Hemoadsorption in Complex Cardiac Surgery—A Single Center Experience
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ball, L.; Costantino, F.; Pelosi, P. Postoperative complications of patients undergoing cardiac surgery. Curr. Opin. Crit. Care 2016, 22, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, M. Modifying risks to improve outcome in cardiac surgery: An anesthesiologist’s perspective. Ann. Card Anaesth. 2017, 20, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Yadava, O.P.; Narayan, P.; Padmanabhan, C.; Sajja, L.R.; Sarkar, K.; Varma, P.K.; Jawali, V. IACTS position statement on “2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization”: Section 7.1—A consensus document. Indian J. Thorac. Cardiovasc. Surg. 2022, 38, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Aittokallio, J.; Uusalo, P.; Kallioinen, M.; Jarvisalo, M.J. Markers of Poor Prognosis in Patients Requiring Continuous Renal Replacement Therapy After Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3329–3335. [Google Scholar] [CrossRef] [PubMed]
- Busse, L.W.; Barker, N.; Petersen, C. Vasoplegic syndrome following cardiothoracic surgery-review of pathophysiology and update of treatment options. Crit. Care 2020, 24, 36. [Google Scholar] [CrossRef]
- Laffey, J.G.; Boylan, J.F.; Cheng, D.C. The systemic inflammatory response to cardiac surgery: Implications for the anesthesiologist. Anesthesiology 2002, 97, 215–252. [Google Scholar] [CrossRef]
- CytoSorbents. CytoSorbents. CytoSorb 300mL Device. In Instructions for Use; CytoSorbents Inc.: Monmouth Junction, NJ, USA, 2021; Available online: www.cytosorb.com (accessed on 8 August 2022).
- Poli, E.C.; Rimmele, T.; Schneider, A.G. Hemoadsorption with CytoSorb ((R)). Intensive Care Med. 2019, 45, 236–239. [Google Scholar] [CrossRef]
- Barton, H.; Zechendorf, E.; Ostareck, D.; Ostareck-Lederer, A.; Stoppe, C.; Zayat, R.; Philipp, T.-S.; Marx, G.; Bickenbach, J. Prognostic Value of GDF-15 in Predicting Prolonged Intensive Care Stay following Cardiac Surgery: A Pilot Study. Dis. Markers 2021, 2021, 5564334. [Google Scholar] [CrossRef]
- Crawford, T.C.; Magruder, J.T.; Grimm, J.C.; Suarez-Pierre, A.; Sciortino, C.M.; Mandal, K.; Zehr, K.J.; Conte, J.V.; Higgings, R.S.; Cameron, D.E.; et al. Complications After Cardiac Operations: All Are Not Created Equal. Ann. Thorac. Surg. 2017, 103, 32–40. [Google Scholar] [CrossRef]
- Bianco, V.; Kilic, A.; Gleason, T.G.; Aranda-Michel, E.; Habertheuer, A.; Wang, Y.; Navid, F.; Kacin, A.; Sultan, I. Reoperative Cardiac Surgery Is a Risk Factor for Long-Term Mortality. Ann. Thorac. Surg. 2020, 110, 1235–1242. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, S.; Tiemuerniyazi, X.; Song, Y.; Feng, W. A Meta-Analysis of Early, Mid-term and Long-Term Mortality of On-Pump vs. Off-Pump in Redo Coronary Artery Bypass Surgery. Front. Cardiovasc. Med. 2022, 9, 869987. [Google Scholar] [CrossRef] [PubMed]
- Nierhaus, A.; Morales, J.; Wendt, D.; Scheier, J.; Gutzler, D.; Jarczak, D.; Born, F.; Hagl, C.; Deliargyris, E.; Mehta, Y. Comparison of the CytoSorb ((R)) 300 mL and Jafron HA380 hemoadsorption devices: An in vitro study. Minim. Invasive Ther. Allied Technol. 2022, 31, 1058–1065. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Crapelli, G.B.; Rahe-Meyer, N.; Menicanti, L.; Frigiola, A.; Surgical Clinical Outcome REsearch (SCORE) Group. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J. Am. Heart Assoc. 2015, 4, e002066. [Google Scholar] [CrossRef] [PubMed]
- Park, C.B.; Suri, R.M.; Burkhart, H.M.; Greason, K.L.; Dearani, J.A.; Schaff, H.V.; Sundt, T.M., III. Identifying patients at particular risk of injury during repeat sternotomy: Analysis of 2555 cardiac reoperations. J. Thorac. Cardiovasc. Surg. 2010, 140, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, R.; Dharmadjati, B.B.; Mulia, E.P.B.; Rachmi, D.A. Vasoplegia: Mechanism and Management Following Cardiopulmonary Bypass. Eurasian J. Med. 2022, 54, 92–99. [Google Scholar] [CrossRef]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb (R) sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef]
- Haidari, Z.; Wendt, D.; Thielmann, M.; Mackowiak, M.; Neuhauser, M.; Jakob, H.; Ruhparwar, A.; El-Gabry, M. Intraoperative Hemoadsorption in Patients with Native Mitral Valve Infective Endocarditis. Ann. Thorac. Surg. 2020, 110, 890–896. [Google Scholar] [CrossRef]
- Mehta, Y.; Singh, A.; Singh, A.; Gupta, A.; Bhan, A. Modulating the Inflammatory Response with Hemadsorption (CytoSorb) in Patients Undergoing Major Aortic Surgery. J. Cardiothorac. Vasc. Anesth. 2021, 35, 673–675. [Google Scholar] [CrossRef]
- Saller, T.; Hagl, C.; Woitsch, S.; Li, Y.; Niedermayer, S.; Born, F.; Luehr, M.; Kammerer, T.; Pichlmaier, M.; Scheiermann, P.; et al. Haemadsorption improves intraoperative haemodynamics and metabolic changes during aortic surgery with hypothermic circulatory arrest. Eur. J. Cardiothorac. Surg. 2019, 56, 731–737. [Google Scholar] [CrossRef]
- Nemeth, E.; Kovacs, E.; Racz, K.; Soltesz, A.; Szigeti, S.; Kiss, N.; Csikos, G.; Koritsanszky, K.B.; Berzsenyi, V.; Trembickij, G.; et al. Impact of intraoperative cytokine adsorption on outcome of patients undergoing orthotopic heart transplantation-an observational study. Clin. Transpl. 2018, 32, e13211. [Google Scholar] [CrossRef]
- Boss, K.; Jahn, M.; Wendt, D.; Haidari, Z.; Demircioglu, E.; Thielmann, M.; Ruhparwar, A.; Kribben, A.; Bartosz, T. Extracorporeal cytokine adsorption: Significant reduction of catecholamine requirement in patients with AKI and septic shock after cardiac surgery. PLoS ONE 2021, 16, e0246299. [Google Scholar] [CrossRef] [PubMed]
- Traeger, K.; Skrabal, C.; Fischer, G.; Datzmann, T.; Schroeder, J.; Fritzler, D.; Hartmann, J.; Liebold, A.; Reinelt, H. Hemoadsorption treatment of patients with acute infective endocarditis during surgery with cardiopulmonary bypass–a case series. Int. J. Artif. Organs 2017, 40, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Traeger, K.; Skrabal, C.; Fischer, G.; Schroeder, J.; Marenski, L.; Liebold, A.; Reinelt, H.; Datzmann, T. Hemoadsorption treatment with CytoSorb ((R)) in patients with extracorporeal life support therapy: A case series. Int. J. Artif. Organs 2020, 43, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Chen, Q.; Zhu, X.; Shen, X.; Zou, L.; Mu, X.; Sun, X. Correlation Between Vasoactive-Inotropic Score and Postoperative Acute Kidney Injury after Cardiovascular Surgery. Heart Surg. Forum 2021, 24, E282–E292. [Google Scholar] [CrossRef] [PubMed]
- Baysal, P.K.; Guzelmeric, F.; Kahraman, E.; Gurcu, M.E.; Erkilinc, A.; Orki, T. Is Vasoactive-Inotropic Score a Predictor for Mortality and Morbidity in Patients Undergoing Coronary Artery Bypass Surgery? Braz. J. Cardiovasc. Surg. 2021, 36, 802–806. [Google Scholar] [CrossRef]
- Belletti, A.; Lerose, C.C.; Zangrillo, A.; Landoni, G. Vasoactive-Inotropic Score: Evolution, Clinical Utility, and Pitfalls. J. Cardiothorac. Vasc. Anesth. 2021, 35, 3067–3077. [Google Scholar] [CrossRef]
- Kalisnik, J.M.; Leiler, S.; Mamdooh, H.; Zibert, J.; Bertsch, T.; Vogt, F.A.; Bagaev, E.; Fittkau, M.; Fischlein, T. Single-Centre Retrospective Evaluation of Intraoperative Hemoadsorption in Left-Sided Acute Infective Endocarditis. J. Clin. Med. 2022, 11, 3954. [Google Scholar] [CrossRef]
- Haidari, Z.; Demircioglu, E.; Boss, K.; Tyczynski, B.; Thielmann, M.; Schmack, B.; Kribben, A.; Weymann, A.; El Gabry, M.; Ruhparwar, A.; et al. Intraoperative hemoadsorption in high-risk patients with infective endocarditis. PLoS ONE 2022, 17, e0266820. [Google Scholar] [CrossRef]
- Calabro, M.G.; Febres, D.; Recca, G.; Lembo, R.; Fominskiy, E.; Scandroglio, A.M.; Zangrillo, A.; Pappalardo, F. Blood Purification with CytoSorb in Critically Ill Patients: Single-Center Preliminary Experience. Artif. Organs 2019, 43, 189–194. [Google Scholar] [CrossRef]
- Tripathi, R.; Morales, J.; Lee, V.; Gibson, C.M.; Mack, M.J.; Schneider, D.J.; Douketis, J.; Sellke, F.W.; Ohman, M.E.; Thourani, V.H.; et al. Antithrombotic Drug Removal from Whole Blood Using Haemoadsorption with a Porous Polymer Bead Sorbent. Eur. Heart J. Cardiovasc. Pharmacother. 2022, pvac036. [Google Scholar] [CrossRef]
- Matejic-Spasic, M.; Hassan, K.; Thielmann, M.; Geidel, S.; Storey, R.F.; Schmoeckel, M.; Adamson, H.; Deliargyris, E.N.; Wendt, D. Management of perioperative bleeding risk in patients on antithrombotic medications undergoing cardiac surgery—A systematic review. J. Thorac. Dis. 2022, 14, 3030–3044. [Google Scholar] [CrossRef] [PubMed]

| Mean (CI) or Median [IQR] | ||||
|---|---|---|---|---|
| Variables | Overall (n = 52) | CS Group (n = 23) | Control Group (n = 29) | p-Value |
| Age, years | 61 [51.5, 65.5] | 64 [60.0, 68.0] | 57 [47.0, 61.0] | 0.01 |
| Sex–male, % (n) | 63.5 (33) | 60.9 (14) | 65.5 (19) | 0.73 |
| Redo procedures, % (n) | 34.6 (18) | 21.7 (5) | 44.8 (13) | 0.08 |
| Lactate mmol/L | 1.36 [1.0, 2.1] | 1.25 [0.8, 2.1] | 1.40 [1.1, 2.1] | 0.38 |
| EuroSCORE II, % | 4.6 [2.2, 14.2] | 7.3 [2.7, 21.4] | 4.4 [1.5, 9.2] | 0.17 |
| Mechanical ventilation, % (n) | 15.4 (8) | 21.7 (5) | 10.3 (3) | 0.26 |
| Intra-aortic balloon pump (IABP), % (n) | 9.6 (5) | 21.7 (5) | 0 | <0.01 |
| Continuous renal replacement therapy (CRRT), % (n) | 1.9 (1) | 4.3 (1) | 0 | 0.26 |
| Inotropes, % (n) | 25.0 (13) | 34.8 (8) | 17.2 (5) | 0.15 |
| Hemoglobin (Hb), g/dL | 12.0 [10.2, 13.5] | 11.5 [9.5, 12.8] | 12.6 [11.2, 13.8] | 0.08 |
| White blood cell count (WBC), N × 109/L | 9.2 (6.3, 11.9) | 8.2 (5.9, 11.4) | 10.6 (6.4, 12.0) | 0.64 |
| Vasoactive-inotropic score (VIS) | 1.6 (0.7, 2.5) | 2.2 (0.7, 3.7) | 1.1 (0.1, 2.1) | 0.20 |
| CPB time, min | 211 [167.5, 275.5] | 217 [171.0, 264.0] | 211 [164.0, 278.0] | 0.99 |
| Cross-clamp time, min | 143 [115.0, 181.0] | 122 [100.0, 156.0] | 154 [131.5, 195.5] | 0.04 |
| CPB temperature, °C | 31 [28, 32] | 30 [28, 32] | 32 [28, 32] | 0.30 |
| Mean (CI Difference) | ||||
|---|---|---|---|---|
| Variable | CS Group | Control Group | Delta | p-Value |
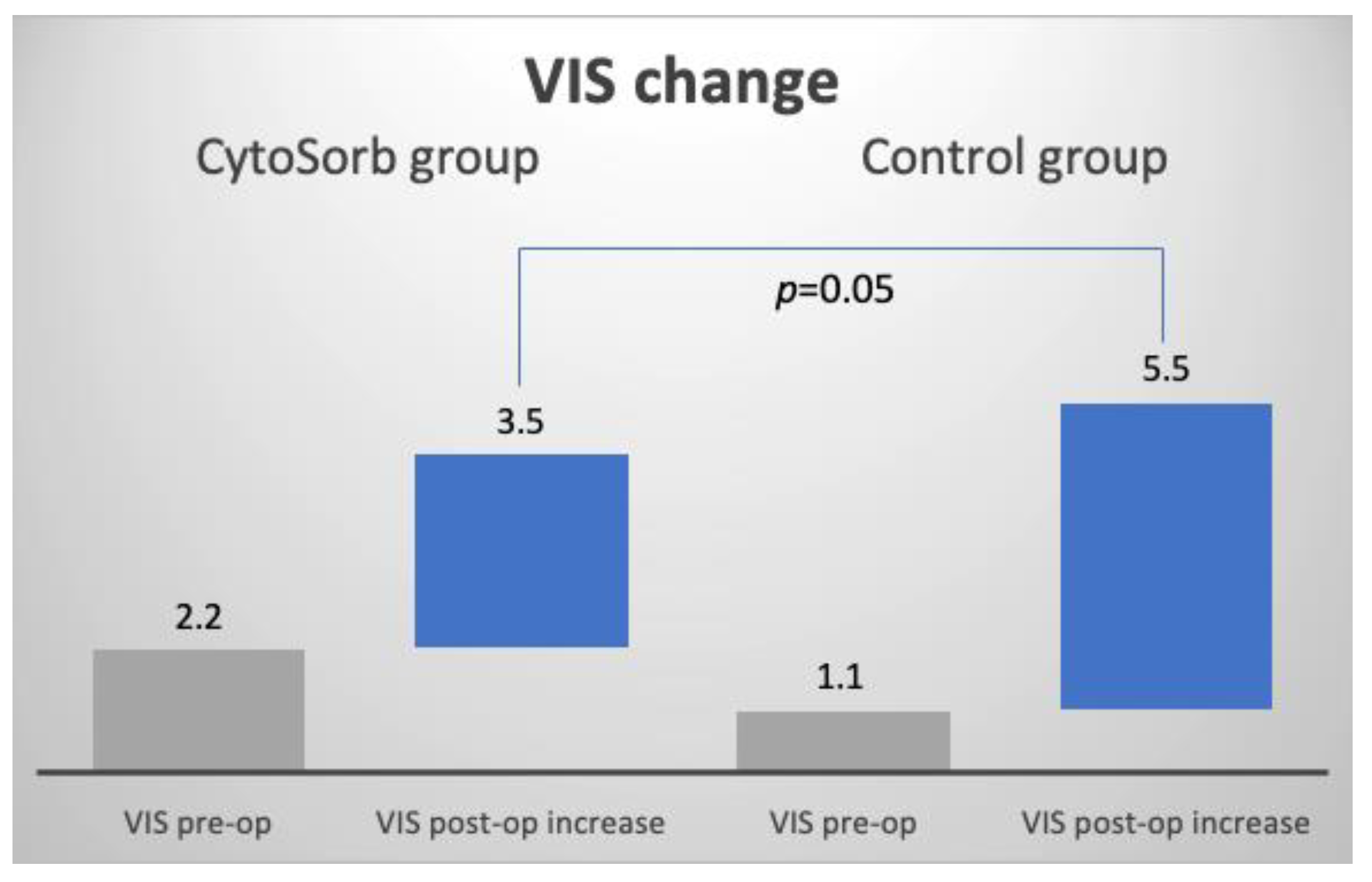
| VIS mean change 1 | 3.5 (2.2, 4.8) | 5.5 (3.6, 7.4) | 2 (−0.4, 4.4) | 0.05 |
| Median [IQR], Mean (CI), or Proportion % (n) | ||||
|---|---|---|---|---|
| Variable | Overall | CS Group | Control Group | p-Value |
| Mortality, % (n) | 15.7 (8) * | 9.1 (2) * | 20.7 (6) | 0.26 |
| VIS post-op | 6.2 (4.8, 7.5) | 5.7 (4.2, 7.2) | 6.6 (4.5, 8.7) | 0.53 |
LoS, days
| ||||
| 3 [2, 5] | 4 [3, 6] | 3 [2, 4] | 0.28 | |
| 2.5 [1, 4] | 3 [2, 4] | 2 [1, 3] | 0.14 | |
| 6 [5, 8] | 6 [5, 8] | 6 [5, 8] | 0.74 | |
| CRRT post-op, % (n) | 9.6 (5) | 8.7 (2) | 10.3 (3) | 0.84 |
| Mechanical ventilation post-op, % (n) | 96.2 (50) | 95.7 (22) | 96.6 (28) | 0.87 |
| Duration of mechanical ventilation, days | 1 [1, 2] | 1 [1, 2] | 1 [1, 2] | 0.83 |
Lactates post-op
| ||||
| 2.3 [1.6, 3.1] | 1.8 [1.3, 3.1] | 2.7 [−3.1, 1.1] | 0.13 | |
| 3.5 [2.0, 6.0] | 3.8 [2.5, 7.8] | 3.2 [1.9, 5.8] | 0.24 | |
| 2.3 [1.4, 4.6] | 2.9 [1.9, 4.6] | 1.8 [1.4, 3.0] | 0.15 | |
| 1.8 [1.3, 3.7] | 1.9 [1.4, 3.2] | 1.6 [1.1, 3.8] | 0.52 | |
Total blood loss, mL
| ||||
| 759 [591, 1181] | 903 [647, 1200] | 715 [445, 1089] | 0.08 | |
| 570 [400, 920] | 700 [460, 990] | 510 [400, 640] | 0.12 | |
| 1512 [1046, 2019] | 1475 [998, 1941] | 1677 [1115, 2337] | 0.23 | |
Blood product usage, units
| ||||
| 4 [3, 5] | 4 [3, 6] | 4 [2, 5] | 0.26 | |
| 0 [0, 4] | 0 [0, 3] | 1 [0, 4] | 0.38 | |
| 0 [0, 0] | 0 [0, 0] | 0 [0, 4] | 0.48 | |
| 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0.28 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manohar, M.; Jawali, V.; Neginahal, S.; GT, S.; Muniraj, G.; Chakravarthy, M. Hemoadsorption in Complex Cardiac Surgery—A Single Center Experience. J. Clin. Med. 2022, 11, 7005. https://doi.org/10.3390/jcm11237005
Manohar M, Jawali V, Neginahal S, GT S, Muniraj G, Chakravarthy M. Hemoadsorption in Complex Cardiac Surgery—A Single Center Experience. Journal of Clinical Medicine. 2022; 11(23):7005. https://doi.org/10.3390/jcm11237005
Chicago/Turabian StyleManohar, Murali, Vivek Jawali, Siddu Neginahal, Sudarshan GT, Geetha Muniraj, and Murali Chakravarthy. 2022. "Hemoadsorption in Complex Cardiac Surgery—A Single Center Experience" Journal of Clinical Medicine 11, no. 23: 7005. https://doi.org/10.3390/jcm11237005
APA StyleManohar, M., Jawali, V., Neginahal, S., GT, S., Muniraj, G., & Chakravarthy, M. (2022). Hemoadsorption in Complex Cardiac Surgery—A Single Center Experience. Journal of Clinical Medicine, 11(23), 7005. https://doi.org/10.3390/jcm11237005







