Intraoperative Hemoadsorption (Cytosorb™) during Open Thoracoabdominal Aortic Repair: A Pilot Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgery
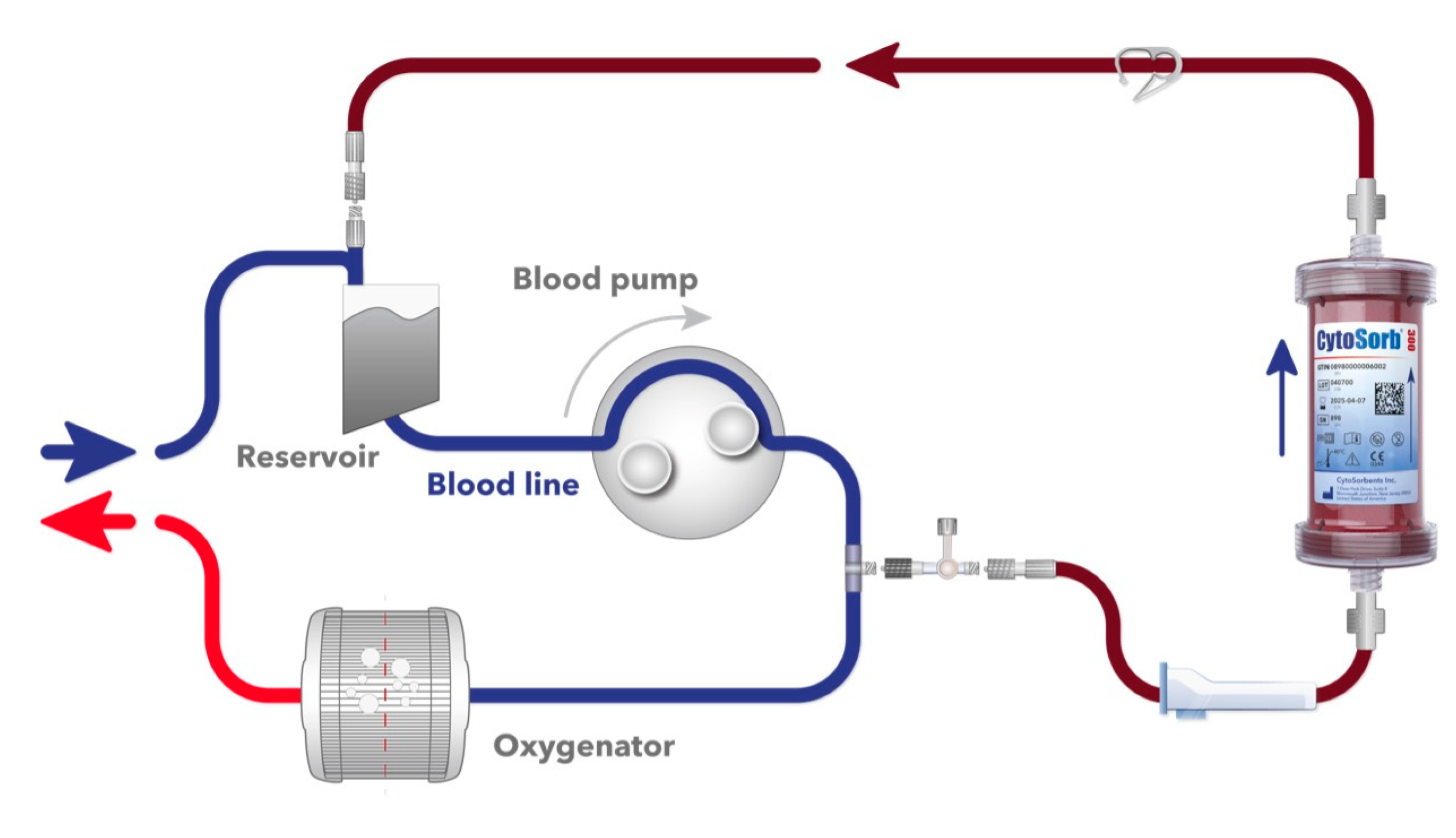
2.3. Device
2.4. Endpoint Definition
2.5. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Operative and Postoperative Outcomes
4. Discussion
5. Perspectives
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riambau, V.; Böckler, D.; Brunkwall, J.; Cao, P.; Chiesa, R.; Coppi, G.; Czerny, M.; Fraedrich, G.; Haulon, S.; Jacobs, M.J.; et al. Editor’s Choice–Management of Descending Thoracic Aorta Diseases: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2017, 53, 4–52. [Google Scholar] [CrossRef]
- Gombert, A.; Frankort, J.; Keszei, A.; Müller, O.; Benning, J.; Kotelis, D.; Jacobs, M.J. Outcome of Elective and Emergency Open Thoraco-Abdominal Aortic Aneurysm Repair in 255 Cases: A Retrospective Single Centre Study. Eur. J. Vasc. Endovasc. Surg. 2022, 63, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Coselli, J.S.; LeMaire, S.A.; Preventza, O.; de la Cruz, K.I.; Cooley, D.A.; Price, M.D.; Stolz, A.P.; Green, S.Y.; Arredondo, C.N.; Rosengart, T.K. Outcomes of 3309 thoracoabdominal aortic aneurysm repairs. J. Thorac. Cardiovasc. Surg. 2016, 151, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.-F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Millar, J.E.; Fanning, J.P.; McDonald, C.I.; McAuley, D.F.; Fraser, J.F. The inflammatory response to extracorporeal membrane oxygenation (ECMO): A review of the pathophysiology. Crit. Care 2016, 20, 387. [Google Scholar] [CrossRef]
- Kalder, J.; Keschenau, P.; Hanssen, S.J.; Greiner, A.; Vermeulen Windsant, I.C.; Kennes, L.N.; Tolba, R.; Prinzen, F.W.; Buurman, W.A.; Jacobs, M.J.; et al. The impact of selective visceral perfusion on intestinal macrohemodynamics and microhemodynamics in a porcine model of thoracic aortic cross-clamping. J. Vasc. Surg. 2012, 56, 149–158. [Google Scholar] [CrossRef]
- Hanssen, S.J.; Derikx, J.P.; Vermeulen Windsant, I.C.; Heijmans, J.H.; Koeppel, T.A.; Schurink, G.W.; Buurman, W.A.; Jacobs, M.J. Visceral injury and systemic inflammation in patients undergoing extracorporeal circulation during aortic surgery. Ann. Surg. 2008, 248, 117–125. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis—Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Inflammation and coagulation. Crit. Care Med. 2010, 38, S26–S34. [Google Scholar] [CrossRef]
- MEDURI, G.U.; YATES, C.R. Systemic Inflammation-Associated Glucocorticoid Resistance and Outcome of ARDS. Ann. New York Acad. Sci. 2004, 1024, 24–53. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Gruda, M.C.; Ruggeberg, K.G.; O’Sullivan, P.; Guliashvili, T.; Scheirer, A.R.; Golobish, T.D.; Capponi, V.J.; Chan, P.P. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb(R) sorbent porous polymer beads. PLoS ONE 2018, 13, e0191676. [Google Scholar] [CrossRef] [PubMed]
- Boss, K.; Jahn, M.; Wendt, D.; Haidari, Z.; Demircioglu, E.; Thielmann, M.; Ruhparwar, A.; Kribben, A.; Tyczynski, B. Extracorporeal cytokine adsorption: Significant reduction of catecholamine requirement in patients with AKI and septic shock after cardiac surgery. PLoS ONE 2021, 16, e0246299. [Google Scholar] [CrossRef]
- Diab, M.; Lehmann, T.; Bothe, W.; Akhyari, P.; Platzer, S.; Wendt, D.; Deppe, A.C.; Strauch, J.; Hagel, S.; Gunther, A.; et al. Cytokine Hemoadsorption During Cardiac Surgery Versus Standard Surgical Care for Infective Endocarditis (REMOVE): Results From a Multicenter Randomized Control. Trial. Circulation 2022, 145, 959–968. [Google Scholar] [CrossRef]
- Mommertz, G.; Sigala, F.; Langer, S.; Koeppel, T.A.; Mess, W.H.; Schurink, G.W.; Jacobs, M.J. Thoracoabdominal aortic aneurysm repair in patients with marfan syndrome. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.J.; Elenbaas, T.W.; Schurink, G.W.; Mess, W.H.; Mochtar, B. Assessment of spinal cord integrity during thoracoabdominal aortic aneurysm repair. Ann. Thorac. Surg. 2002, 74, S1864–S1866; discussion S1892–S1868. [Google Scholar] [CrossRef]
- Song, J.; Cho, H.; Park, D.W.; Moon, S.; Kim, J.Y.; Ahn, S.; Lee, S.G.; Park, J. Vasoactive-Inotropic Score as an Early Predictor of Mortality in Adult Patients with Sepsis. J. Clin. Med. 2021, 10, 495. [Google Scholar] [CrossRef]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E.; Winearls, C.G. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Bosco, E.; Hsueh, L.; McConeghy, K.W.; Gravenstein, S.; Saade, E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: A systematic review. BMC Med. Res. Methodol. 2021, 21, 241. [Google Scholar] [CrossRef]
- Ards Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.; McGinlay, M.; Amin, R.; Burns, K.E.; Connolly, B.; Hart, N.; Jouvet, P.; Katz, S.; Leasa, D.; Mawdsley, C.; et al. Variation in Definition of Prolonged Mechanical Ventilation. Respir. Care 2017, 62, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Preventza, O.; Orozco-Sevilla, V.; Coselli, J.S. Critical care management after open thoracoabdominal aortic aneurysm repair. J. Cardiovasc. Surg. 2021, 62, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Moulakakis, K.G.; Karaolanis, G.; Antonopoulos, C.N.; Kakisis, J.; Klonaris, C.; Preventza, O.; Coselli, J.S.; Geroulakos, G. Open repair of thoracoabdominal aortic aneurysms in experienced centers. J. Vasc. Surg. 2018, 68, 634–645.e612. [Google Scholar] [CrossRef] [PubMed]
- Gombert, A.; Barbati, M.; Kotelis, D.; Simon, T.P.; Breuer, T.; Hartmann, O.; Santos, K.; Bergmann, D.; Schulte, J.; Marx, G.; et al. In-hospital mortality and organ failure after open and endovascular thoraco-abdominal aortic surgery can be predicted by increased levels of circulating dipeptidyl peptidase. Eur. J. Cardio–Thorac. Surg. 2021, 59, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Magoon, R.; Loona, M.; Kohli, J.K.; Kashav, R. Cytokine Adsorption in Cardiac Surgery: Where do we stand? Braz. J. Cardiovasc. Surg. 2020, 35, XV–XVI. [Google Scholar] [CrossRef] [PubMed]
- Heunks, L.M.; van der Hoeven, J.G. Clinical review: The ABC of weaning failure—A structured approach. Crit. Care 2010, 14, 245. [Google Scholar] [CrossRef]
- Nierhaus, A.; Morales, J.; Wendt, D.; Scheier, J.; Gutzler, D.; Jarczak, D.; Born, F.; Hagl, C.; Deliargyris, E.; Mehta, Y. Comparison of the CytoSorb((R)) 300 mL and Jafron HA380 hemoadsorption devices: An in vitro study. Minim. Invasive Ther. Allied Technol. 2022, 31, 1058–1065. [Google Scholar] [CrossRef]
- Brouwer, W.P.; Duran, S.; Kuijper, M.; Ince, C. Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: A propensity-score-weighted retrospective study. Crit. Care 2019, 23, 317. [Google Scholar] [CrossRef]
- Soltesz, A.; Molnar, Z.A.; Szakal-Toth, Z.; Tamaska, E.; Katona, H.; Fabry, S.; Csikos, G.; Berzsenyi, V.; Tamas, C.; Edes, I.F.; et al. Influence of Venoarterial Extracorporeal Membrane Oxygenation Integrated Hemoadsorption on the Early Reversal of Multiorgan and Microcirculatory Dysfunction and Outcome of Refractory Cardiogenic Shock. J. Clin. Med. 2022, 11, 6517. [Google Scholar] [CrossRef]
- Hawchar, F.; Rao, C.; Akil, A.; Mehta, Y.; Rugg, C.; Scheier, J.; Adamson, H.; Deliargyris, E.; Molnar, Z. The Potential Role of Extracorporeal Cytokine Removal in Hemodynamic Stabilization in Hyperinflammatory Shock. Biomedicines 2021, 9, 768. [Google Scholar] [CrossRef] [PubMed]
- Paul, R.; Sathe, P.; Kumar, S.; Prasad, S.; Aleem, M.; Sakhalvalkar, P. Multicentered prospective investigator initiated study to evaluate the clinical outcomes with extracorporeal cytokine adsorption device (CytoSorb(®)) in patients with sepsis and septic shock. World J. Crit. Care Med. 2021, 10, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Kalisnik, J.M.; Leiler, S.; Mamdooh, H.; Zibert, J.; Bertsch, T.; Vogt, F.A.; Bagaev, E.; Fittkau, M.; Fischlein, T. Single-Centre Retrospective Evaluation of Intraoperative Hemoadsorption in Left-Sided Acute Infective Endocarditis. J. Clin. Med. 2022, 11, 3954. [Google Scholar] [CrossRef] [PubMed]
- Haidari, Z.; Demircioglu, E.; Boss, K.; Tyczynski, B.; Thielmann, M.; Schmack, B.; Kribben, A.; Weymann, A.; El Gabry, M.; Ruhparwar, A.; et al. Intraoperative hemoadsorption in high-risk patients with infective endocarditis. PLoS ONE 2022, 17, e0266820. [Google Scholar] [CrossRef] [PubMed]
- Iscimen, R.; Cartin-Ceba, R.; Yilmaz, M.; Khan, H.; Hubmayr, R.D.; Afessa, B.; Gajic, O. Risk factors for the development of acute lung injury in patients with septic shock: An observational cohort study. Crit. Care Med. 2008, 36, 1518–1522. [Google Scholar] [CrossRef]

| Variable | HA-Group n = 10 | Control Group n = 17 | p |
|---|---|---|---|
| Demographics | |||
| Age, years | 49.7 ± 13.1 | 50.5 ± 11.1 | 0.71 |
| Gender, male | 7 (70) | 11 (64.7) | 1 |
| BMI, m2 | 23.5 ± 5.4 | 24.4 ± 4.3 | 0.69 |
| COPD | 2 (20) | 3 (17.6) | 1 |
| Systemic hypertension | 8 (80) | 13 (76.4) | 1 |
| Smoking | 4 (40) | 4 (23.5) | 0.64 |
| Prior stroke | 1 (10) | 3 (17.6) | 1 |
| Diabetes | 2 (20) | 0 (0) | 0.12 |
| Atrial fibrillation | 2 (20) | 0 (0) | 0.12 |
| Emergency | 2 (20) | 1 (5.8) | 0.53 |
| Renal insufficiency | 6 (60) | 8 (47.1) | 0.69 |
| Reoperation | 6 (60) | 11 (64.7) | 1 |
| Cardiac re-operation | 1 (10) | 5 (29.4) | 0.36 |
| Heart failure (EF < 30%) | 2 (20) | 3 (29.4) | 1 |
| Aortic aneurysm diameter | 5.8 ± 1.6 | 6.1 ± 1.2 | 0.68 |
| Aortic pathology | |||
| True aneurysm | 1 (10) | 4 (23.5) | 0.62 |
| Mycotic including rupture | 2 (20) | 2 (11.8) | 0.61 |
| Post-dissection | 4 (40) | 9 (52.9) | 0.69 |
| Dissection | 1 (10) | 0 (0) | 0.37 |
| Marfan syndrome | 1 (10) | 2 (11.8) | 1 |
| Anastomotic aneurysm | 1 (10) | 0 (0) | 0.37 |
| Variable | HA-Group n = 10 | Control Group n = 17 | p |
|---|---|---|---|
| Operative characteristics | |||
| CPB, min. | 149 ± 46 | 152 ± 46 | 0.90 |
| Total OR time, min. | 489 ± 125 | 502 ± 89 | 0.92 |
| Y-prosthesis | 2 (20) | 5 (29.4) | 0.67 |
| Mild hypothermia (down to 35 °C) | 10 (100) | 17 100) | 1 |
| Thoraco-laparotomy approach | 10 (100) | 17 (100) | 1 |
| Crawford classification | |||
| Type 1 | 0 (0) | 1 (5.8) | 1 |
| Type 2 | 5 (50) | 6 (35.3) | 0.68 |
| Type 3 | 3 (30) | 5 (29.4) | 1 |
| Type 4 | 1 (10) | 4 (23.5) | 0.62 |
| Type 5 | 1 (10) | 0 (0) | 0.37 |
| Blood products | |||
| Packed red blood cells, bags | 22.5 ± 18.3 | 19.8 ± 11.1 | 0.63 |
| Fresh-frozen plasma, bags | 30.3 ± 19.8 | 23.4 ± 10.8 | 0.25 |
| Platelet concentrate, bags | 6.6 ± 3.7 | 4.6 ± 1.8 | 0.07 |
| Postoperative outcomes | |||
| Rethoracotomy | 4 (40) | 9 (52.3) | 0.69 |
| Dialysis | 5 (50) | 12 (70.1) | 0.41 |
| Sepsis | 3 (30) | 7 (41.1) | 0.69 |
| Ventilation time, h | 88 [9–337] | 510 [14–954.5] | 0.08 |
| Prolonged ventilation | 1 (10) | 9 (52.9) | 0.03 * |
| Tracheostomy | 4 (40) | 11 (64.7) | 0.23 |
| Pneumonia | 7 (70) | 11 (64.7) | 0.79 |
| ARDS (severe) | 0 (0) | 7 (41.1) | 0.02 * |
| New stroke | 0 (0) | 5 (29.4) | 0.12 |
| Myocardial infarction | 0 (0) | 0 (0) | 1 |
| Cardiac-related death | 0 (0) | 0 (0) | 1 |
| MACE (composite) | 0 (0) | 5 (29.4) | 0.12 |
| Septic shock | 1 (10) | 4 (23.5) | 0.62 |
| Hospital stay, days | 46.4 ± 30.1 | 49.7 ± 34.6 | 0.51 |
| VISmax | 35.9 ± 45.5 | 32.1 ± 35.8 | 0.58 |
| VISmax 24 h | 42.8 ± 64 | 32.12 ± 35.8 | 0.64 |
| Liver failure | 3 (30) | 2 (11.8) | 0.32 |
| 30-day mortality | 2 (20) | 2 (11.8) | 0.61 |
| In-hospital mortality | 3 (30) | 2 (11.8) | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doukas, P.; Hellfritsch, G.; Wendt, D.; Magliani, M.; Barbati, M.E.; Jalaie, H.; Jacobs, M.J.; Gombert, A. Intraoperative Hemoadsorption (Cytosorb™) during Open Thoracoabdominal Aortic Repair: A Pilot Randomized Controlled Trial. J. Clin. Med. 2023, 12, 546. https://doi.org/10.3390/jcm12020546
Doukas P, Hellfritsch G, Wendt D, Magliani M, Barbati ME, Jalaie H, Jacobs MJ, Gombert A. Intraoperative Hemoadsorption (Cytosorb™) during Open Thoracoabdominal Aortic Repair: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(2):546. https://doi.org/10.3390/jcm12020546
Chicago/Turabian StyleDoukas, Panagiotis, Gabriel Hellfritsch, Daniel Wendt, Mirko Magliani, Mohammad E. Barbati, Houman Jalaie, Michael J. Jacobs, and Alexander Gombert. 2023. "Intraoperative Hemoadsorption (Cytosorb™) during Open Thoracoabdominal Aortic Repair: A Pilot Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 2: 546. https://doi.org/10.3390/jcm12020546
APA StyleDoukas, P., Hellfritsch, G., Wendt, D., Magliani, M., Barbati, M. E., Jalaie, H., Jacobs, M. J., & Gombert, A. (2023). Intraoperative Hemoadsorption (Cytosorb™) during Open Thoracoabdominal Aortic Repair: A Pilot Randomized Controlled Trial. Journal of Clinical Medicine, 12(2), 546. https://doi.org/10.3390/jcm12020546







