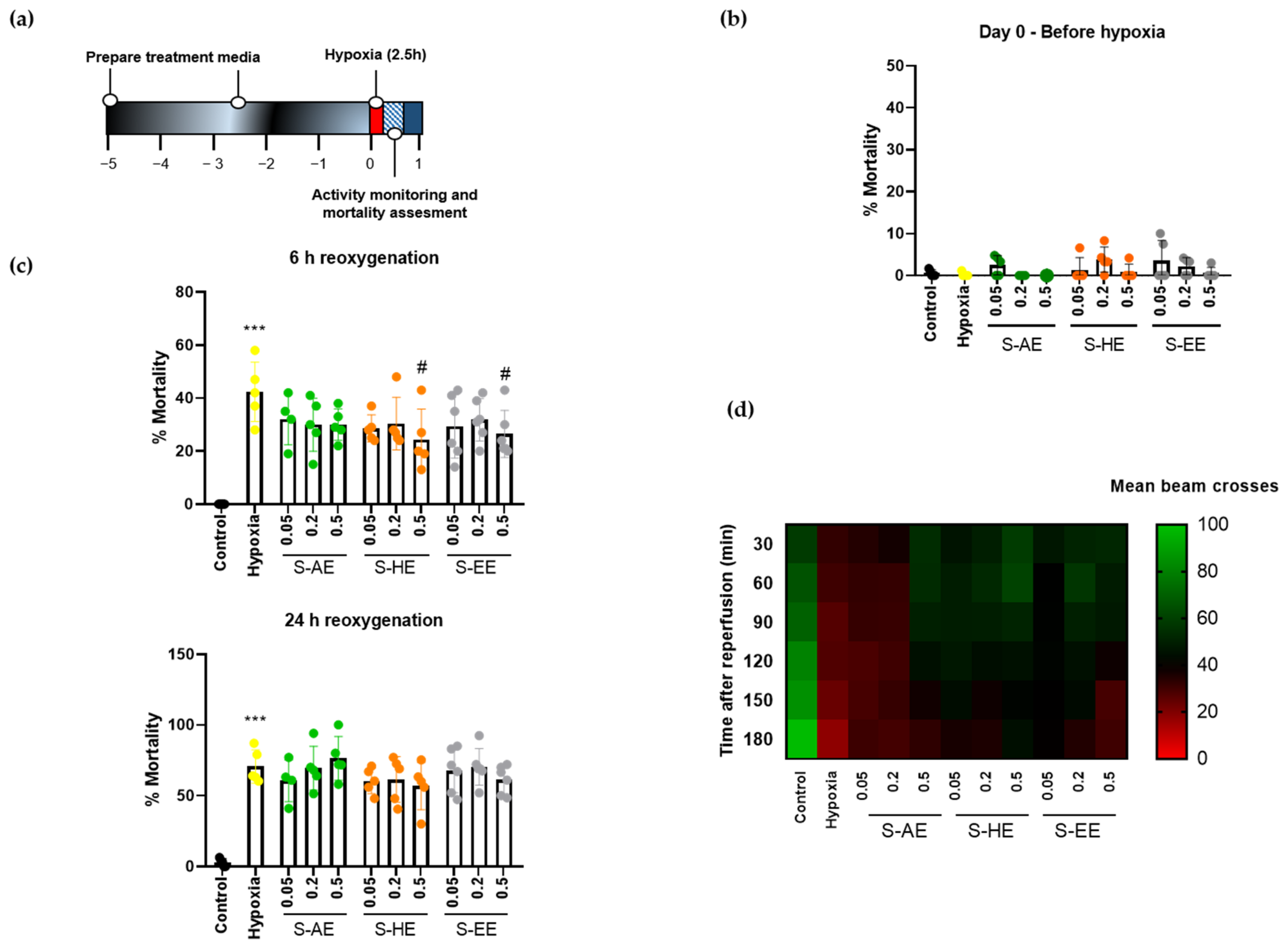
Then, we studied the effect of the three Salicornia extracts on hypoxia performance of Drosophila. Flies were treated for 5 days with different doses of S-AE, S-HE, or S-EE and subjected to severe hypoxia for 2.5 h followed by reoxygenation (Figure 2a). No signs of toxicity were observed for any of the extracts, since mortality rates after 5 days of treatment at the highest doses under normoxic conditions were similar to the control group (Figure 2b). Treatment with S-AE for 5 days did not protect flies from hypoxia-induced mortality. On the contrary, S-HE and S-EE at their highest dose (0.5 mg/mL) significantly reduced mortality rate after hypoxia (Figure 2c, upper panel). However, the protective effect of both extracts was lost 24 h after reoxygenation in standard non-supplemented media (Figure 2c, bottom panel). Finally, the effect of Salicornia extracts on hypoxia-induced locomotor deficit was studied after reperfusion. As expected, hypoxia-exposed flies showed a decline in locomotor activity. On the other hand, pre-treatment with S-AE at the highest dose, as well as the three different doses of S-HE and S-EE greatly improved general locomotor activity after hypoxia exposure and postponed the locomotor decline compared to the hypoxia group (Figure 2d).
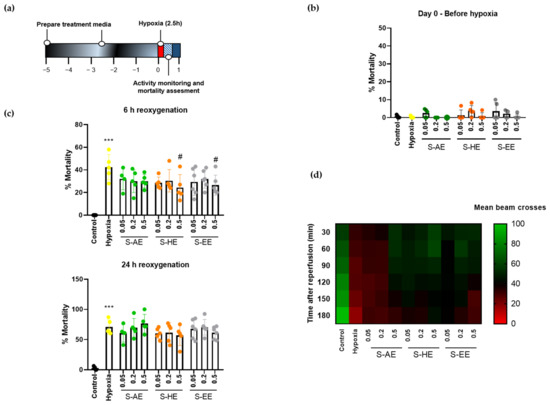
Figure 2.
Effect of different concentrations of S-AE, S-HE, and S-EE pre-treatment (mg/mL) on flies exposed to acute hypoxia. (a) Schematic illustration of the hypoxia protocol in Drosophila males. (b) Mortality rate assessment after 5 days of treatment in normoxia (n = 5 experiments; 150–180 individuals per condition). (c) Mortality rate of control and treated flies 6 and 24 h after hypoxia exposure (n = 5 experiments; 136–181 individuals per condition). (d) Heatmap displaying the locomotor activity of treated flies (n = 3 experiments; 79–90 individuals per condition). Flies were transferred into the DAM system immediately after hypoxia and activity was recorded for 180 min after reoxygenation. Each cell shows the mean beam crosses per fly. Data were analyzed using one-way ANOVA test followed by Dunnett´s multiple comparisons test *** p < 0.001 vs. control; # p < 0.05 vs. hypoxia.
3.4. Oral Supplementation with S-EE Prevented Brain Damage after Experimental Stroke in Mice
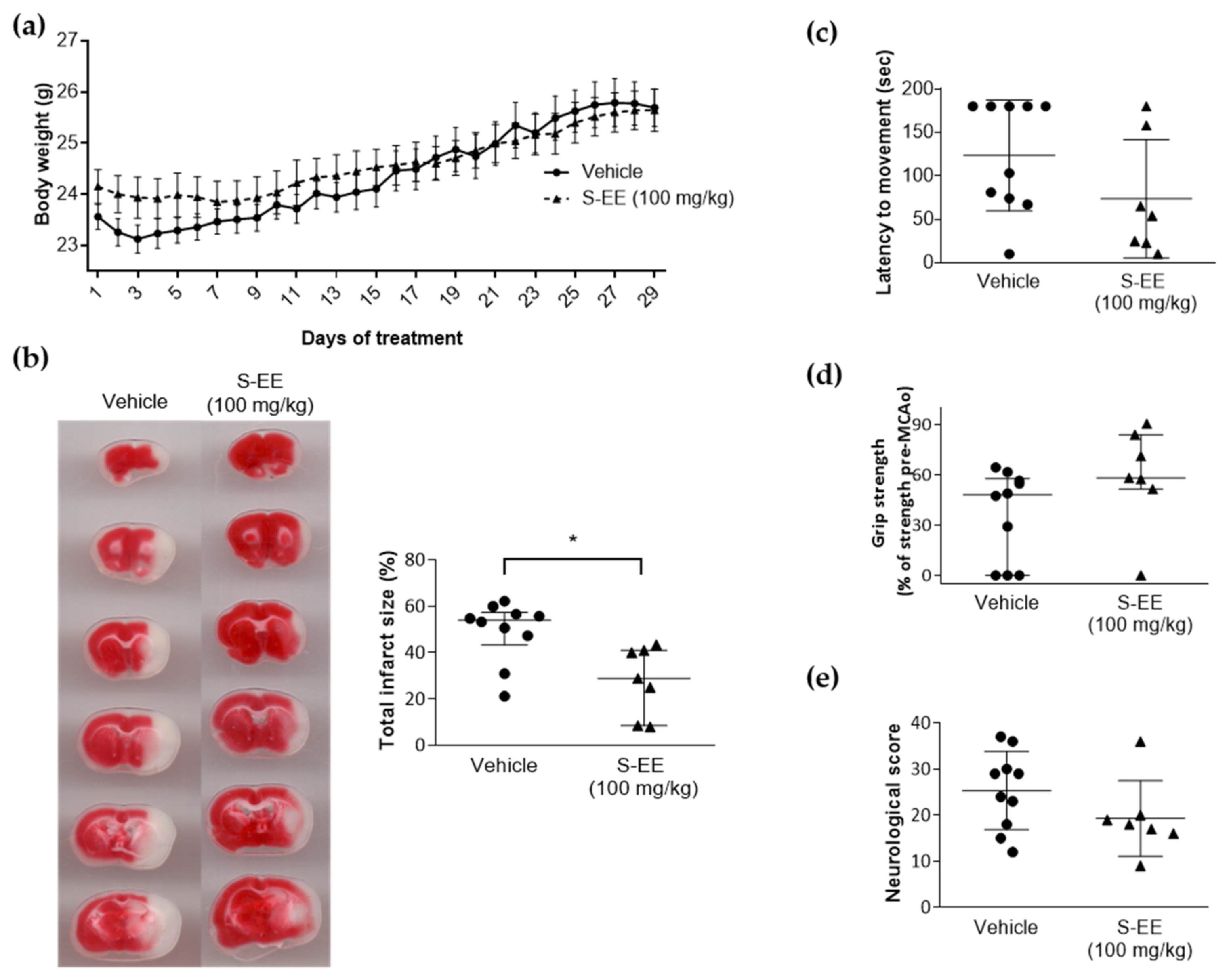
We investigated whether the protective effect observed for S-EE, the extract richest in polyphenols, was translated to mice brain ischemia. To this end, mice were given oral gavage supplementation with S-EE (100 mg/kg) or vehicle for 28 days prior to the induction of experimental stroke. No signs of toxicity or significant weight loss were observed for S-EE treatment during the experimental period (Figure 3a). Three out of ten mice treated with the extract died after the surgery before scarification, while no mortality was found in animals treated with vehicle. No significant difference was found between groups (p > 0.1, Chi-square test). As shown in brain images, oral supplementation with S-EE significantly reduced brain infarct volume after 90 min of t-pMCAo.
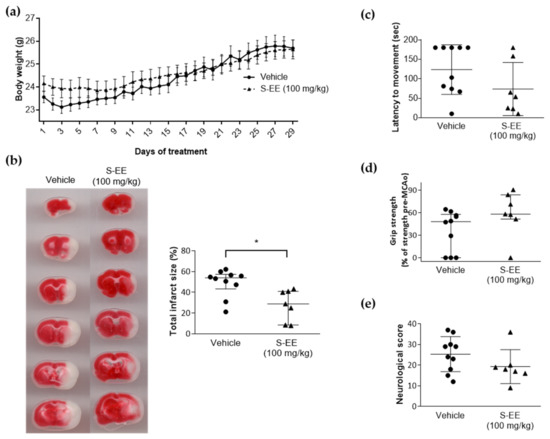
Figure 3.
Effect of oral supplementation with S-EE for 28 days on experimental strokes in mice. (a) Daily bodyweight monitoring after 28 days of treatment with S-EE or vehicle. (b) Representative images of TTC brain staining and quantification of percentage of infarct volume 24 h after t-pMCAo. Neurological and functional evaluation were performed 24 h after surgery including latency–movement test (c), grip strength test (d) and neurological score (e). Sample size: vehicle n = 10; S-EE n = 7. Data were analyzed using Student’s t-test. * p < 0.05 vs. vehicle.
To determine the effect of S-EE on functional outcomes, a latency–movement test and a grip test were performed, as well as a neurological scale for general and focal deficit evaluation. Compared to the vehicle group, animals treated with S-EE showed a tendency towards reduced time of latency to movement and increased grip strength (Figure 3c,d). No difference was found regarding neurological score (Figure 3e).
3.5. Effect of S-EE Supplementation Oxidative Stress Markers in Plasma and the Brain
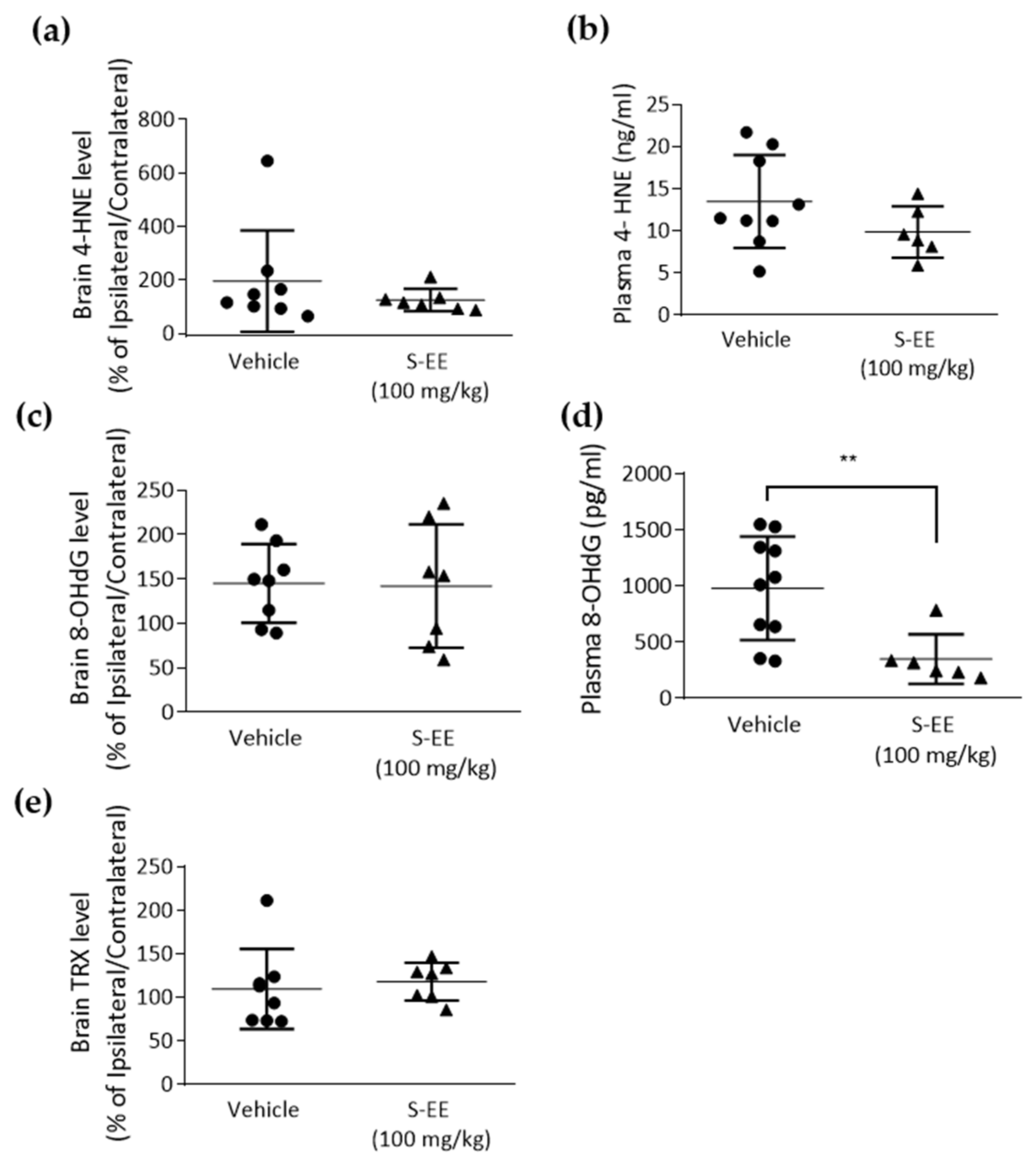
The brain is characterized by a high requirement of oxygen and relatively low antioxidant capacity, so it is very vulnerable to ischemia. A major role of oxidative stress in stroke injury has been suggested and animal models of the disease have shown decreased brain and plasma antioxidant capacity [38]. Therefore, we analyzed plasma and brain levels of different tissue oxidation markers. First, we measured the level of the lipid peroxidant product 4-hydroxynonenal (4-HNE) in the brain and found no effect in S-EE-treated mice compared to vehicle (Figure 4a). When measuring 4-HNE in plasma, we observed a trend of a reduced level of 4-HNE after S-EE supplementation, although it did not reach statistical significance (Figure 4b). We also assessed 8-hydroxydeoxyguanosine (8-OHdG), an oxidized nucleoside of DNA. Again, no significant changes were observed in the brain (Figure 4c), while plasma levels of 8-OHdG were significantly reduced when mice were treated with S-EE for 28 days prior to t-pMCAo (Figure 4d). Finally, levels of thioredoxin (TRX), a redox-regulating protein with antioxidant activity, were measured in brain tissue, but no effect was observed in S-EE-treated mice (Figure 4e).
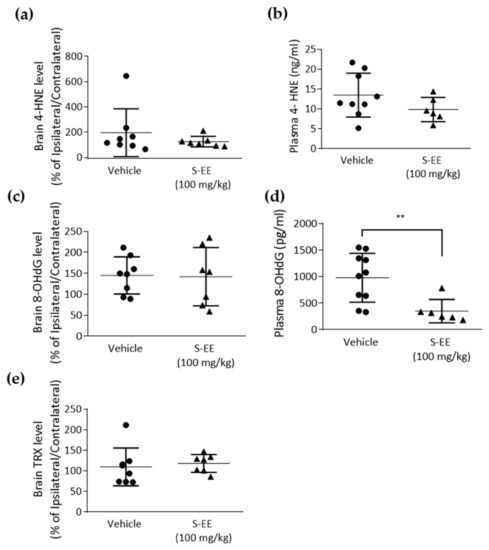
Figure 4.
Oxidative stress markers measurement in mice plasma and brain tissue. Samples were collected 24 h after t-pMCAo and analyzed for 4-HNE (a,b), 8-OHdG (c,d) or TRX (e) levels. Data were analyzed using Student’s t-test. Sample size: brain samples (vehicle n = 8; S-EE n = 7), plasma samples (vehicle n = 8; S-E E n = 6). ** p < 0.01 vs. vehicle.
4. Discussion
Current evidence suggests the presence of important bioactive compounds in halophyte plants. Here, we investigated S. ramosissima as a source of polyphenols for the treatment of brain ischemia. The preparation of aqueous, hydroalcoholic, and ethanolic S. ramosissima extracts proved that ethanol concentration in the extraction solvent affected the recovery of phenolic compounds, with S-EE extraction being the most suitable for the highest recovery of polyphenols.
To our knowledge, this is the first study reporting the neuroprotective effect of S. ramosissima against ischemic brain damage. The three extracts were screened for protective potential using a Drosophila model of hypoxia–reoxygenation. We observed that pre-treatment with S-HE and S-EE reduced hypoxia-induced mortality and locomotor impairment. Moreover, we showed here that oral supplementation with S-EE (100 mg/kg) for four weeks before ischemia protected mice brains from experimental strokes, as shown by a significant reduction in t-pMCAo-induced infarction, a tendency to improve functional outcome and a reduction in plasma 8-OHdG levels.
Salicornia has been extensively studied in Chinese medicine and numerous reports have attributed this halophyte antioxidant [39,40], antithrombotic [41], anti-inflammatory, or analgesic [42] properties. In fact, a S. herbacea ethanolic extract was found to be neuroprotective in vitro through the induction of antioxidant defense enzymes [17] and an ethyl acetate extract of S. europaea protected mice from a Parkinson´s disease-like model by regulating proinflammatory molecules [43]. In our study, dietetic supplementation with S-EE protected fruit flies from global hypoxia so a beneficial effect against ischemia in other tissues different from the brain may also be plausible. Moreover, using the t-pMCAo model in mice, a systemic effect of S-EE was also observed, as shown by a reduction in plasma levels of the oxidative stress markers, 4-HNE and 8-OHdG, 24 h after reperfusion.
Salicornia extracts represent a complex source of biomolecules with neuroprotective or antioxidant activities [10]. As expected by previous reports [26,27,28,29,30], we found many different compounds that can be responsible for the therapeutic effect of S-EE, mainly including caffeoylquinic acid derivatives, flavonoids, and fatty acids. Although the exact therapeutic mechanisms of S-EE were not revealed in this study, several phytochemicals present in the plant might have contributed to the protective effect in combination. Among them, quercetin administration prior to the onset of ischemia has been reported in several studies to reduce brain infarct volume and neuronal loss, and to improve neurological outcome [44,45,46]. Another major component of S. ramosissima, caffeic acid, ameliorated neurological dysfunction and reduced infarct volume after focal and global ischemia [47,48]. In addition, the presence of caffeoylquinic acid and its derivatives in Artemisia princeps Pampanini extract showed a neuroprotective effect on PC-12 cells under the insult of amyloid ß peptide by reducing oxidative stress [49]. Several works have reported that ferulic acid, a derivative of cinnamic acid also present in S-EE, protects against experimental cerebral ischemia-reperfusion in vivo, reducing brain infarct through antioxidant mechanisms [50,51]. Interestingly, we found cannabidiolic acid (CBDA) to be present in S-EE. CBDA is a less stable carboxylated precursor of CBD, which has been broadly studied as a neuroprotectant against many conditions, including brain ischemia [52]. Additionally, the main fatty acids found in our Salicornia extracts, linoleic and linolenic acids, are widely known for their role in cardiovascular protection [53,54,55].
As suggested by the Stroke Therapy Academic Industry Roundtable (STAIR) [56], our data should be replicated in new studies with the inclusion of female, aged animals and comorbid conditions, such as hypertension, diabetes, and hypercholesterolemia in order to increase the translational potential of the treatment, but also to identify feasible therapeutic windows. In our experimental approach, S-EE reduced flies’ death at 6 h after reoxygenation when given before hypoxia. However, the protective effect was lost at 24 h after reoxygenation, suggesting that prophylactic treatment is not enough for improving long-term outcomes, and, therefore, treatment might need to be extended and continued after hypoxia. In mice, a neuroprotective effect was also observed when S-EE treatment was performed before ischemia onset. Despite this strategy being challenging to translate into the clinic, many strokes can be prevented through the control of risk factors. In this sense, a S. herbacea extract was shown to control hyperlipidemia in diabetic mice through inhibition of pancreatic lipase [57]. Moreover, other authors have suggested that Salicornia extracts would be a useful dietary supplement to avoid abnormal vascular events that could precede stroke, such as vascular dysfunction and remodeling [14,15]. For this reason, Salicornia is being studied as a salt substitute in fermented food [58].
The current interest in nutraceuticals from both industry and consumers is notably rising due to their safety and therapeutic effects. A randomized controlled trial has recently shown that oral administration of desalted S.europaea ethanolic extract for 12 weeks is well tolerated by subjects complaining of memory dysfunction [59]. In agreement with this, some major constituents described in Salicornia extracts are marketed as ingredients of dietary supplements for different health conditions [60], including quercetin [61], apigenin [62], or chrysin [63]. In this respect, our group is currently evaluating the long-term safety and tolerability of S-EE in healthy volunteers.
Taken together, the results shown in this study highlight the protective effect of S-EE against hypoxia/ischemia in both fruit flies and mice models. Considering the rich composition of bioactive substances described in Salicornia and their safety profile, S-EE might serve as a potential candidate for the long-term prevention of neurovascular disease. Further studies are needed to elucidate the mechanism of action of isolated compounds and the synergic effects of S-EE components underlying its protective effect against ischemia.
Author Contributions
Conceptualization, funding acquisition, review, and editing, J.M.; methodology, writing—original draft preparation and supervision, C.d.R.; methodology, formal analysis, data curation, especially on the mice models, writing—review and editing, P.G.-R., F.M. and L.R.; methodology, formal analysis, data curation, especially on the drosophila models, review and editing, M.R.-B., P.M.-V. and C.d.R.; methodology, review and editing, especially future project validation: A.M.N. and S.P.-S.; methodology, formal analysis, data curation, especially on the extracts preparation, writing—review and editing, M.d.l.L.C.-G., F.J.L.-J. and A.S.-C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received financial support from “CSF-Proyectos estratégicos de I+D+i. Proyectos cofinanciados en un 80% por fondos del Programa Operativo FEDER de Andalucía 2014–2020”, grant number PE-0527-2019. This research was partially funded by “Consejería de Transformación Económica, Industria, Conocimiento y Universidades (CTEICU) y 80% cofinanciados por la UE, PO FEDER Andalucía 2014-2020”, grant number [PY20_01351]. C.d.R. received financial support from the Sara Borrell program funded by ISCIII, grant number [CD21/00148].
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of The Vall d´Hebrón Institute (protocol code 03/19).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are provided within the manuscript.
Acknowledgments
We are thankful to Manuel Díaz and Demófilo Vitorique from Halofitas Onuba SL. at Isla Cristina, Huelva, Spain, for collecting and sharing Salicornia ramosissima for our experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bejot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. Presse Med. 2016, 45, e391–e398. [Google Scholar] [CrossRef] [PubMed]
- Saver, J.L. Time is brain—Quantified. Stroke 2006, 37, 263–266. [Google Scholar] [CrossRef] [PubMed]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; van der Worp, B.H.; Howells, D.W. 1026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef] [PubMed]
- NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Chao, T.F.; Nedeljkovic, M.A.; Lip, G.Y.H.; Potpara, T.S. Stroke prevention in atrial fibrillation: Comparison of recent international guidelines. Eur. Heart J. Suppl. 2020, 22, O53–O60. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Montaner, J. Advanced neuroprotection for brain ischemia: An alternative approach to minimize stroke damage. Expert Opin. Investig. Drugs 2015, 24, 1137–1142. [Google Scholar] [CrossRef]
- Chelluboina, B.; Vemuganti, R. Therapeutic potential of nutraceuticals to protect brain after stroke. Neurochem. Int. 2021, 142, 104908. [Google Scholar] [CrossRef]
- Surget, G.; Stiger-Pouvreau, V.; Le Lann, K.; Kervarec, N.; Couteau, C.; Coiffard, L.J.; Gaillard, F.; Cahier, K.; Guerard, F.; Poupart, N. Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J. Photochem. Photobiol. B 2015, 143, 52–60. [Google Scholar] [CrossRef]
- Parrella, E.; Gussago, C.; Porrini, V.; Benarese, M.; Pizzi, M. From Preclinical Stroke Models to Humans: Polyphenols in the Prevention and Treatment of Stroke. Nutrients 2020, 13, 85. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Salicornia: Evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Roberto, V.P.; Surget, G.; Le Lann, K.; Mira, S.; Tarasco, M.; Guerard, F.; Poupart, N.; Laize, V.; Stiger-Pouvreau, V.; Cancela, M.L. Antioxidant, Mineralogenic and Osteogenic Activities of Spartina alterniflora and Salicornia fragilis Extracts Rich in Polyphenols. Front. Nutr. 2021, 8, 719438. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective Effect of Salicornia europaea Extracts on High Salt Intake-Induced Vascular Dysfunction and Hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef] [PubMed]
- Won, K.J.; Lee, K.P.; Baek, S.; Cui, L.; Kweon, M.H.; Jung, S.H.; Ryu, Y.K.; Hong, J.M.; Cho, E.A.; Shin, H.S.; et al. Desalted Salicornia europaea extract attenuated vascular neointima formation by inhibiting the MAPK pathway-mediated migration and proliferation in vascular smooth muscle cells. Biomed. Pharmacother. 2017, 94, 430–438. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, J.Y.; Oh, J.; Jang, Y.K.; Lee, C.H.; Kim, J.S. Neuroprotective Effect of Halophyte Salicornia herbacea L. Is Mediated by Activation of Heme Oxygenase-1 in Mouse Hippocampal HT22 Cells. J. Med. Food 2017, 20, 140–151. [Google Scholar] [CrossRef]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef]
- Chung, Y.C.; Choi, J.H.; Oh, K.N.; Chun, H.K.; Jeong, H.G. OhTungtungmadic acid isolated from Salicornia herbacea suppresses the progress of carbon tetrachloride-induced hepatic fibrosis. Off. J. Korean Soc. Toxicol. 2006, 22, 267–273. [Google Scholar]
- Clark, W.M.; Lessov, N.S.; Dixon, M.P.; Eckenstein, F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol. Res. 1997, 19, 641–648. [Google Scholar] [CrossRef]
- Ma, F.; Martinez-San Segundo, P.; Barcelo, V.; Morancho, A.; Gabriel-Salazar, M.; Giralt, D.; Montaner, J.; Rosell, A. Matrix metalloproteinase-13 participates in neuroprotection and neurorepair after cerebral ischemia in mice. Neurobiol. Dis. 2016, 91, 236–246. [Google Scholar] [CrossRef]
- Orsini, F.; Villa, P.; Parrella, S.; Zangari, R.; Zanier, E.R.; Gesuete, R.; Stravalaci, M.; Fumagalli, S.; Ottria, R.; Reina, J.J.; et al. Targeting mannose-binding lectin confers long-lasting protection with a surprisingly wide therapeutic window in cerebral ischemia. Circulation 2012, 126, 1484–1494. [Google Scholar] [CrossRef]
- De Simoni, M.G.; Storini, C.; Barba, M.; Catapano, L.; Arabia, A.M.; Rossi, E.; Bergamaschini, L. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J. Cereb. Blood Flow Metab. 2003, 23, 232–239. [Google Scholar] [CrossRef]
- Balkaya, M.G.; Trueman, R.C.; Boltze, J.; Corbett, D.; Jolkkonen, J. Behavioral outcome measures to improve experimental stroke research. Behav. Brain Res. 2018, 352, 161–171. [Google Scholar] [CrossRef]
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, ahead of print, 1–24. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Cacador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima J. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Ferreira, D.; Isca, V.M.S.; Leal, P.; Seca, A.M.L.; Silva, H.; de Lourdes Pereira, M.; Silva, A.M.S.; Pinto, D.C.G.A. Salicornia ramosissima: Secondary metabolites and protective effect against acute testicular toxicity. Arab. J. Chem. 2018, 11, 70–80. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]
- Alfheeaid, H.A.; Raheem, D.; Ahmed, F.; Alhodieb, F.S.; Alsharari, Z.D.; Alhaji, J.H.; BinMowyna, M.N.; Saraiva, A.; Raposo, A. Salicornia bigelovii, S. brachiata and S. herbacea: Their Nutritional Characteristics and an Evaluation of Their Potential as Salt Substitutes. Foods 2022, 11, 3402. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef]
- Gupta, M.L.; Tewari, J.P.; Khanna, S.N.; Gupta, P.C.; Srivastava, M.C.; Mishra, S.S. Phytopharmacologic studies of Ipomoea muricata seeds. J. Pharm. Sci. 1967, 56, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Lv, Q.; Liu, L.; Zhang, Y.; Yang, X. New bakuchiol dimers from Psoraleae Fructus and their inhibitory activities on nitric oxide production. Chin. Med. 2021, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.C.; Choi, H.S.; Kim, J.H.; Kim, S.L.; Yun, B.S.; Lee, D.S. Coriolic Acid (13-(S)-Hydroxy-9Z, 11E-octadecadienoic Acid) from Glasswort (Salicornia herbacea L.) Suppresses Breast Cancer Stem Cell through the Regulation of c-Myc. Molecules 2020, 25, 4950. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, B.; Jiang, M.; Wang, H.; Hu, Y.; Wang, H.; Xu, X.; Gao, X.; Yang, W. Integrating Enhanced Profiling and Chemometrics to Unveil the Potential Markers for Differentiating among the Leaves of Panax ginseng, P. quinquefolius, and P. notoginseng by Ultra-High Performance Liquid Chromatography/Ion Mobility-Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2022, 27, 5549. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Kinoshita, M.; Ohnishi, M. Chemical Characterization of Glycerolipids and Cerebrosides in a Halophytic Plant, Salicornia europaea L. J. Oleo Sci. 2004, 53, 337–341. [Google Scholar] [CrossRef][Green Version]
- Liu, P. Application of FT-ICR Mass Spectrometry in Hydrogen Deuterium Exchange and Lipidomics. Ph.D. Thesis, Florida State University, Tallahassee, FL, USA, 2018. [Google Scholar]
- Pandey, U.B.; Nichols, C.D. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol. Rev. 2011, 63, 411–436. [Google Scholar] [CrossRef]
- Awooda, H.A.; Lutfi, M.F.; Sharara, G.G.; Saeed, A.M. Oxidative/nitrosative stress in rats subjected to focal cerebral ischemia/reperfusion. Int. J. Health Sci. 2015, 9, 17–24. [Google Scholar] [CrossRef]
- Kim, Y.A.; Kong, C.S.; Um, Y.R.; Lim, S.Y.; Yea, S.S.; Seo, Y. Evaluation of Salicornia herbacea as a potential antioxidant and anti-inflammatory agent. J. Med. Food 2009, 12, 661–668. [Google Scholar] [CrossRef]
- Ha, B.J.; Lee, S.H.; Kim, H.J.; Lee, J.Y. The role of Salicornia herbacea in ovariectomy-induced oxidative stress. Biol. Pharm. Bull. 2006, 29, 1305–1309. [Google Scholar] [CrossRef]
- Jang, H.S.; Kim, K.R.; Choi, S.W.; Woo, M.H.; Choi, J.H. Antioxidant and antithrombus activities of enzyme-treated Salicornia herbacea extracts. Ann. Nutr. Metab. 2007, 51, 119–125. [Google Scholar] [CrossRef]
- Giordano, R.; Aliotta, G.E.; Johannesen, A.S.; Voetmann-Jensen, D.; Laustsen, F.H.; Andersen, L.A.; Rezai, A.; Fredsgaard, M.; Vecchio, S.L.; Arendt-Nielsen, L.; et al. Effects of Salicornia-Based Skin Cream Application on Healthy Humans’ Experimental Model of Pain and Itching. Pharmaceuticals 2022, 15, 150. [Google Scholar] [CrossRef]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The Ameliorative Effects of the Ethyl Acetate Extract of Salicornia europaea L. and Its Bioactive Candidate, Irilin B, on LPS-Induced Microglial Inflammation and MPTP-Intoxicated PD-Like Mouse Model. Oxid. Med. Cell. Longev. 2019, 2019, 6764756. [Google Scholar] [CrossRef]
- Park, D.J.; Kang, J.B.; Shah, M.A.; Koh, P.O. Quercetin alleviates the injury-induced decrease of protein phosphatase 2A subunit B in cerebral ischemic animal model and glutamate-exposed HT22 cells. J. Vet. Med. Sci. 2019, 81, 1047–1054. [Google Scholar] [CrossRef]
- Park, D.J.; Shah, F.A.; Koh, P.O. Quercetin attenuates neuronal cells damage in a middle cerebral artery occlusion animal model. J. Vet. Med. Sci. 2018, 80, 676–683. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, M.M.; Hoda, M.N.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.E.; Safhi, M.M.; et al. Quercetin protects against oxidative stress associated damages in a rat model of transient focal cerebral ischemia and reperfusion. Neurochem. Res. 2011, 36, 1360–1371. [Google Scholar] [CrossRef]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, S.H.; Ye, Y.L.; Chu, L.S.; Zhang, W.P.; Wang, M.L.; Wei, E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006, 27, 1103–1110. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, H.; Nam, T.G.; Eom, S.H.; Heo, H.J.; Lee, C.Y.; Kim, D.O. Neuroprotective effect of caffeoylquinic acids from Artemisia princeps Pampanini against oxidative stress-induced toxicity in PC-12 cells. J. Food Sci. 2011, 76, C250–C256. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Ho, T.Y.; Lee, E.J.; Su, S.Y.; Tang, N.Y.; Hsieh, C.L. Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am. J. Chin. Med. 2008, 36, 1105–1119. [Google Scholar] [CrossRef]
- Ceprian, M.; Jimenez-Sanchez, L.; Vargas, C.; Barata, L.; Hind, W.; Martinez-Orgado, J. Cannabidiol reduces brain damage and improves functional recovery in a neonatal rat model of arterial ischemic stroke. Neuropharmacology 2017, 116, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Fretts, A.M.; Mozaffarian, D.; Siscovick, D.S.; Sitlani, C.; Psaty, B.M.; Rimm, E.B.; Song, X.; McKnight, B.; Spiegelman, D.; King, I.B.; et al. Plasma phospholipid and dietary alpha-linolenic acid, mortality, CHD and stroke: The Cardiovascular Health Study. Br. J. Nutr. 2014, 112, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Chen, M.; Chowdhury, R.; Wu, J.H.; Sun, Q.; Campos, H.; Mozaffarian, D.; Hu, F.B. Alpha-Linolenic acid and risk of cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2012, 96, 1262–1273. [Google Scholar] [CrossRef] [PubMed]
- Veno, S.K.; Schmidt, E.B.; Jakobsen, M.U.; Lundbye-Christensen, S.; Bach, F.W.; Overvad, K. Substitution of Linoleic Acid for Other Macronutrients and the Risk of Ischemic Stroke. Stroke 2017, 48, 3190–3195. [Google Scholar] [CrossRef] [PubMed]
- Lapchak, P.A.; Zhang, J.H.; Noble-Haeusslein, L.J. RIGOR guidelines: Escalating STAIR and STEPS for effective translational research. Transl. Stroke Res. 2013, 4, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.Y.; Lee, S.K.; Jo, J.R.; Kim, M.E.; So, H.A.; Cho, C.W.; Seo, Y.W.; Kim, J.I. Hypolipidemic effect of Salicornia herbacea in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2007, 1, 371–375. [Google Scholar] [CrossRef]
- Pires-Cabral, P.; Pires-Cabral, P.; Quintas, C. Salicornia ramosissima as a salt substitute in the fermentation of white cabbage. J. Food Sci. Technol. 2022, 59, 597–605. [Google Scholar] [CrossRef]
- Lee, W.J.; Shin, Y.W.; Kim, D.E.; Kweon, M.H.; Kim, M. Effect of desalted Salicornia europaea L. ethanol extract (PM-EE) on the subjects complaining memory dysfunction without dementia: A 12 week, randomized, double-blind, placebo-controlled clinical trial. Sci. Rep. 2020, 10, 19914. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Andres, S.; Pevny, S.; Ziegenhagen, R.; Bakhiya, N.; Schafer, B.; Hirsch-Ernst, K.I.; Lampen, A. Safety Aspects of the Use of Quercetin as a Dietary Supplement. Mol. Nutr. Food Res. 2018, 62, 1700447. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. Biomed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Stompor-Goracy, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).