Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress—Mediated PERK Signaling Pathway Impairs Placental Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals and Experimental Design
2.3. Observation of Placental Structure
2.3.1. Hematoxylin-Eosin (HE) Staining
2.3.2. Transmission Electron Microscopy (TEM)
2.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5. Immunohistochemistry (IHC) Staining
2.6. TUNEL Staining
2.7. Western Blotting
2.8. Total RNA Isolation and Real-Time Quantitative PCR (qPCR)
2.9. Statistical Analysis
3. Results
3.1. Gestational Exposure to Cyfluthrin Disturbs Pregnancy Outcomes and Fetal Development
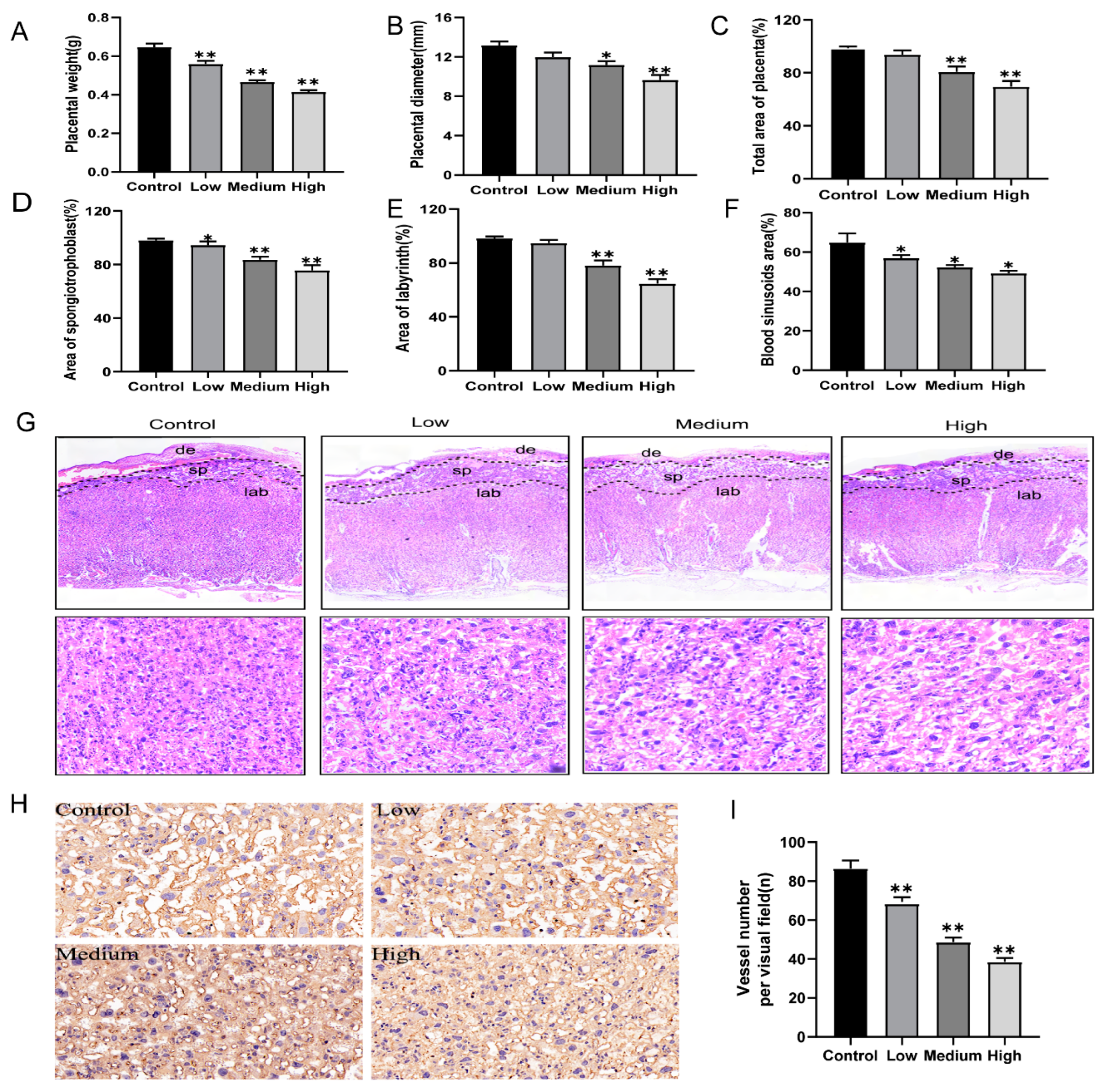
3.2. Gestational Exposure to Cyfluthrin Impairs Placental Development
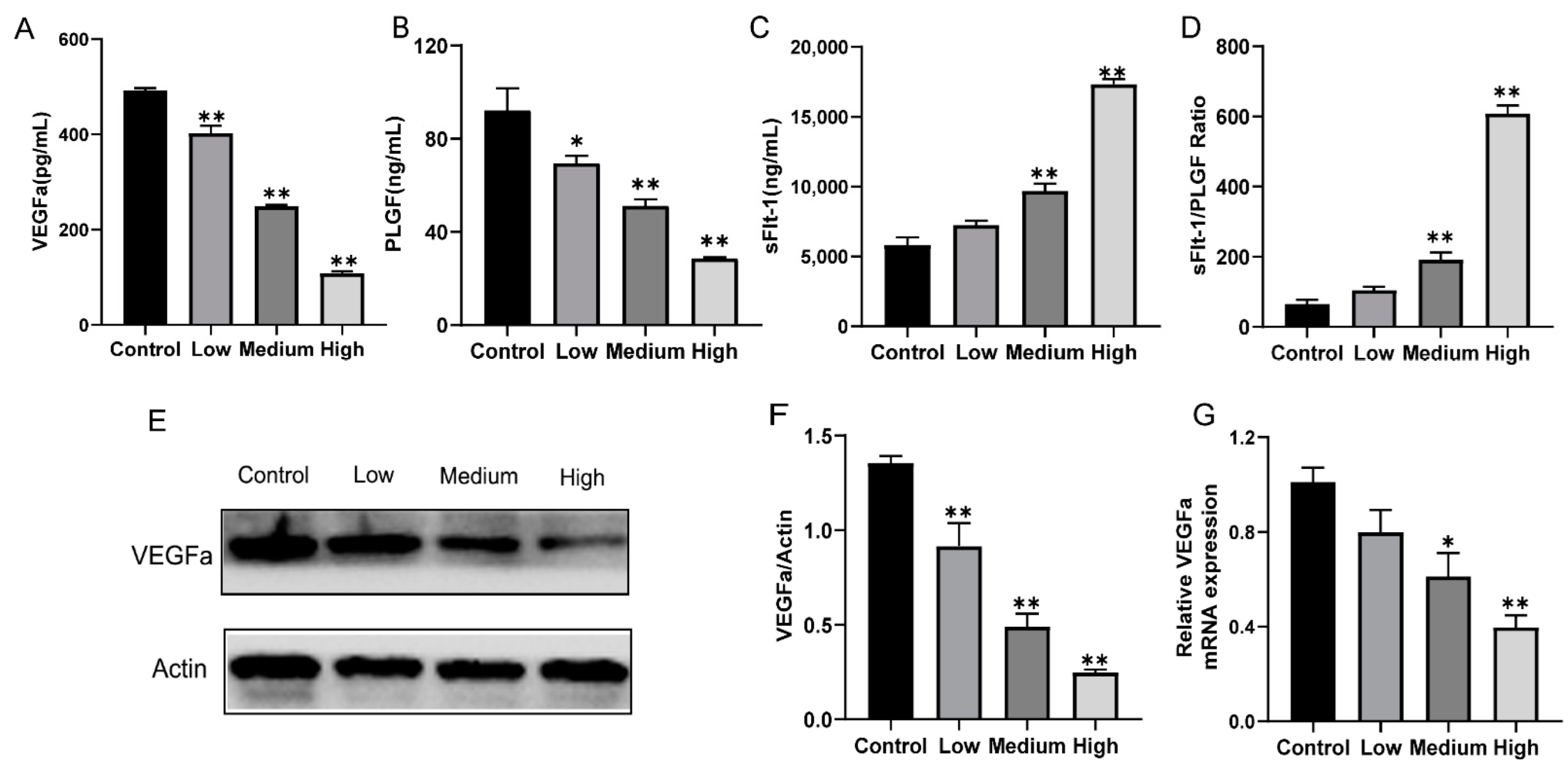
3.3. Gestational Exposure to Cyfluthrin Alters the Expression Levels of Pro-Angiogenic and Anti-Angiogenic Factors in the Placenta
3.4. Gestational Exposure to Cyfluthrin Inhibits Cell Proliferation and Induces Cell Apoptosis in the Placenta
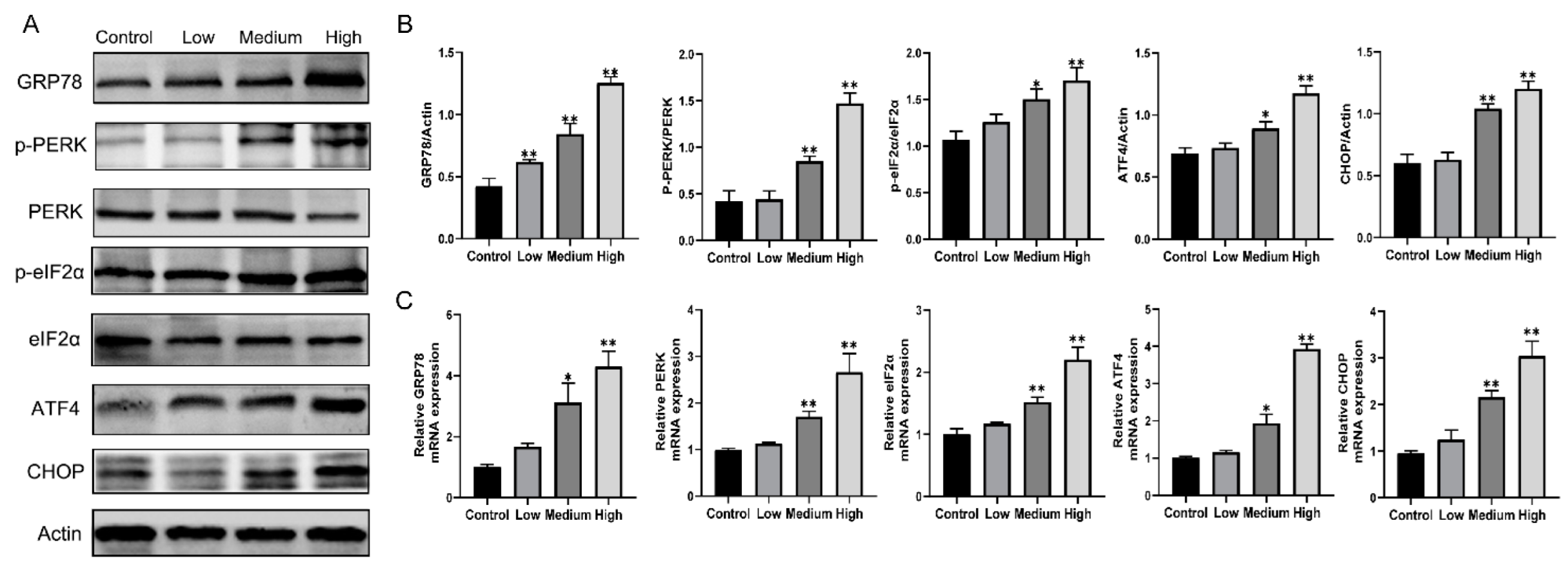
3.5. Gestational Exposure to Cyfluthrin Triggers ER Stress-Mediated PERK/eIF2α/ATF4/CHOP Signaling in the Placenta
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| de | decidua |
| sp | spongiotrophoblast |
| lab | labyrinth |
| TEM | transmission electron microscope |
| N | nuclear |
| MIT | mitochondrion |
| SER | smooth endoplasmic reticulum |
| RER | rough endoplasmic reticulum |
References
- Richardson, A.X., Jr.; Fitsanakis, V.; Westerink, R.H.S.; Kanthasamy, A.G. Neurotoxicity of Pesticides. Acta Neuropathol. 2019, 138, 343–362. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cheng, F.; Wei, Y.; Lydy, M.J.; You, J. Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: An overview. J. Hazard. Mater. 2017, 324, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Wang, D.; Wang, J.; Wu, Z.; Li, L.; Huang, M.; Xu, S.; Yan, D. Pyrethroid pesticide residues in the global environment: An overview. Chemosphere 2018, 191, 990–1007. [Google Scholar] [CrossRef] [PubMed]
- Coker, E.; Chevrier, J.; Rauch, S.; Bradman, A.; Obida, M.; Crause, M.; Bornman, R.; Eskenazi, B. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the VHEMBE South African birth cohort. Environ. Int. 2018, 113, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Dereumeaux, C.; Saoudi, A.; Goria, S.; Wagner, V.; De Crouy-Chanel, P.; Pecheux, M.; Berat, B.; Zaros, C.; Guldner, L. Urinary levels of pyrethroid pesticides and determinants in pregnant French women from the Elfe cohort. Environ. Int. 2018, 119, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Cui, C.; Chen, L.; Gao, Y.; Zhou, Y.; Shi, R.; Tian, Y. Prenatal exposure to pyrethroid insecticides and birth outcomes in Rural Northern China. J. Expo. Sci. Environ. Epidemiol. 2014, 25, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yoshinaga, J.; Hisada, A.; Shiraishi, H.; Shimodaira, K.; Okai, T.; Koyama, M.; Watanabe, N.; Suzuki, E.; Shirakawa, M.; et al. Prenatal pyrethroid insecticide exposure and thyroid hormone levels and birth sizes of neonates. Sci. Total Environ. 2014, 488, 275–279. [Google Scholar] [CrossRef]
- Bouwman, H.; Sereda, B.; Meinhardt, H.M. Meinhardt. Simultaneous Presence of Ddt and Pyrethroid Residues in Human Breast Milk from a Malaria Endemic Area in South Africa. Environ. Pollut. 2006, 144, 902–917. [Google Scholar] [CrossRef] [PubMed]
- Wren, M.; Liu, M.; Vetrano, A.; Richardson, J.R.; Shalat, S.L.; Buckley, B. Analysis of six pyrethroid insecticide metabolites in cord serum using a novel gas chromatography-ion trap mass spectrometry method. J. Chromatogr. B 2021, 1173, 122656. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ma, H.; Lydy, M.J.; You, J. Occurrence, Seasonal Variation and Inhalation Exposure of Atmospheric Organophosphate and Pyrethroid Pesticides in an Urban Community in South China. Chemosphere 2014, 95, 363–369. [Google Scholar] [CrossRef]
- Peris, A.; Barbieri, M.; Postigo, C.; Rambla-Alegre, M.; de Alda, M.L.; Eljarrat, E. Pesticides in sediments of the Ebro River Delta cultivated area (NE Spain): Occurrence and risk assessment for aquatic organisms. Environ. Pollut. 2022, 305, 119239. [Google Scholar] [CrossRef]
- Furlong, M.A.; Barr, D.B.; Wolff, M.S.; Engel, S.M. Prenatal exposure to pyrethroid pesticides and childhood behavior and executive functioning. Neurotoxicology 2017, 62, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, X.; Su, Q.; Xu, L.; Zhang, P.; Kong, Z.; Xu, J.; Teng, J. Effect of Synthetic Pyrethroid Pesticide Exposure During Pregnancy on the Growth and Development of Infants. Asia Pac. J. Public Health 2013, 25, 72S–79S. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.F.; Geraghty, E.M.; Tancredi, D.J.; Delwiche, L.D.; Schmidt, R.J.; Ritz, B.; Hansen, R.L.; Hertz-Picciotto, I. Neurodevelopmental Disorders and Prenatal Residential Proximity to Agricultural Pesticides: The Charge Study. Environ. Health Perspect. 2014, 122, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef]
- Barker, D.J.; Gelow, J.; Thornburg, K.; Osmond, C.; Kajantie, E.; Eriksson, J.G. The Early Origins of Chronic Heart Failure: Impaired Placental Growth and Initiation of Insulin Resistance in Childhood. Eur. J. Heart Fail. 2010, 12, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, Y.; Shi, M.-X.; Wang, B.; Liu, J.-J.; Xu, D.-X.; Meng, X.-H. Critical time window of fenvalerate-induced fetal intrauterine growth restriction in mice. Ecotoxicol. Environ. Saf. 2019, 172, 186–193. [Google Scholar] [CrossRef]
- Soni, I.; Syed, F.; Bhatnagar, P.; Mathur, R. Perinatal Toxicity of Cyfluthrin in Mice: Developmental and Behavioral Effects. Hum. Exp. Toxicol. 2011, 30, 1096–1105. [Google Scholar] [CrossRef]
- Syed, F.; John, P.J.; Soni, I. Neurodevelopmental consequences of gestational and lactational exposure to pyrethroids in rats. Environ. Toxicol. 2015, 31, 1761–1770. [Google Scholar] [CrossRef]
- Murphy, V.E.; Smith, R.; Giles, W.B.; Clifton, V.L. Endocrine Regulation of Human Fetal Growth: The Role of the Mother, Placenta, and Fetus. Endocr. Rev. 2006, 27, 141–169. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.-X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Pharmacol. 2019, 67, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.; Ji, X.; Zhang, Y.; Li, G.; Sang, N. Gestational Exposure to Pm(2.5) Impairs Vascularization of the Placenta. Sci. Total Environ. 2019, 665, 153–161. [Google Scholar] [CrossRef]
- Walter, F.; Schmid, J.; Düssmann, H.; Concannon, C.G.; Prehn, J.H.M. Imaging of Single Cell Responses to Er Stress Indicates That the Relative Dynamics of Ire1/Xbp1 and Perk/Atf4 Signalling Rather Than a Switch between Signalling Branches Determine Cell Survival. Cell Death Differ. 2015, 22, 1502–1516. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Diehl, J.A. PERK-dependent Activation of Nrf2 Contributes to Redox Homeostasis and Cell Survival following Endoplasmic Reticulum Stress. J. Biol. Chem. 2004, 279, 20108–20117. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Nie, P.; Yao, L.; Tang, Y.; Hong, W.; Liu, W.; Fu, F.; Xu, H. TiO2 NPs induce the reproductive toxicity in mice with gestational diabetes mellitus through the effects on the endoplasmic reticulum stress signaling pathway. Ecotoxicol. Environ. Saf. 2021, 226, 112814. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, S.; Wang, F.; Wang, H.; Xu, D.; Li, G. Microcystin-LR exposure decreased the fetal weight of mice by disturbance of placental development and ROS-mediated endoplasmic reticulum stress in the placenta. Environ. Pollut. 2019, 256, 113362. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez, M.A.; Martínez, M.; Castellano, V.; Ares, I.; Romero, A.; Fernández, R.; Martínez-Larrañaga, M.R. Differential Induction of Cytochrome P450 Isoforms and Peroxisomal Proliferation by Cyfluthrin in Male Wistar Rats. Toxicol. Lett. 2013, 220, 135–142. [Google Scholar] [CrossRef]
- Rodríguez, J.L.; Ares, I.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Effects of Exposure to Pyrethroid Cyfluthrin on Serotonin and Dopamine Levels in Brain Regions of Male Rats. Environ. Res. 2016, 146, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.M.; Green, J.A.; Schulz, L.C. The evolution of the placenta. Reproduction 2016, 152, R179–R189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, C.; Zhao, S.; Wang, B.; Wang, H.; Zhang, J.; Wang, Y.; Cheng, H.; Zhu, L.; Shen, R.; et al. Exposure to DEHP or its metabolite MEHP promotes progesterone secretion and inhibits proliferation in mouse placenta or JEG-3 cells. Environ. Pollut. 2019, 257, 113593. [Google Scholar] [CrossRef] [PubMed]
- Rossant, J.; Cross, J.C. Placental development: Lessons from mouse mutants. Nat. Rev. Genet. 2001, 2, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, T.; Li, Q.; Clementi, C.; Lydon, J.P.; DeMayo, F.J.; Matzuk, M.M. Bmpr2 Is Required for Postimplantation Uterine Function and Pregnancy Maintenance. J. Clin. Investig. 2013, 123, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.; Jauniaux, E. Pathophysiology of placental-derived fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S745–S761. [Google Scholar] [CrossRef]
- Albers, R.E.; Kaufman, M.R.; Natale, B.V.; Keoni, C.; Kulkarni-Datar, K.; Min, S.; Williams, C.R.; Natale, D.R.C.; Brown, T.L. Trophoblast-Specific Expression of Hif-1α Results in Preeclampsia-Like Symptoms and Fetal Growth Restriction. Sci. Rep. 2019, 9, 2742. [Google Scholar] [CrossRef]
- Natale, B.V.; Mehta, P.; Vu, P.; Schweitzer, C.; Gustin, K.; Kotadia, R.; Natale, D.R.C. Reduced Uteroplacental Perfusion Pressure (RUPP) causes altered trophoblast differentiation and pericyte reduction in the mouse placenta labyrinth. Sci. Rep. 2018, 8, 17162. [Google Scholar] [CrossRef] [PubMed]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.; Cross, J. Development of Structures and Transport Functions in the Mouse Placenta. Physiology 2005, 20, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Holloway, A.C.; Salomon, A.; Soares, M.J.; Garnier, V.; Raha, S.; Sergent, F.; Nicholson, C.J.; Feige, J.J.; Benharouga, M.; Alfaidy, N. Characterization of the Adverse Effects of Nicotine on Placental Development: In Vivo and in Vitro Studies. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E443–E456. [Google Scholar] [CrossRef] [PubMed]
- Jardim, L.L.; Rios, D.R.A.; Perucci, L.O.; de Sousa, L.P.; Gomes, K.B.; Dusse, L.M.S. Is the Imbalance between Pro-Angiogenic and Anti-Angiogenic Factors Associated with Preeclampsia? Clin. Chim. Acta 2015, 447, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating Angiogenic Factors and the Risk of Preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Longo, M.; Tamayo, E.; Maner, W.; Al-Hendy, A.; Anderson, G.D.; Hankins, G.D.; Saade, G.R. The Effect of over-Expression of Sflt-1 on Blood Pressure and the Occurrence of Other Manifestations of Preeclampsia in Unrestrained Conscious Pregnant Mice. Am. J. Obstet. Gynecol. 2007, 196, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Bastida-Ruiz, D.; Aguilar, E.; Ditisheim, A.; Yart, L.; Cohen, M. Endoplasmic reticulum stress responses in placentation-A true balancing act. Placenta 2017, 57, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C.; Baker, P.N.; Symonds, E.M. Increased Placental Apoptosis in Intrauterine Growth Restriction. Am. J. Obstet. Gynecol. 1997, 177, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Xu, Z.M.; Ji, Y.L.; Chen, Y.H.; Zhang, Z.H.; Zhang, C.; Meng, X.H.; Zhao, M.; Xu, D.X. Cadmium-Induced Teratogenicity: Association with Ros-Mediated Endoplasmic Reticulum Stress in Placenta. Toxicol. Appl. Pharmacol. 2012, 259, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Yoshimi, M.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S. Prolonged endoplasmic reticulum stress alters placental morphology and causes low birth weight. Toxicol. Appl. Pharmacol. 2014, 275, 134–144. [Google Scholar] [CrossRef]
- Yung, H.W.; Hemberger, M.; Watson, E.D.; Senner, C.E.; Jones, C.P.; Kaufman, R.J.; Charnock-Jones, D.S.; Burton, G.J. Endoplasmic Reticulum Stress Disrupts Placental Morphogenesis: Implications for Human Intrauterine Growth Restriction. J. Pathol. 2012, 228, 554–564. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Nishitoh, H. CHOP is a multifunctional transcription factor in the ER stress response. J. Biochem. 2012, 151, 217–219. [Google Scholar] [CrossRef]



| Indexes | Control | Low | Medium | High | |
|---|---|---|---|---|---|
| Groups | |||||
| Birth defect per litter (n) | 0 | 0 | 0.29 ± 0.76 | 0.14 ± 0.38 | |
| Dead fetuses per litter (n) | 0 | 0.14 ± 0.38 | 0.29 ± 0.49 | 0.43 ± 0.79 | |
| Resorptions per litter (n) | 0.14 ± 0.14 | 0.29 ± 0.29 | 0.57 ± 0.29 | 0.86 ± 0.40 | |
| Implantation sites per litter (n) | 12.14 ± 0.43 | 12.00 ± 0.38 | 11.57 ± 0.53 | 10.57 ± 0.69 * | |
| Live fetuses per litter (n) | 12.86 ± 0.51 | 12.57 ± 0.37 | 11.57 ± 0.48 | 10.43 ± 0.72 * | |
| Survival to PND4 (%) | 100 | 100 | 95.97 | 98.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W.; Gao, H.; Wu, B.; Zhao, J.; Sun, J.; Song, Y.; Sun, Y.; Yang, H. Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress—Mediated PERK Signaling Pathway Impairs Placental Development. Toxics 2022, 10, 733. https://doi.org/10.3390/toxics10120733
Ni W, Gao H, Wu B, Zhao J, Sun J, Song Y, Sun Y, Yang H. Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress—Mediated PERK Signaling Pathway Impairs Placental Development. Toxics. 2022; 10(12):733. https://doi.org/10.3390/toxics10120733
Chicago/Turabian StyleNi, Wensi, Haoxuan Gao, Bing Wu, Ji Zhao, Jian Sun, Yanan Song, Yiping Sun, and Huifang Yang. 2022. "Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress—Mediated PERK Signaling Pathway Impairs Placental Development" Toxics 10, no. 12: 733. https://doi.org/10.3390/toxics10120733
APA StyleNi, W., Gao, H., Wu, B., Zhao, J., Sun, J., Song, Y., Sun, Y., & Yang, H. (2022). Gestational Exposure to Cyfluthrin through Endoplasmic Reticulum (ER) Stress—Mediated PERK Signaling Pathway Impairs Placental Development. Toxics, 10(12), 733. https://doi.org/10.3390/toxics10120733








