Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-𝜅B and mTOR Pathways
Abstract
1. Introduction
2. Results
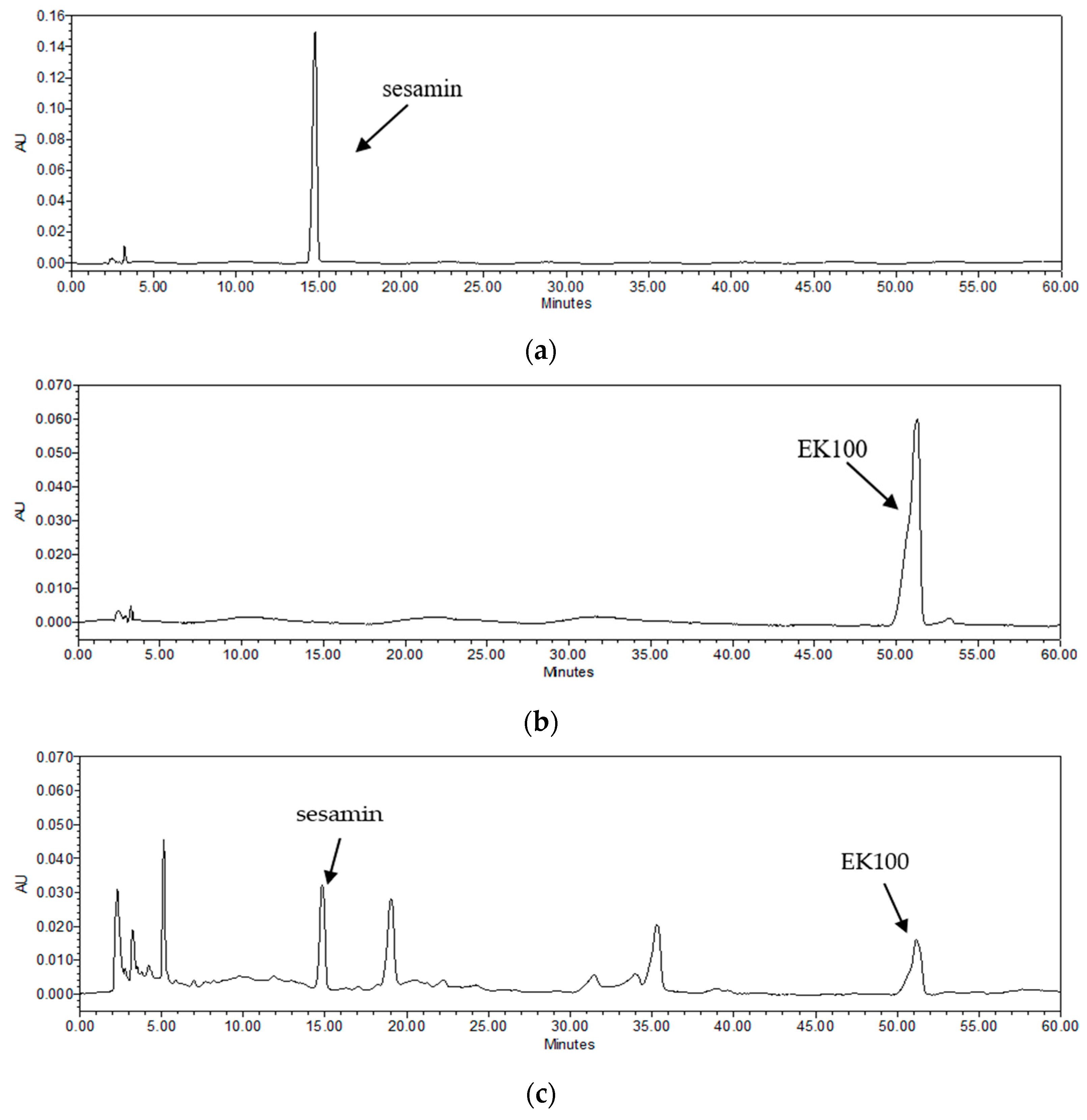
2.1. Quantitation of Bioactive Components in TC
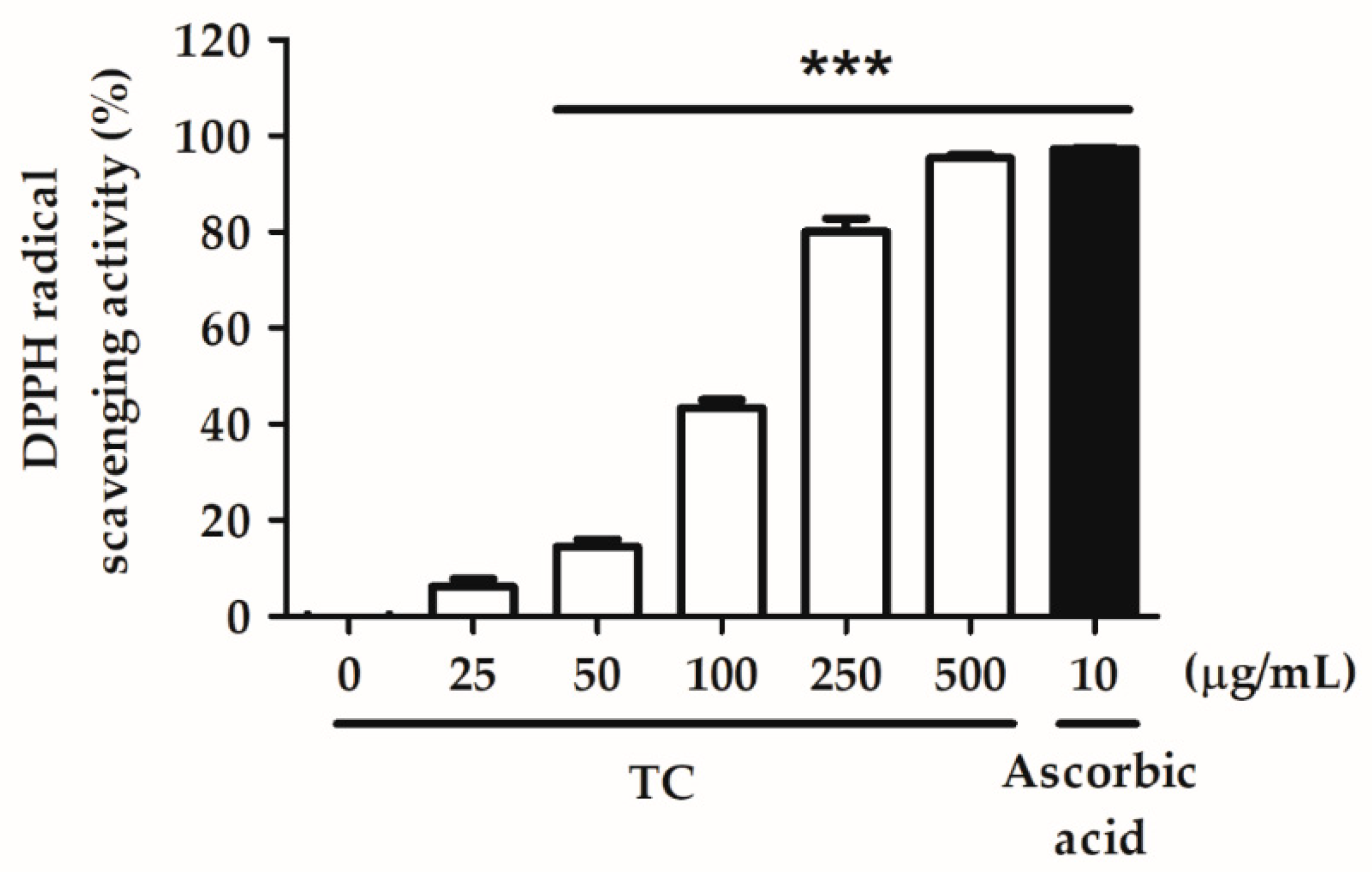
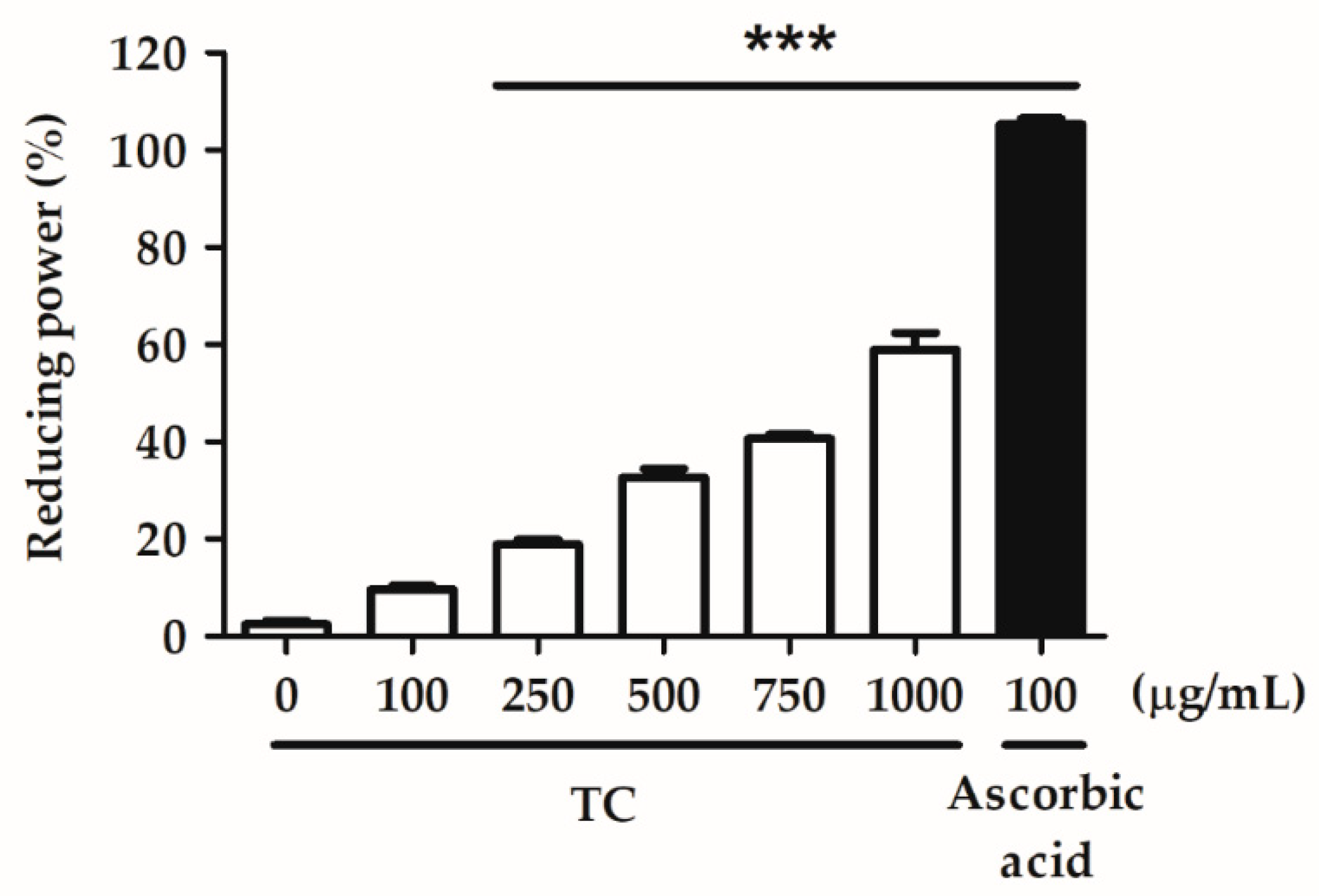
2.2. Evaluation of the Antioxidant Ability of TC
2.2.1. DPPH Radical Scavenging Activity
2.2.2. Reducing Power
2.2.3. H2O2 Inhibition Activity
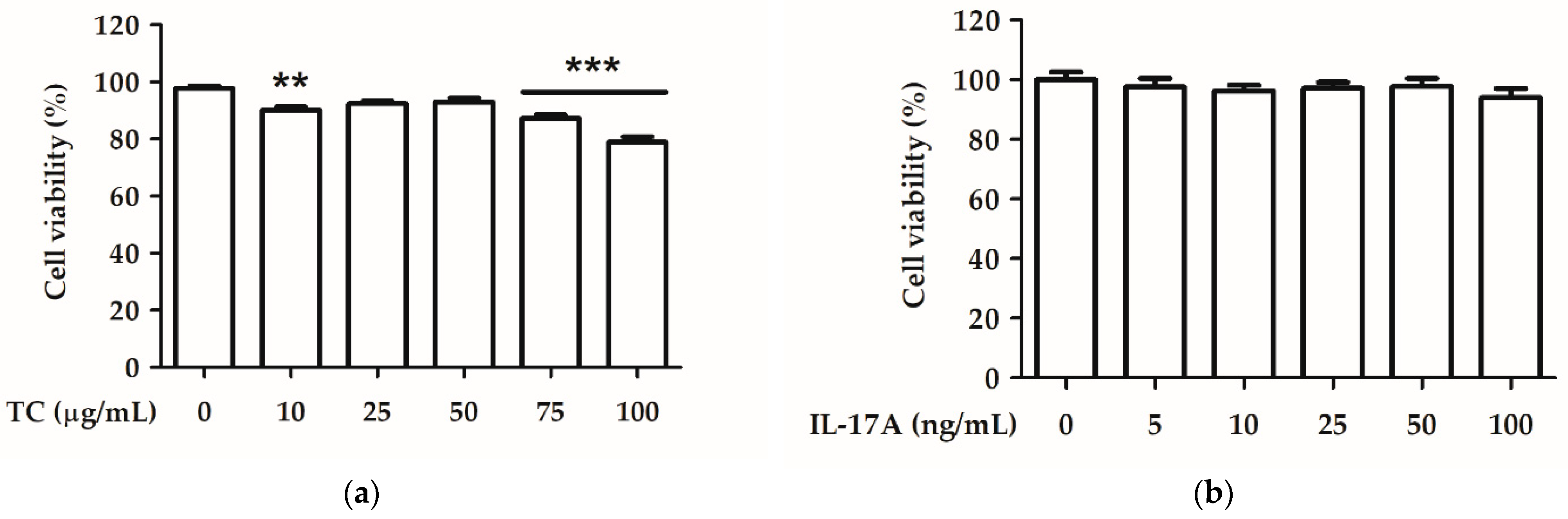
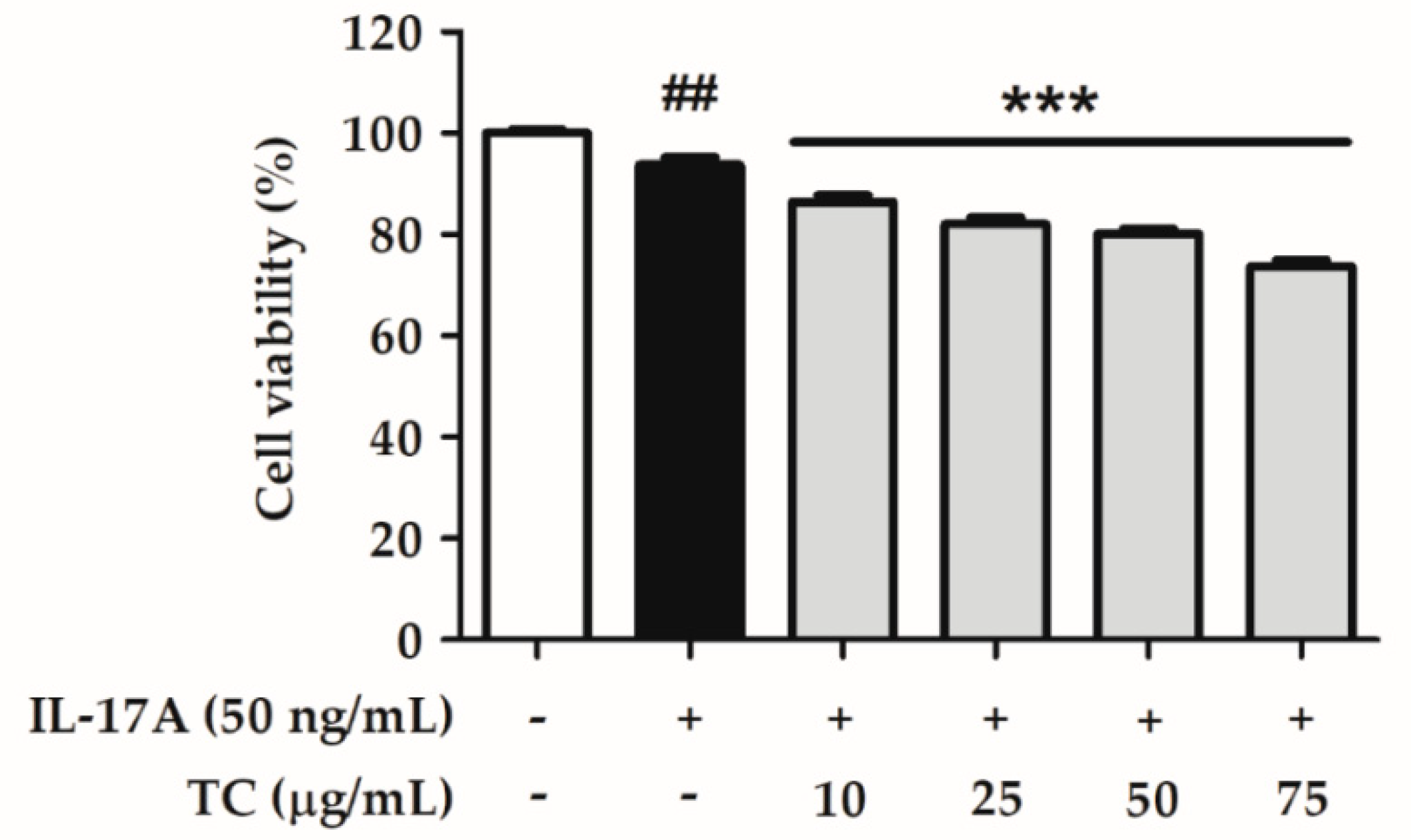
2.3. Effect of TC on Cell Viability
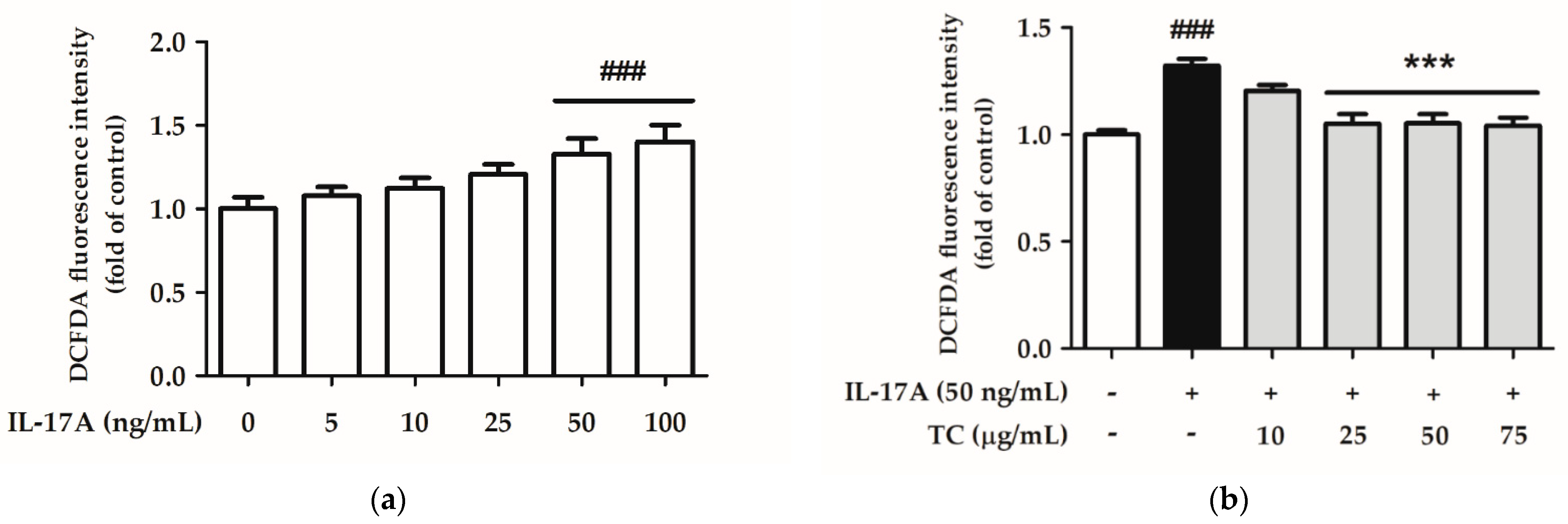
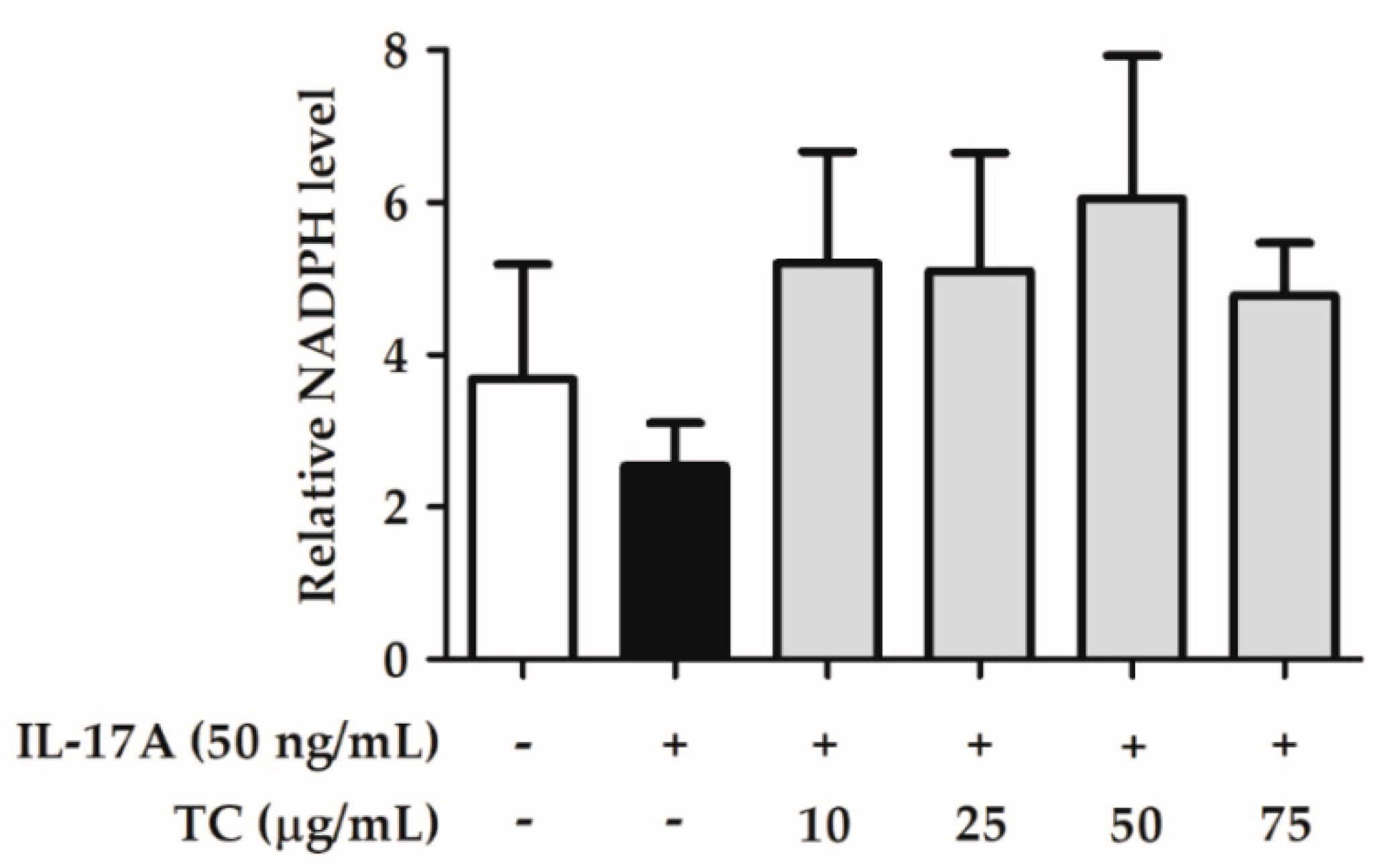
2.4. Effect of TC on Intracellular Oxidative Stress
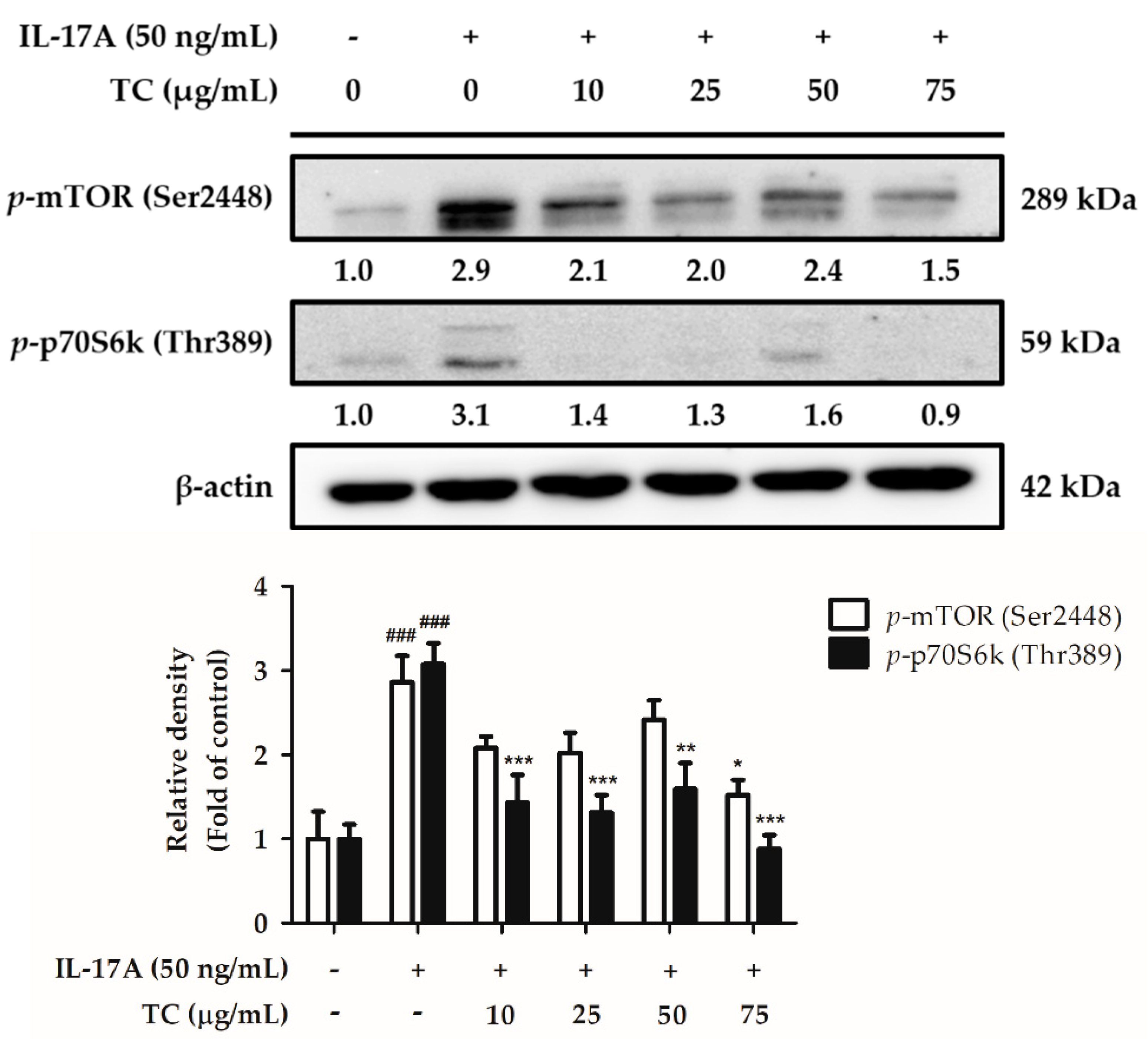
2.5. Influence of TC on the Activation of the Cell Proliferation Signaling Pathway
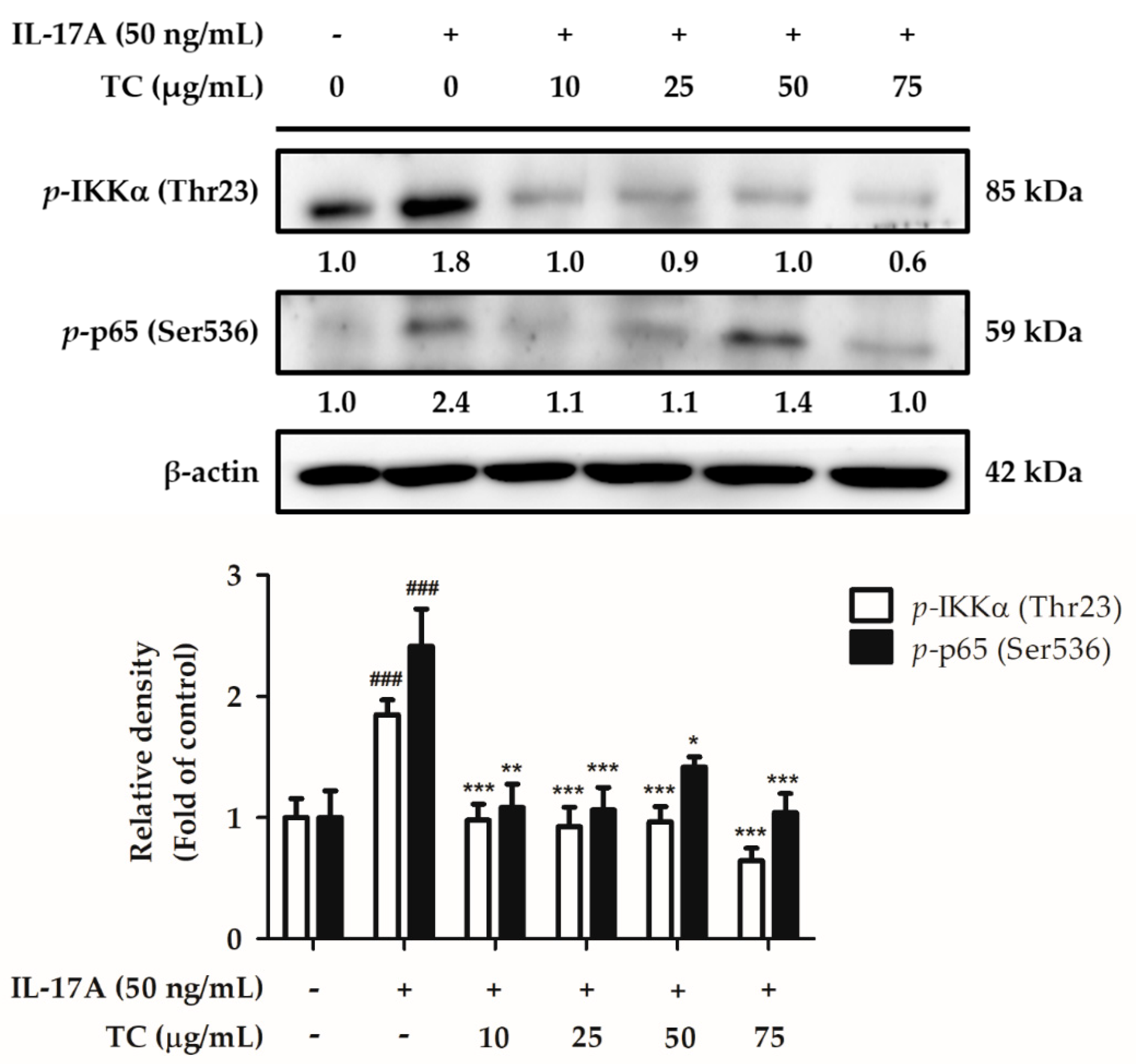
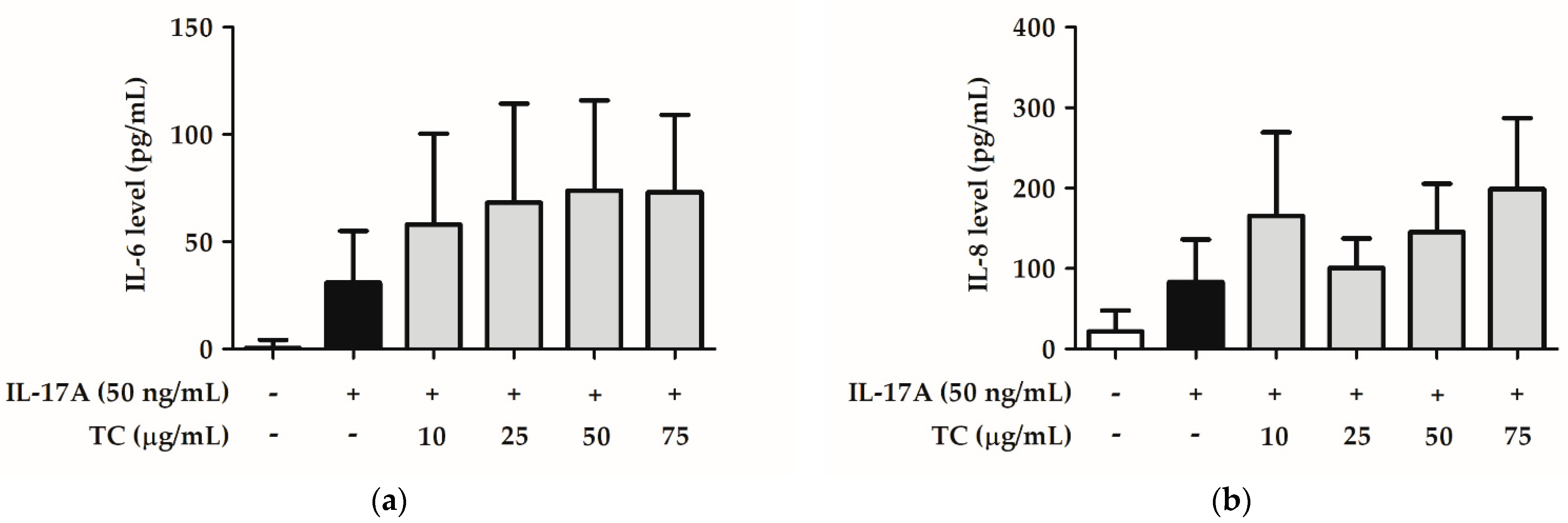
2.6. Influence of TC on the Inflammatory Signaling Pathway
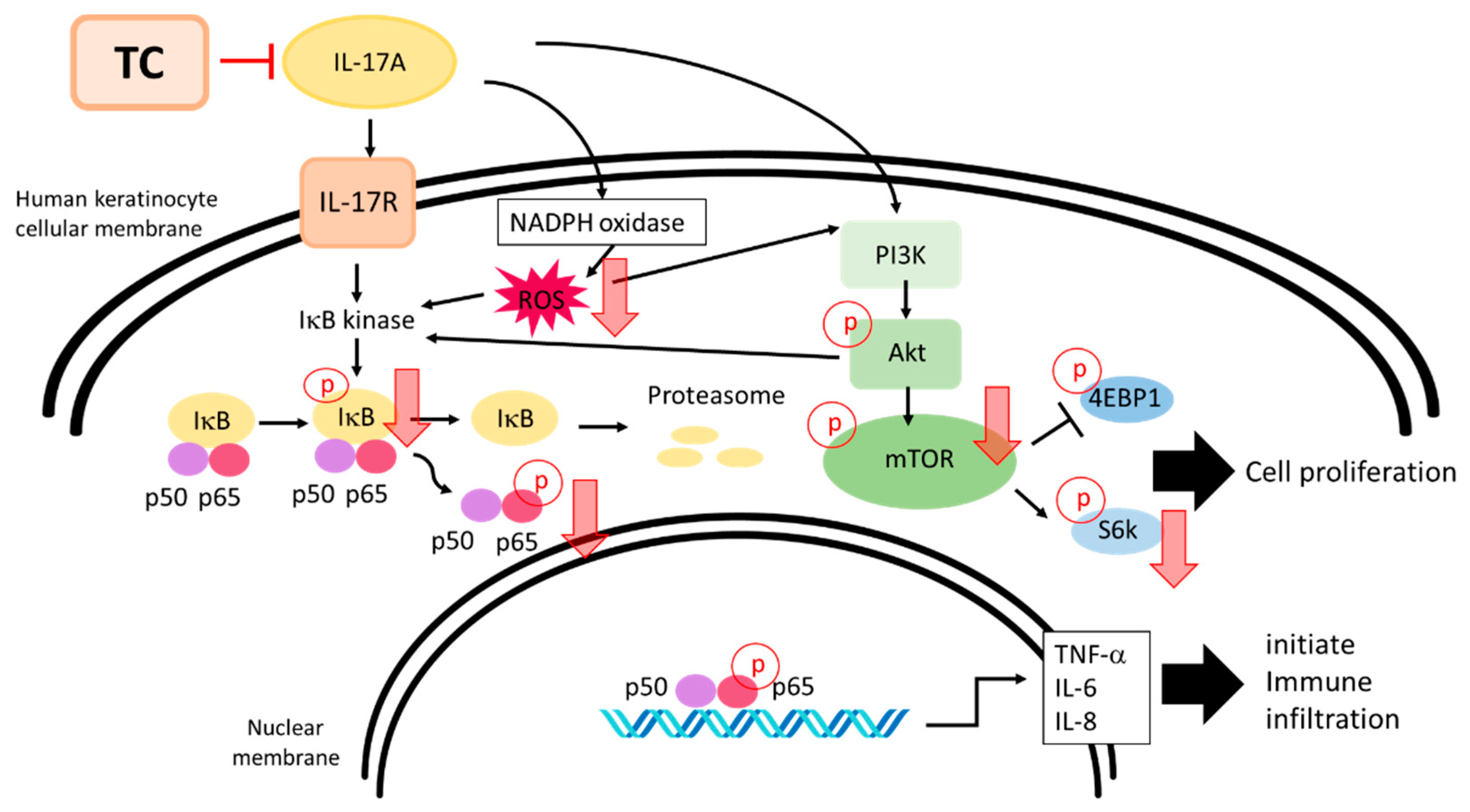
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Fermented TC Extract
4.3. High-Performance Liquid Chromatography
4.4. Antioxidant Assays
4.4.1. DPPH Radical Scavenging Assay
4.4.2. Reducing Power Assay
4.4.3. H2O2 Inhibition Assay
4.5. Cell Culture and MTT Assay
4.6. DCFDA Assay
4.7. Western Blotting
4.8. NADPH Assay
4.9. ELISA
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayala-Fontánez, N.; Soler, D.C.; McCormick, T.S. Current knowledge on psoriasis and autoimmune diseases. Psoriasis 2016, 6, 7–32. [Google Scholar]
- Nestle, F.O.; Di Meglio, P.; Qin, J.Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Yamamoto, O.; Honda, T. Pathophysiology of psoriasis: A review. J. Dermatol. 2021, 48, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Discov. 2022, 13, 81. [Google Scholar] [CrossRef]
- Mahil, S.K.; Capon, F.; Barker, J.N. Genetics of psoriasis. Dermatol. Clin. 2015, 33, 1–11. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting deregulated oxidative stress in skin inflammatory diseases: An update on clinical importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Holmannova, D.; Borska, L.; Andrys, C.; Borsky, P.; Kremlacek, J.; Hamakova, K.; Rehacek, V.; Malkova, A.; Svadlakova, T.; Palicka, V.; et al. The Impact of Psoriasis and Metabolic Syndrome on the Systemic Inflammation and Oxidative Damage to Nucleic Acids. J. Immunol. Res. 2020, 2020, 7352637. [Google Scholar] [CrossRef]
- Hawkes, J.E.; Chan, T.C.; Krueger, J.G. Psoriasis pathogenesis and the development of novel targeted immune therapies. J. Allergy Clin. Immunol. 2017, 140, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Goldminz, A.M.; Au, S.C.; Kim, N.; Gottlieb, A.B.; Lizzul, P.F. NF-κB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.A.; Suh, J.W.; Lee, K.H.; Kang, J.L.; Woo, S.Y. IL-17 and IL-22 enhance skin inflammation by stimulating the secretion of IL-1β by keratinocytes via the ROS-NLRP3-caspase-1 pathway. Int. Immunol. 2012, 24, 147–158. [Google Scholar] [CrossRef]
- Schüler, R.; Brand, A.; Klebow, S.; Wild, J.; Veras, F.P.; Ullmann, E.; Roohani, S.; Kolbinger, F.; Kossmann, S.; Wohn, C.; et al. Antagonization of IL-17A Attenuates Skin Inflammation and Vascular Dysfunction in Mouse Models of Psoriasis. J. Investig. Dermatol. 2019, 139, 638–647. [Google Scholar] [CrossRef]
- Endo, M.; Yamamoto, H.; Setsu, N.; Kohashi, K.; Takahashi, Y.; Ishii, T.; Iida, K.; Matsumoto, Y.; Hakozaki, M.; Aoki, M.; et al. Prognostic significance of AKT/mTOR and MAPK pathways and antitumor effect of mTOR inhibitor in NF1-related and sporadic malignant peripheral nerve sheath tumors. Clin. Cancer Res. 2013, 19, 450–461. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes. Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef]
- Chen, H.; Liu, H.; Tang, B.; Chen, Y.; Han, L.; Yu, J.; Yan, Y.; Lu, C. The Protective Effects of 18β-Glycyrrhetinic Acid on Imiquimod-Induced Psoriasis in Mice via Suppression of mTOR/STAT3 Signaling. J. Immunol. Res. 2020, 2020, 1980456. [Google Scholar] [CrossRef]
- Nguyen, U.T.; Nguyen, L.T.H.; Kim, B.A.; Choi, M.J.; Yang, I.J.; Shin, H.M. Natural Compound Mixture, Containing Emodin, Genipin, Chlorogenic Acid, Cimigenoside, and Ginsenoside Rb1, Ameliorates Psoriasis-Like Skin Lesions by Suppressing Inflammation and Proliferation in Keratinocytes. Evid. Based Complement. Altern. Med. 2020, 2020, 9416962. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Tsai, M.J.; Weng, C.F. Antroquinonol Targets FAK-Signaling Pathway Suppressed Cell Migration, Invasion, and Tumor Growth of C6 Glioma. PLoS ONE 2015, 10, e0141285. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea-An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef]
- Cheng, J.J.; Chao, C.H.; Lu, M.K. Large-scale preparation of sulfated polysaccharides with anti-angionenic and anti-inflammatory properties from Antrodia cinnamomia. Int. J. Biol. Macromol. 2018, 113, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Tung, Y.T.; Kuo, Y.H.; Liao, J.W.; Tsai, H.C.; Chong, K.Y.; Chen, H.L.; Chen, C.M. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 2015, 159, 113–121. [Google Scholar] [CrossRef]
- Chiu, H.W.; Hua, K.F. Hepatoprotective Effect of Wheat-Based Solid-State Fermented Antrodia cinnamomea in Carbon Tetrachloride-Induced Liver Injury in Rat. PLoS ONE 2016, 11, e0153087. [Google Scholar] [CrossRef]
- Lee, I.H.; Huang, R.L.; Chen, C.T.; Chen, H.C.; Hsu, W.C.; Lu, M.K. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol. Lett. 2002, 209, 63–67. [Google Scholar] [CrossRef]
- Song, T.Y.; Yen, G.C. Protective effects of fermented filtrate from Antrodia camphorata in submerged culture against CCl4-induced hepatic toxicity in rats. J. Agric. Food Chem. 2003, 51, 1571–1577. [Google Scholar] [CrossRef]
- Huang, G.J.; Deng, J.S.; Chen, C.C.; Huang, C.J.; Sung, P.J.; Huang, S.S.; Kuo, Y.H. Methanol extract of Antrodia camphorata protects against lipopolysaccharide-induced acute lung injury by suppressing NF-κB and MAPK pathways in mice. J. Agric. Food Chem. 2014, 62, 5321–5329. [Google Scholar] [CrossRef]
- Kuo, Y.H.; Lin, T.Y.; You, Y.J.; Wen, K.C.; Sung, P.J.; Chiang, H.M. Antiinflammatory and Antiphotodamaging Effects of Ergostatrien-3β-ol, Isolated from Antrodia camphorata, on Hairless Mouse Skin. Molecules 2016, 21, 1213. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.T.; Kuo, Y.H.; Wang, S.D.; Hong, H.J.; Lin, L.J. Analogous corticosteroids, 9A and EK100, derived from solid-state-cultured mycelium of Antrodia camphorata inhibit proinflammatory cytokine expression in macrophages. Cytokine 2018, 108, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Lin, S.S.; Shao, Y.Y.; Chen, C.C.; Hou, W.C.; Kuo, Y.H. Analgesic effects and the mechanisms of anti-inflammation of ergostatrien-3beta-ol from Antrodia camphorata submerged whole broth in mice. J. Agric. Food Chem. 2010, 58, 7445–7452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Guan, Y.Y.; Hu, P.F.; Chen, L.; Xu, G.R.; Liu, L.; Cheung, P.C.K. Production of bioactive metabolites by submerged fermentation of the medicinal mushroom Antrodia cinnamomea: Recent advances and future development. Crit. Rev. Biotechnol. 2019, 39, 541–554. [Google Scholar] [CrossRef]
- Meng, L.; Luo, B.; Yang, Y.; Faruque, M.O.; Zhang, J.; Li, X.; Hu, X. Addition of Vegetable Oil to Improve Triterpenoids Production in Liquid Fermentation of Medicinal Fungus Antrodia cinnamomea. J. Fungi 2021, 7, 926. [Google Scholar] [CrossRef]
- Andargie, M.; Vinas, M.; Rathgeb, A.; Möller, E.; Karlovsky, P. Lignans of Sesame (Sesamum indicum L.): A Comprehensive Review. Molecules 2021, 26, 883. [Google Scholar] [CrossRef]
- Kuo, J.T.; Lin, E.S.; Yang, C.T. Effect of cultivating conditions on the superoxide and free radical-scavenging activities of Antrodia cinnamomea. J. Food Biochem. 2011, 35, 1493–1500. [Google Scholar] [CrossRef]
- Yang, F.C.; Yang, Y.H.; Lu, H.C. Enhanced antioxidant and antitumor activities of Antrodia cinnamomea cultured with cereal substrates in solid state fermentation. Biochem. Eng. J. 2013, 78, 108–113. [Google Scholar] [CrossRef]
- Chu, Y.; Wu, P.Y.; Chen, C.W.; Lyu, J.L.; Liu, Y.J.; Wen, K.C.; Lin, C.Y.; Kuo, Y.H.; Chiang, H.M. Protective Effects and Mechanisms of N-Phenethyl Caffeamide from UVA-Induced Skin Damage in Human Epidermal Keratinocytes through Nrf2/HO-1 Regulation. Int. J. Mol. Sci. 2019, 20, 164. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Liao, J.W.; Xiao, J.H.; Gokila Vani, M.; Wang, S.Y. Hepatoprotective effect of lucidone against alcohol-induced oxidative stress in human hepatic HepG2 cells through the up-regulation of HO-1/Nrf-2 antioxidant genes. Toxicol. In Vitro 2012, 26, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Wu, H.C.; Yao, H.J.; Lin, C.C.; Wen, S.F.; Pan, I.H. Antrodia cinnamomea Extract Inhibits Th17 Cell Differentiation and Ameliorates Imiquimod-Induced Psoriasiform Skin Inflammation. Am. J. Chin. Med. 2015, 43, 1401–1417. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Rao, Y.K.; Wu, C.C.; Huang, C.Y.; Geethangili, M.; Hsu, S.L.; Tzeng, Y.M. Methyl antcinate A from Antrodia camphorata induces apoptosis in human liver cancer cells through oxidant-mediated cofilin- and Bax-triggered mitochondrial pathway. Chem. Res. Toxicol. 2010, 23, 1256–1267. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- He, Y.C.; He, K.Z.; Pu, Q.; Li, J.; Zhao, Z.J. Optimization of Cultivating Conditions for Triterpenoids Production from Antrodia cinnmomea. Indian J. Microbiol. 2012, 52, 648–653. [Google Scholar] [CrossRef]
- Yang, F.; Ke, Y.; Kuo, S. Effect of fatty acids on the mycelial growth and polysaccharide formation by Ganoderma lucidum in shake flask cultures. Enzyme Microb. Technol. 2000, 27, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kiso, Y. Antioxidative roles of sesamin, a functional lignan in sesame seed, and it’s effect on lipid- and alcohol-metabolism in the liver: A DNA microarray study. Biofactors 2004, 21, 191–196. [Google Scholar] [CrossRef]
- Lin, T.Y.; Wu, P.Y.; Hou, C.W.; Chien, T.Y.; Chang, Q.X.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. Protective Effects of Sesamin against UVB-Induced Skin Inflammation and Photodamage In Vitro and In Vivo. Biomolecules 2019, 9, 479. [Google Scholar] [CrossRef] [PubMed]
- Tsuruta, D. NF-kappaB links keratinocytes and lymphocytes in the pathogenesis of psoriasis. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 40–48. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, B.; Dang, E.; Jin, L.; Fan, X.; Wang, G. Impaired function of regulatory T cells in patients with psoriasis is mediated by phosphorylation of STAT3. J. Dermatol. Sci. 2016, 81, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Nadiv, O.; Beer, Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: A possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis. Rheum. 2001, 44, 2176–2184. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Bakry, O.A.; Samaka, R.M.; Shoeib, M.A.; Abdel Aal, S.M. Nuclear factor kappa B and cyclo-oxygenase-2: Two concordant players in psoriasis pathogenesis. Ultrastruct. Pathol. 2015, 39, 49–61. [Google Scholar] [CrossRef]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef]
- Lee, K.E.; Khoi, P.N.; Xia, Y.; Park, J.S.; Joo, Y.E.; Kim, K.K.; Choi, S.Y.; Jung, Y.D. Helicobacter pylori and interleukin-8 in gastric cancer. World J. Gastroenterol. 2013, 19, 8192. [Google Scholar] [CrossRef] [PubMed]
- Varshney, P.; Saini, N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 1795–1803. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.N.; Walker, A.L.; Liu, Y.Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.M.; Lin, T.J.; Chiu, C.Y.; Chang, C.W.; Hsu, K.C.; Fan, P.C.; Wen, K.C. Coffea arabica extract and its constituents prevent photoaging by suppressing MMPs expression and MAP kinase pathway. Food Chem. Toxicol. 2011, 49, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe(III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chiu, H.H.; Liao, S.T.; Chen, Y.T.; Chang, H.C.; Wen, K.C. Isoflavonoid-Rich Flemingia macrophylla Extract Attenuates UVB-Induced Skin Damage by Scavenging Reactive Oxygen Species and Inhibiting MAP Kinase and MMP Expression. Evid. Based Complement. Altern. Med. 2013, 2013, 696879. [Google Scholar] [CrossRef]
- Wu, P.Y.; Lin, T.Y.; Hou, C.W.; Chang, Q.X.; Wen, K.C.; Lin, C.Y.; Chiang, H.M. 1,2-Bis[(3-Methoxyphenyl)Methyl]Ethane-1,2-Dicarboxylic Acid Reduces UVB-Induced Photodamage In Vitro and In Vivo. Antioxidants 2019, 8, 452. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.-W.; Wu, P.-Y.; Kuo, Y.-H.; Chang, Q.-X.; Wen, K.-C.; Chiang, H.-M. Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-𝜅B and mTOR Pathways. Int. J. Mol. Sci. 2022, 23, 14623. https://doi.org/10.3390/ijms232314623
Shen J-W, Wu P-Y, Kuo Y-H, Chang Q-X, Wen K-C, Chiang H-M. Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-𝜅B and mTOR Pathways. International Journal of Molecular Sciences. 2022; 23(23):14623. https://doi.org/10.3390/ijms232314623
Chicago/Turabian StyleShen, Jia-Wei, Po-Yuan Wu, Yueh-Hsiung Kuo, Qiao-Xin Chang, Kuo-Ching Wen, and Hsiu-Mei Chiang. 2022. "Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-𝜅B and mTOR Pathways" International Journal of Molecular Sciences 23, no. 23: 14623. https://doi.org/10.3390/ijms232314623
APA StyleShen, J.-W., Wu, P.-Y., Kuo, Y.-H., Chang, Q.-X., Wen, K.-C., & Chiang, H.-M. (2022). Fermented Taiwanofungus camphoratus Extract Ameliorates Psoriasis-Associated Response in HaCaT Cells via Modulating NF-𝜅B and mTOR Pathways. International Journal of Molecular Sciences, 23(23), 14623. https://doi.org/10.3390/ijms232314623







