Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree
Abstract
1. Introduction
2. Materials and Methods
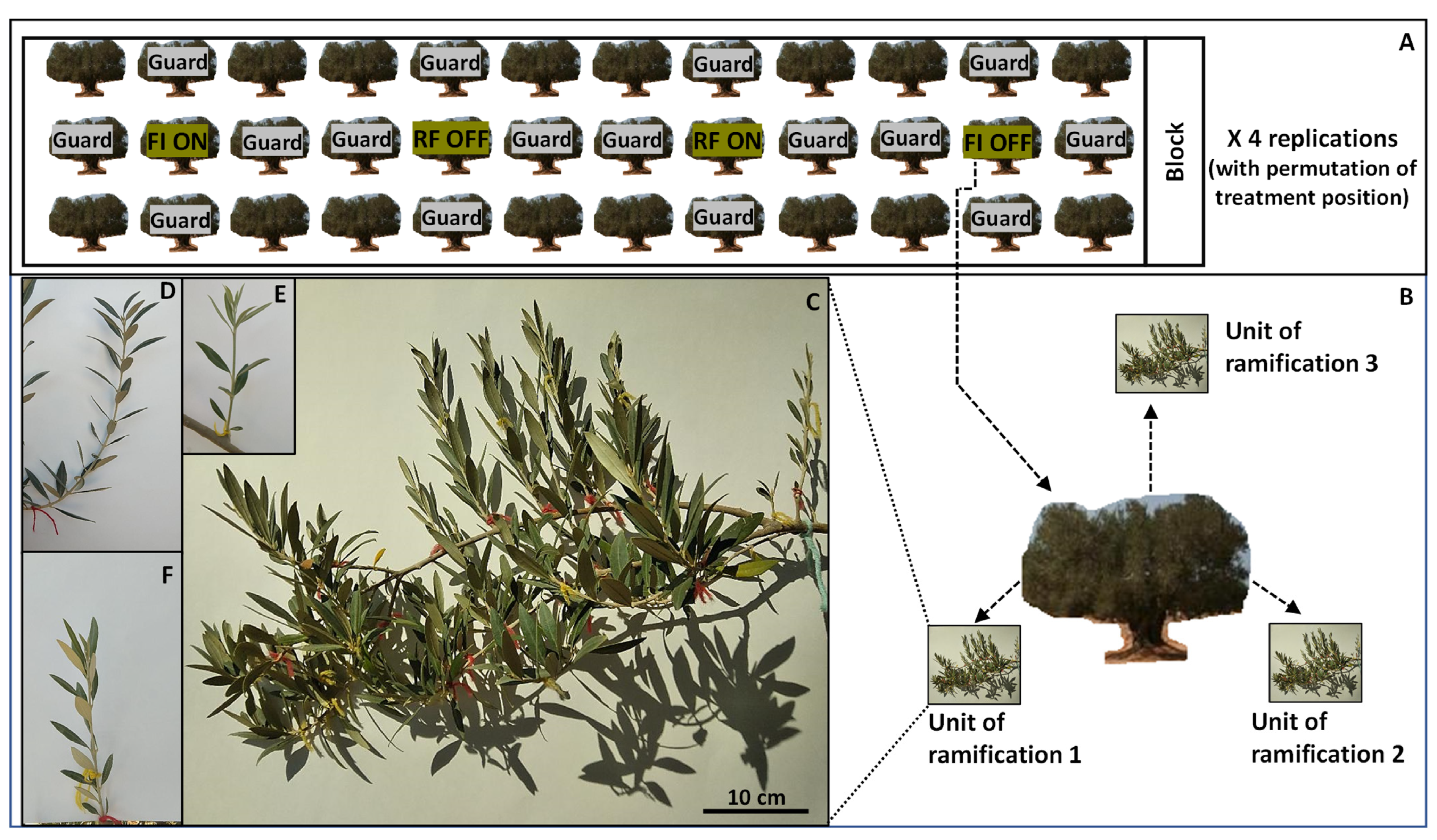
2.1. Experimental Site and Treatments
2.2. Assessed Parameters
2.3. Statistical Analysis
3. Results
3.1. Data Variability
3.2. Meteorological Data
3.3. Effect of Bearing Status and Water Treatments on the RWC
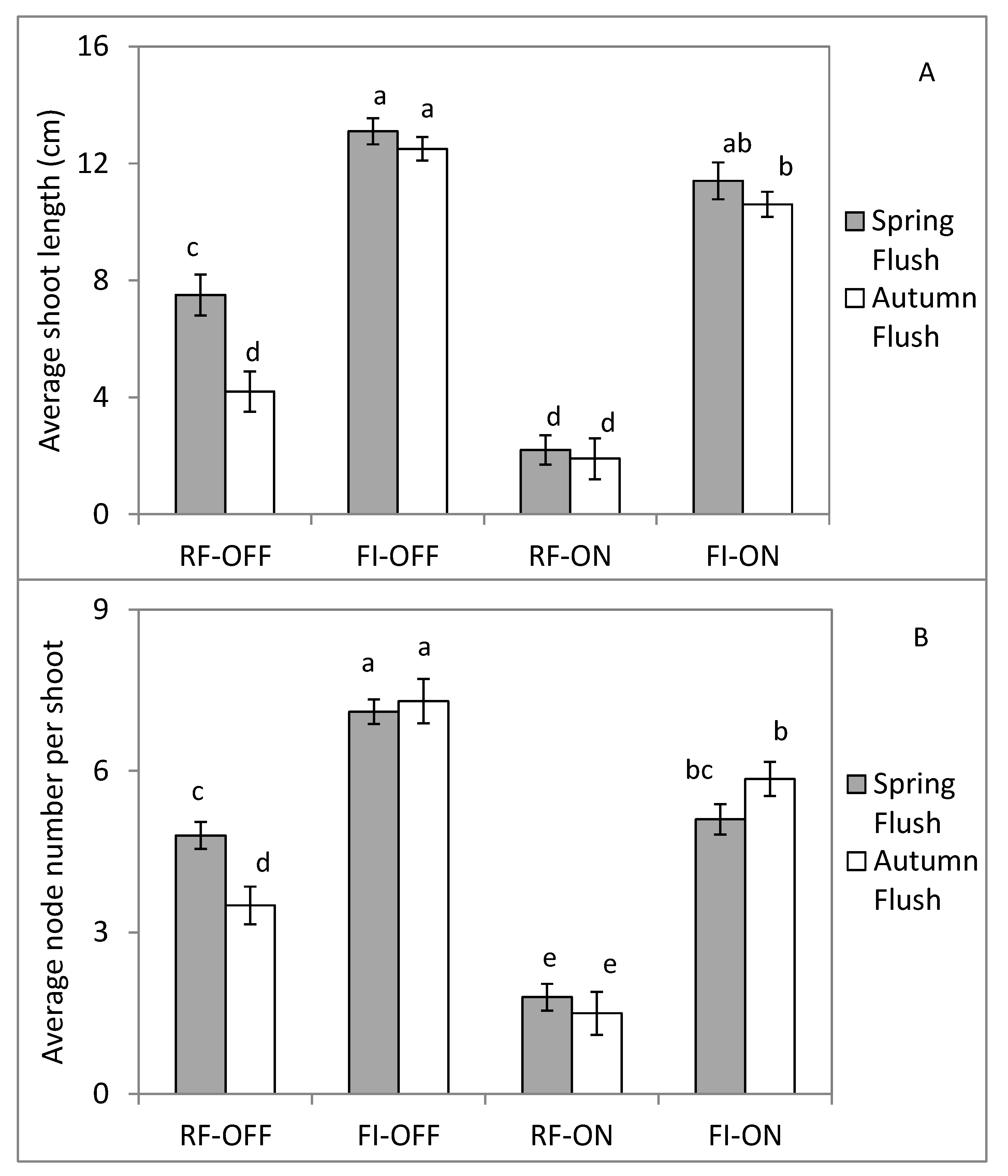
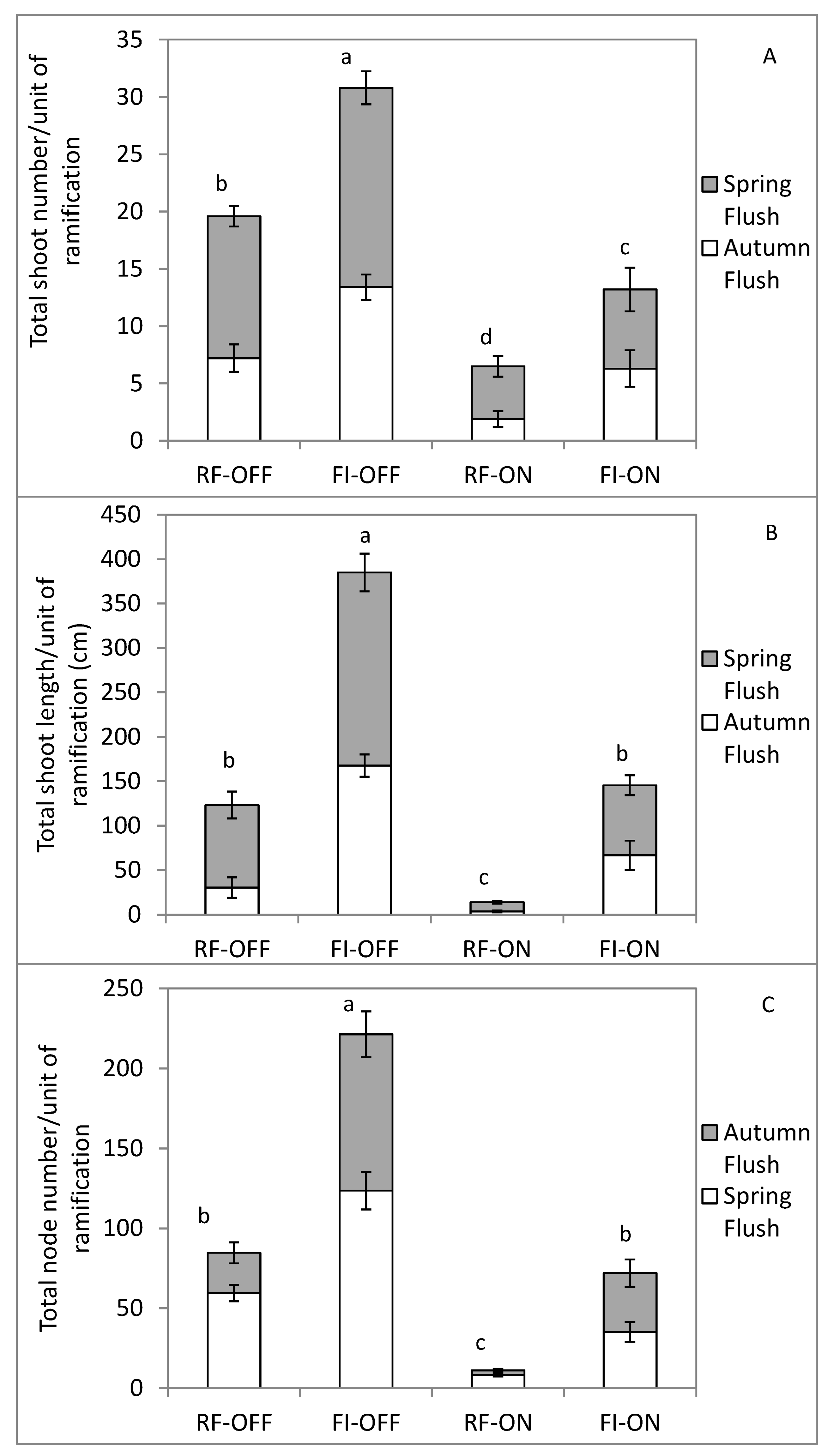
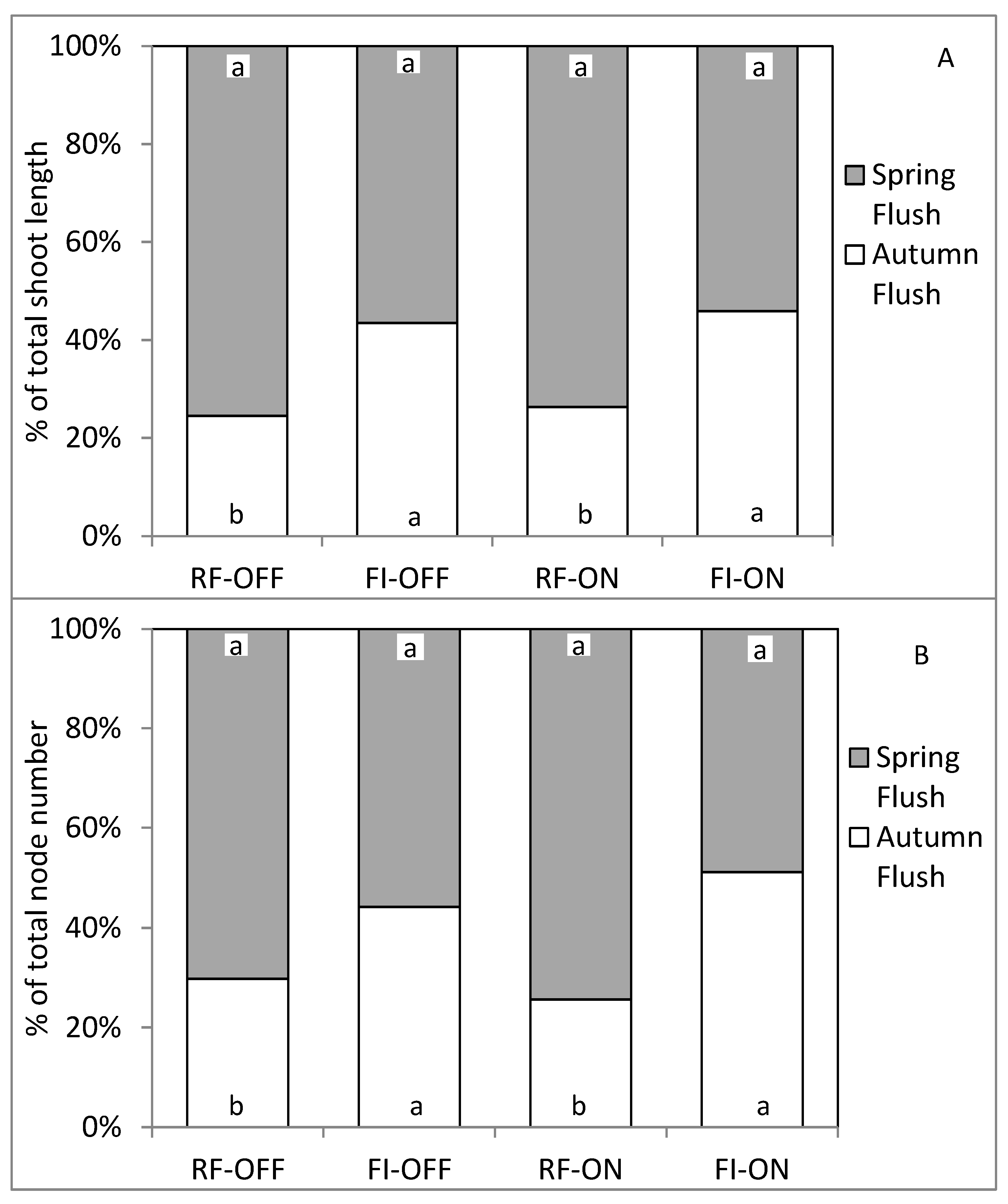
3.4. Effect of Bearing Status and Water Treatments on the Vegetative Growth Parameters
3.5. Effect of Bearing Status and Water Treatments on the Flowering Parameters of the Following Season
4. Discussion
4.1. Effect of Bearing Status and Water Supply on the Tree Water Status
4.2. Effect of Bearing Status and Water Supply on the Vegetative Growth Dynamic and Intensity
4.3. Effect of Bearing Status and Water Supply in the Productive Potential of the Following Season
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, H.M.; Samach, A. Constraints to obtaining consistent annual yields in perennial tree crops. I: Heavy fruit load dominates over vegetative growth. Plant Sci. 2013, 207, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, A.L.; Blanke, M.M.; Kunz, A.; Damerow, L.; Lakso, A.N.; Meland, M. Alternate bearing in fruit tree crops: Past, present and future. In International Symposium on Physiological Principles and Their Application to Fruit Production; ISHS: Leuven, Belgium, 2014; Volume 1177, pp. 241–248. [Google Scholar]
- Sharma, N.; Singh, S.K.; Mahato, A.K.; Ravishankar, H.; Dubey, A.K.; Singh, N.K. Physiological and molecular basis of alternate bearing in perennial fruit crops. Sci. Hortic. 2018, 243, 214–225. [Google Scholar] [CrossRef]
- Goldschmidt, E.E.; Sadka, A. Yield Alternation: Horticulture, Physiology, Molecular Biology, and Evolution. Hortic. Rev. 2021, 48, 363–418. [Google Scholar]
- Rallo, L.; Cuevas, J. Fruiting and Production. In Olive Growing; Barranco, D., Fernández-Escobar, R., Rallo, L., Eds.; RIRDC: Wagga Wagga, Australia, 2010; pp. 113–145. [Google Scholar]
- Castillo-Llanque, F.; Rapoport, H.F. Relationship between reproductive behavior and new shoot development in 5-year-old branches of olive trees (Olea europaea L.). Trees 2011, 25, 823–832. [Google Scholar] [CrossRef]
- Turktas, M.; Inal, B.; Okay, S.; Erkilic, E.G.; Dundar, E.; Hernandez, P.; Dorado, G.; Unver, T. Nutrition Metabolism Plays an Important Role in the Alternate Bearing of the Olive Tree (Olea europaea L.). PLoS ONE 2013, 8, e59876. [Google Scholar] [CrossRef]
- Arji, I. Determining of Growth and Yield Performance in Some Olive Cultivars in Warm Conditions. In Biological Forum; Research Trend: Dewas, India, 2015; Volume 7, p. 1865. [Google Scholar]
- Ramos, A.; Rapoport, H.; Cabello, D.; Rallo, L. Chilling accumulation, dormancy release temperature, and the role of leaves in olive reproductive budburst: Evaluation using shoot explants. Sci. Hortic. 2018, 231, 241–252. [Google Scholar] [CrossRef]
- Wahbi, S.; Wakrim, R.; Aganchich, B.; Tahi, H.; Serraj, R. Effects of partial rootzone drying (PRD) on adult olive tree (Olea europaea) in field conditions under arid climate: I. Physiological and agronomic responses. Agric. Ecosyst. Environ. 2005, 106, 289–301. [Google Scholar] [CrossRef]
- Iniesta, F.; Testi, L.; Orgaz, F.; Villalobos, F. The effects of regulated and continuous deficit irrigation on the water use, growth and yield of olive trees. Eur. J. Agron. 2009, 30, 258–265. [Google Scholar] [CrossRef]
- Martín-Vertedor, A.I.; Rodríguez, J.M.P.; Losada, H.P.; Castiel, E.F. Interactive responses to water deficits and crop load in olive (Olea europaea L., cv. Morisca) I.—Growth and water relations. Agric. Water Manag. 2011, 98, 941–949. [Google Scholar] [CrossRef][Green Version]
- Mezghani, M.A.; Charfi, C.M.; Gouiaa, M.; Labidi, F. Vegetative and reproductive behaviour of some olive tree varieties (Olea europaea L.) under deficit irrigation regimes in semi-arid conditions of Central Tunisia. Sci. Hortic. 2012, 146, 143–152. [Google Scholar] [CrossRef]
- Abboud, S.; Dbara, S.; Abidi, W.; Braham, M. Differential agro-physiological responses induced by partial root-zone drying irrigation in olive cultivars grown in semi-arid conditions. Environ. Exp. Bot. 2019, 167, 103663. [Google Scholar] [CrossRef]
- Naor, A.; Schneider, D.; Ben-Gal, A.; Zipori, I.; Dag, A.; Kerem, Z.; Birger, R.; Peres, M.; Gal, Y. The effects of crop load and irrigation rate in the oil accumulation stage on oil yield and water relations of ‘Koroneiki’ olives. Irrig. Sci. 2012, 31, 781–791. [Google Scholar] [CrossRef]
- Bustan, A.; Dag, A.; Yermiyahu, U.; Erel, R.; Presnov, E.; Agam, N.; Kool, D.; Iwema, J.; Zipori, I.; Ben-Gal, A. Fruit load governs transpiration of olive trees. Tree Physiol. 2016, 36, 380–391. [Google Scholar] [CrossRef] [PubMed]
- Siakou, M.; Bruggeman, A.; Eliades, M.; Djuma, H.; Kyriacou, M.C.; Moriana, A. Phenology, Morphology and Physiology Responses of Deficit Irrigated ‘Koroneiki’ Olive Trees as Affected by Environmental Conditions and Alternate Bearing. Agronomy 2022, 12, 879. [Google Scholar] [CrossRef]
- Grattan, S.; Berenguer, M.; Connell, J.; Polito, V.; Vossen, P. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Gucci, R.; Lodolini, E.; Rapoport, H. Productivity of olive trees with different water status and crop load. J. Hortic. Sci. Biotechnol. 2007, 82, 648–656. [Google Scholar] [CrossRef]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The effects of drought on the water use, fruit development and oil yield from young olive trees. Agric. Water Manag. 2009, 96, 1525–1531. [Google Scholar] [CrossRef]
- Palese, A.M.; Nuzzo, V.; Favati, F.; Pietrafesa, A.; Celano, G.; Xiloyannis, C. Effects of water deficit on the vegetative response, yield and oil quality of olive trees (Olea europaea L., cv Coratina) grown under intensive cultivation. Sci. Hortic. 2010, 125, 222–229. [Google Scholar] [CrossRef]
- Moriana, A.; Perez-Lopez, D.; Prieto, M.H.; Ramírez-Santa-Pau, M.; Pérez Rodríguez, J.M. Midday stem water potential as a useful tool for estimating irrigation requirements in olive trees. Agric. Water Manag. 2012, 112, 43–54. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Calderón, F.J.; Contreras-Zanessi, O.; Galarza, W.; Banco, A.P.; Puertas, C.M. Effect of regulated deficit irrigation during the vegetative growth period on shoot elongation and oil yield components in olive hedgerows (cv. Arbosana) pruned annually on alternate sides in San Juan, Argentina. Irrig. Sci. 2019, 37, 533–546. [Google Scholar] [CrossRef]
- Masmoudi-Charfi, C.; Habaieb, H. Rainfall Distribution Functions for Irrigation Scheduling: Calculation Procedures Following Site of Olive (Olea europaea L.) Cultivation and Growing Periods. Am. J. Plant Sci. 2014, 5, 2094–2133. [Google Scholar] [CrossRef][Green Version]
- Field, C.B.; Barros, V.R. (Eds.) Climate Change 2014-Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sonwa, D.J.; Dieye, A.; El Mzouri, E.-H.; Majule, A.; Mugabe, F.T.; Omolo, N.; Wouapi, H.; Obando, J.; Brooks, N. Drivers of climate risk in African agriculture. Clim. Dev. 2017, 9, 383–398. [Google Scholar] [CrossRef]
- Mukherjee, S.; Mishra, A.; Trenberth, K.E. Climate Change and Drought: A Perspective on Drought Indices. Curr. Clim. Chang. Rep. 2018, 4, 145–163. [Google Scholar] [CrossRef]
- Shukla, P.R.; Skeg, J.; Buendia, E.C.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, S.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; Philpapers: London, UK, 2019. [Google Scholar]
- Arampatzis, G.; Hatzigiannakis, E.; Pisinaras, V.; Kourgialas, N.; Psarras, G.; Kinigopoulou, V.; Panagopoulos, A.; Koubouris, G. Soil water content and olive tree yield responses to soil management, irrigation, and precipitation in a hilly Mediterranean area. J. Water Clim. Chang. 2018, 9, 672–678. [Google Scholar] [CrossRef]
- Fraga, H.; Pinto, J.G.; Santos, J.A. Olive tree irrigation as a climate change adaptation measure in Alentejo, Portugal. Agric. Water Manag. 2020, 237, 106193. [Google Scholar] [CrossRef]
- Pérez, D.; Ribas, F.; Olmedilla, J.N. Influence of irrigation on a traditional rainfed olive orchard (cv Cornicabra). Options Méditerranéennes 2004, 60, 85–89. [Google Scholar]
- Hasegawa, S.; Takeda, H. Functional specialization of current shoots as a reproductive strategy in Japanese alder (Alnus hirsuta var. sibirica). Can. J. Bot. 2001, 79, 38–48. [Google Scholar]
- Suzuki, A. Patterns of vegetative growth and reproduction in relation to branch orders: The plant as a spatially structured population. Trees 2000, 14, 329–333. [Google Scholar] [CrossRef]
- Allen, R.G. Crop Evapotranspiration-Guideline for computing crop water requirements. Irrig. Drain 1998, 56, 300. [Google Scholar]
- Fereres, E.; Goldhamer, D.A.; Sadras, V.O. Yield response to water of fruit trees and vines: Guidelines. FAO Irrig. Drain. Pap. 2012, 66, 246–497. [Google Scholar]
- Romero-Trigueros, C.; Vivaldi, G.A.; Nicolás, E.N.; Paduano, A.; Salcedo, F.P.; Camposeo, S. Ripening indices, olive yield and oil quality in response to irrigation with saline reclaimed water and deficit strategies. Front. Plant Sci. 2019, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Jabloun, M.D.; Sahli, A. Evaluation of FAO-56 methodology for estimating reference evapotranspiration using limited climatic data: Application to Tunisia. Agric. Water Manag. 2008, 95, 707–715. [Google Scholar] [CrossRef]
- Trentacoste, E.R.; Puertas, C.M.; Sadras, V.O. Effect of irrigation and tree density on vegetative growth, oil yield and water use efficiency in young olive orchard under arid conditions in Mendoza, Argentina. Irrig. Sci. 2015, 33, 429–440. [Google Scholar] [CrossRef]
- Pietragalla, J.; Mullan, D. Leaf Relative Water Content. In Physiological Breeding II: A Field Guide to Wheat Genotyping; CIMMYT: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Rapoport, H.; Hammami, S.B.; Martins, P.; Pérez-Priego, O.; Orgaz, F. Influence of water deficits at different times during olive tree inflorescence and flower development. Environ. Exp. Bot. 2012, 77, 227–233. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl Jasmonate-Induced Alteration in Lipid Peroxidation, Antioxidative Defence System and Yield in Soybean Under Drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Torres, I.; Sanchez, M.T.; Benlloch-Gonzalez, M.; Perez-Marin, D. Irrigation decision support based on leaf relative water content determination in olive grove using near infrared spectroscopy. Biosyst. Eng. 2019, 180, 50–58. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, B.; Boujnah, D.; Zarrouk, M. Seasonal changes in water relations and gas exchange in leaves of two Tunisian olive (Olea europaea L.) cultivars under water deficit. J. Hortic. Sci. Biotechnol. 2007, 82, 721–726. [Google Scholar] [CrossRef]
- Rouina, B.B.; Trigui, A.; d’Andria, R.; Boukhris, M.; Chaieb, M. Effects of water stress and soil type on photosynthesis, leaf water potential and yield of olive trees (Olea europaea L. cv. Chemlali Sfax). Aust. J. Exp. Agric. 2007, 47, 1484–1490. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Chaïbi, W.; Zarrouk, M. Impacts of water stress on gas exchange, water relations, chlorophyll content and leaf structure in the two main Tunisian olive (Olea europaea L.) cultivars. Sci. Hortic. 2009, 119, 257–263. [Google Scholar] [CrossRef]
- Tognetti, R.; d’Andria, R.; Morelli, G.; Calandrelli, D.; Fragnito, F. Irrigation effects on daily and seasonal variations of trunk sap flow and leaf water relations in olive trees. Plant Soil 2004, 263, 249–264. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Hammami, S.B.M.; Costagli, G.; Rapoport, H.F. Cell and tissue dynamics of olive endocarp sclerification vary according to water availability. Physiol. Plant. 2013, 149, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Basile, B.; Morelli, G.; D’Andria, R.; Tonutti, P. Effects of irrigation on fruit ripening behavior and metabolic changes in olive. Sci. Hortic. 2012, 144, 201–207. [Google Scholar] [CrossRef]
- Ben-Gal, A.; Yermiyahu, U.; Zipori, I.; Presnov, E.; Hanoch, E.; Dag, A. The influence of bearing cycles on olive oil production response to irrigation. Irrig. Sci. 2010, 29, 253–263. [Google Scholar] [CrossRef]
- Hammami, S.B.; de la Rosa, R.; Sghaier-Hammami, B.; León, L.; Rapoport, H.F. Reliable and relevant qualitative descriptors for evaluating complex architectural traits in olive progenies. Sci. Hortic. 2012, 143, 157–166. [Google Scholar] [CrossRef]
- Strippoli, G.; Vivaldi, G.A.; Camposeo, S.; Contò, F. Sprouts seasonal elongation of two olive cultivars in a high-density orchard. Agric. Sci. 2013, 04, 376–381. [Google Scholar] [CrossRef]
- Fernández, F.J.; Ladux, J.L.; Searles, P. Dynamics of shoot and fruit growth following fruit thinning in olive trees: Same season and subsequent season responses. Sci. Hortic. 2015, 192, 320–330. [Google Scholar] [CrossRef]
- Ramos, A.M.D.S. Inducción Floral y Latencia de Las Yemas Del Olivo (Olea europaea L.); ETSIAM: Córdoba, Spain, 2000. [Google Scholar]
- Lavee, S. Alternate bearing in olive initiated by abiotic induction leading to biotic responses. Adv. Hortic. Sci. 2015, 29, 213–220. [Google Scholar]
- Dag, A.; Bustan, A.; Avni, A.; Tzipori, I.; Lavee, S.; Riov, J. Timing of fruit removal affects concurrent vegetative growth and subsequent return bloom and yield in olive (Olea europaea L.). Sci. Hortic. 2010, 123, 469–472. [Google Scholar] [CrossRef]
- Fernandez-Escobar, R.; Benlloch, M.; Navarro, C.; Martin, G.C. The time of floral induction in the olive. J. Am. Soc. Hortic. Sci. 1992, 117, 304–307. [Google Scholar] [CrossRef]
- Stutte, G.W.; Martin, G.C. Effect of killing the seed on return bloom of olive. Sci. Hortic. 1986, 29, 107–113. [Google Scholar] [CrossRef]
- Gómez-del-Campo, M. Summer deficit-irrigation strategies in a hedgerow olive orchard cv.’Arbequina’: Effect on fruit characteristics and yield. Irrig. Sci. 2013, 31, 259–269. [Google Scholar] [CrossRef]
- Ulger, S.; Sonmez, S.; Karkacier, M.; Ertoy, N.; Akdesir, O.; Aksu, M. Determination of endogenous hormones, sugars and mineral nutrition levels during the induction, initiation and differentiation stage and their effects on flower formation in olive. Plant Growth Regul. 2004, 42, 89–95. [Google Scholar] [CrossRef]
- Kitsaki, C.K.; Drossopoulos, J.B. Environmental effect on ABA concentration and water potential in olive leaves (Olea europaea L. cv “Koroneiki”) under non-irrigated field conditions. Environ. Exp. Bot. 2005, 54, 77–89. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.M.; Diaz-Espejo, A.; Perez-Martin, A.; Hernandez-Santana, V. Role of hydraulic and chemical signals in leaves, stems and roots in the stomatal behaviour of olive trees under water stress and recovery conditions. Tree Physiol. 2015, 35, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Proietti, P. Changes in photosynthesis and fruit characteristics in olive in response to assimilate availability. Photosynthetica 2003, 41, 559–564. [Google Scholar] [CrossRef]
- Tombesi, A. Biologia fiorale e di fruttificazione. Olea. Trattato Di Olivic. 2003, 35–55. [Google Scholar]
- Cherbiy-Hoffmann, S.U.; Hall, A.J.; Searles, P.S.; Rousseaux, M.C. Responses of olive tree yield determinants and components to shading during potentially critical phenological phases. Sci. Hortic. 2015, 184, 70–77. [Google Scholar] [CrossRef]
- Hueso, A.; Trentacoste, E.R.; Ruiz, C.; Meng, L.; Pérez-Pastor, A.; De la Rosa, J.M.; Gómez-del-Campo, M. Effect of deficit irrigation during the oil synthesis period on carbohydrate content in olive ‘Arbequina’ hedgerows. Acta Hortic. 2018, 1199, 75–80. [Google Scholar] [CrossRef]
- Fabbri, A.; Benelli, C. Review Article Flower bud induction and differentiation in olive. J. Hortic. Sci. Biotechnol. 2000, 75, 131–141. [Google Scholar] [CrossRef]
- Andreini, L.; Bartolini, S.; Guivarch, A.; Chriqui, D.; Vitagliano, C. Histological and immunohistochemical studies on flower induction in the olive tree (Olea europaea L.). Plant Biol. 2008, 10, 588–595. [Google Scholar] [CrossRef] [PubMed]


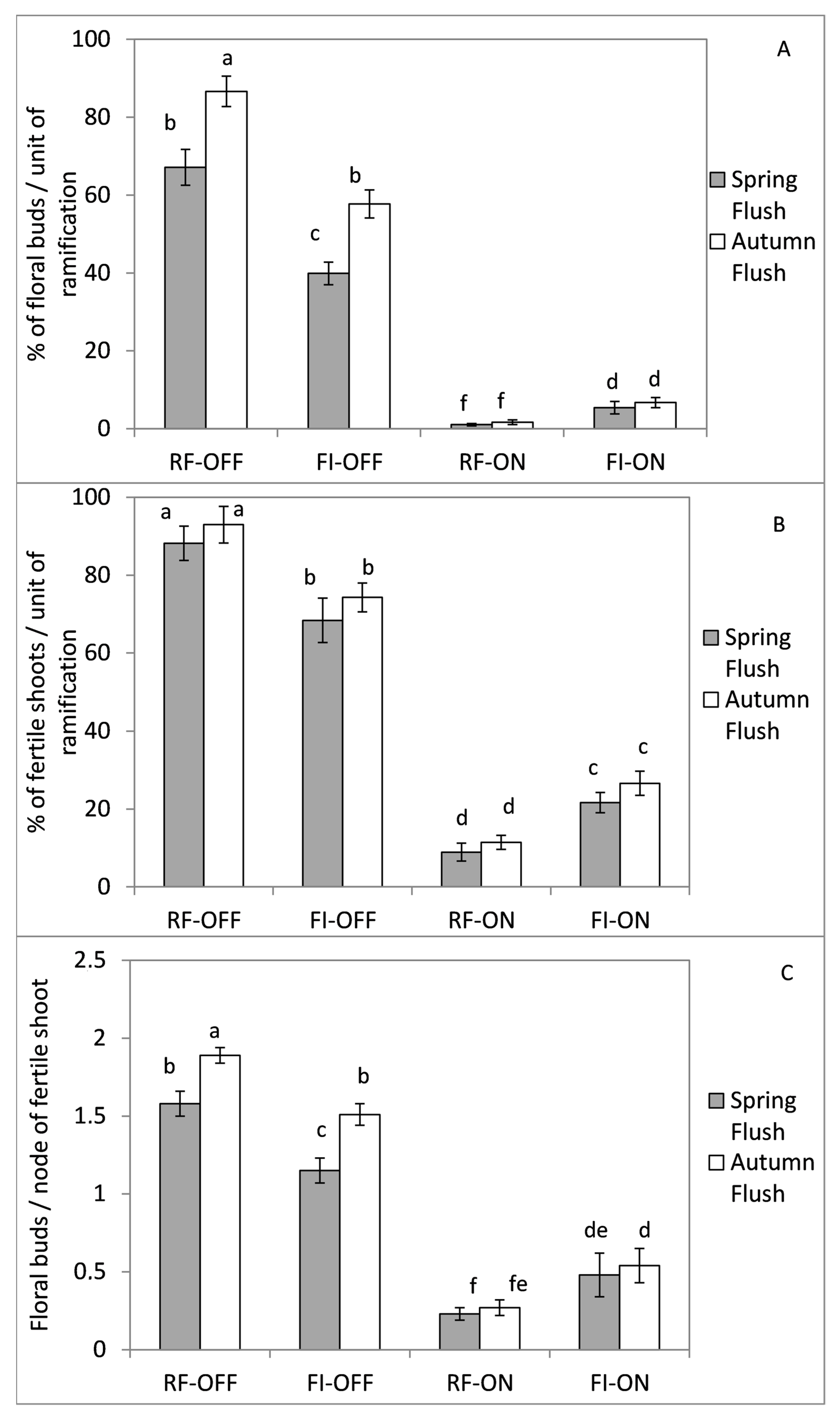
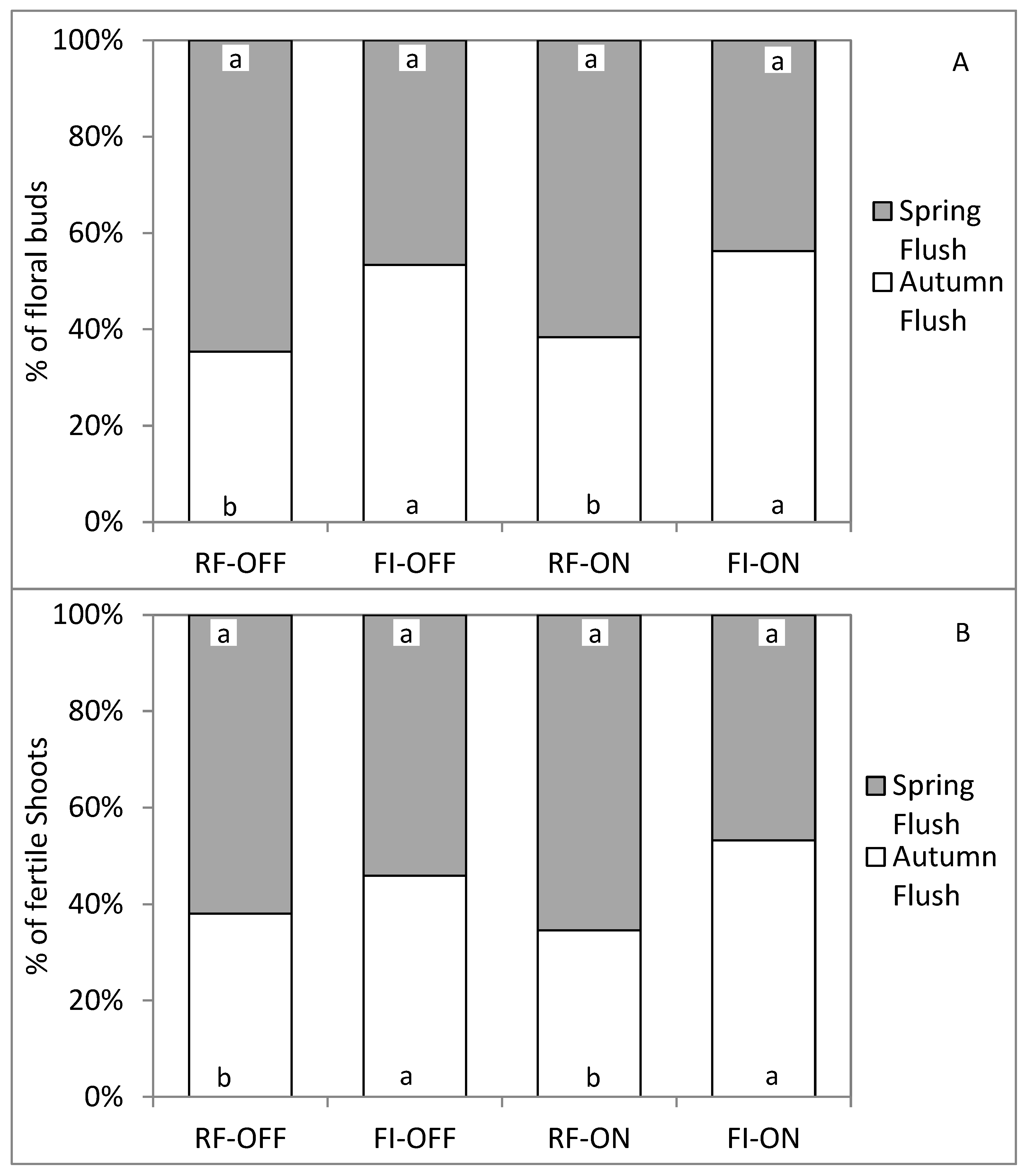
| Parameter | SS of BS (%) | SS of WR (%) | % SS of BS∗WR | SS of Error (%) |
|---|---|---|---|---|
| RWC | 1 n.s. a | 55 *** | 1 n.s. a | 43 |
| Average shoot length | 6 *** | 44 *** | 2 * | 48 |
| Average node number per shoot | 12 *** | 34 *** | 2 * | 52 |
| Shoot number per unit of ramifi. | 26 *** | 8 ** | 0 n.s. | 66 |
| Total shoot length per unit of ramifi. | 20 *** | 24 *** | 1 n.s. | 55 |
| Total node number per unit of ramifi. | 26 ** | 21 *** | 1 n.s. | 52 |
| Total number of floral buds per unit of ramifi. | 53 *** | 10 *** | 6 ** | 31 |
| Number of fertile shoots per unit of ramifi. | 61 *** | 7 ** | 5 * | 27 |
| % of floral buds per unit of ramifi. | 71 *** | 7 ** | 7 ** | 15 |
| % of fertile shoots per unit of ramifi. | 74 *** | 6 ** | 8 ** | 12 |
| Number of floral buds per node of fertile shoot | 72 *** | 6 ** | 7 ** | 15 |
| Month | Rainfall (mm) | ET0 (mm) | Irrigation (mm) | |
|---|---|---|---|---|
| Rainfed | Fully Irrigated | |||
| January | 18.7 | 46.5 | 0 | 0 |
| February | 24.7 | 41.9 | 0 | 0 |
| March | 10.8 | 84.1 | 0 | 15.9 |
| April | 12.4 | 114.8 | 0 | 24.8 |
| May | 8.3 | 145.7 | 0 | 41.4 |
| June | 0.0 | 177.8 | 0 | 53.3 |
| July | 0.0 | 207.2 | 0 | 62.2 |
| August | 12.9 | 189.5 | 0 | 39.6 |
| September | 31.4 | 170.4 | 0 | 19.4 |
| October | 10.8 | 116.2 | 0 | 27.4 |
| November | 82.3 | 65.8 | 0 | 0 |
| December | 4.3 | 54.2 | 0 | 0 |
| Total | 216.6 | 1414.1 | 0 | 284 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammami, S.B.M.; Ben Laya, M.; Baazaoui, N.; Sghaier-Hammami, B. Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree. Sustainability 2022, 14, 15614. https://doi.org/10.3390/su142315614
Hammami SBM, Ben Laya M, Baazaoui N, Sghaier-Hammami B. Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree. Sustainability. 2022; 14(23):15614. https://doi.org/10.3390/su142315614
Chicago/Turabian StyleHammami, Sofiene B. M., Manel Ben Laya, Narjes Baazaoui, and Besma Sghaier-Hammami. 2022. "Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree" Sustainability 14, no. 23: 15614. https://doi.org/10.3390/su142315614
APA StyleHammami, S. B. M., Ben Laya, M., Baazaoui, N., & Sghaier-Hammami, B. (2022). Vegetative Growth Dynamic and Its Impact on the Flowering Intensity of the Following Season Depend on Water Availability and Bearing Status of the Olive Tree. Sustainability, 14(23), 15614. https://doi.org/10.3390/su142315614






