Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study
Abstract
1. Introduction
2. Results
2.1. In Vitro Biocompatibility Analyses of Bioabsorbable ISs
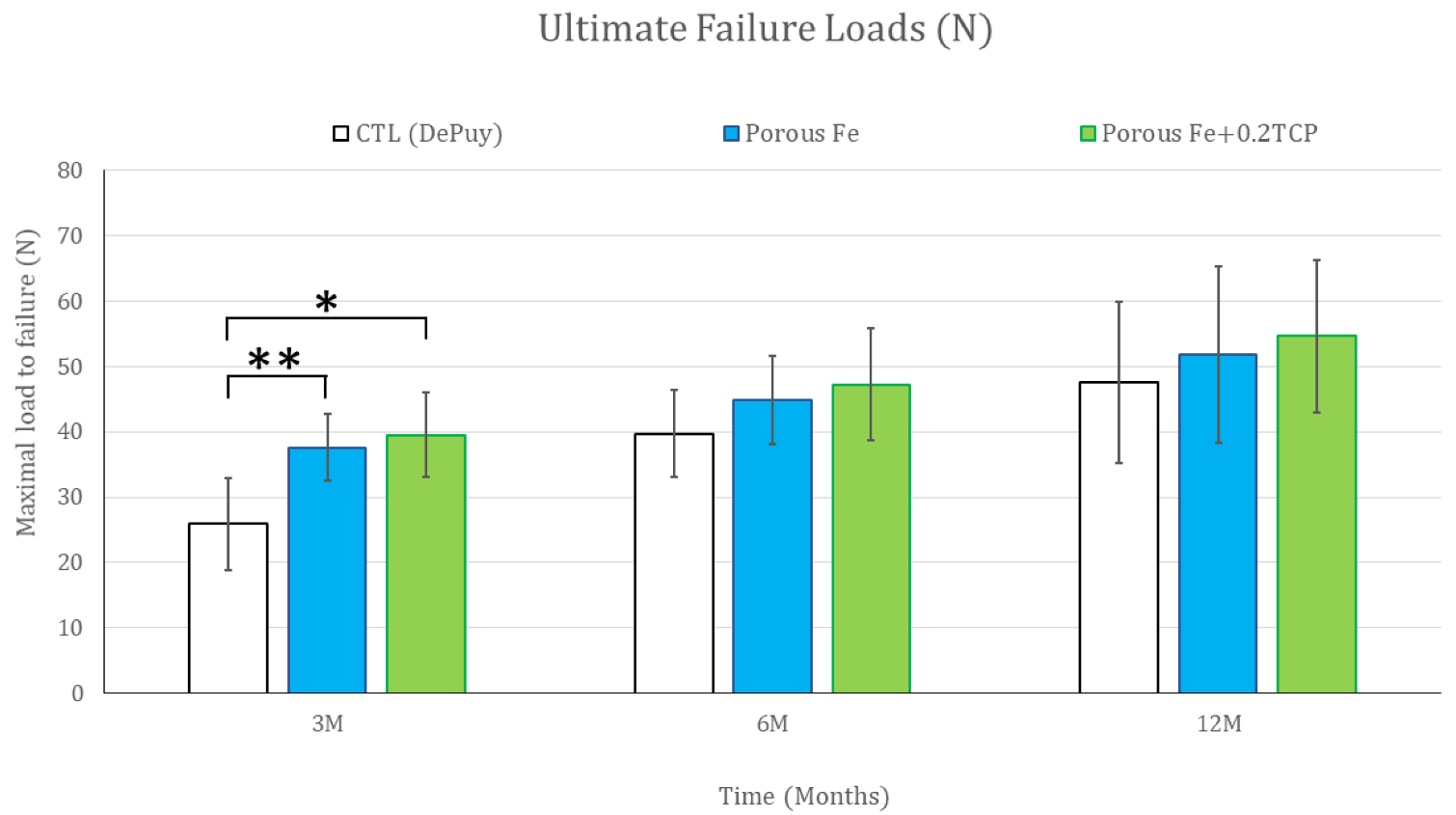
2.2. In Vivo Biomechanical Analysis
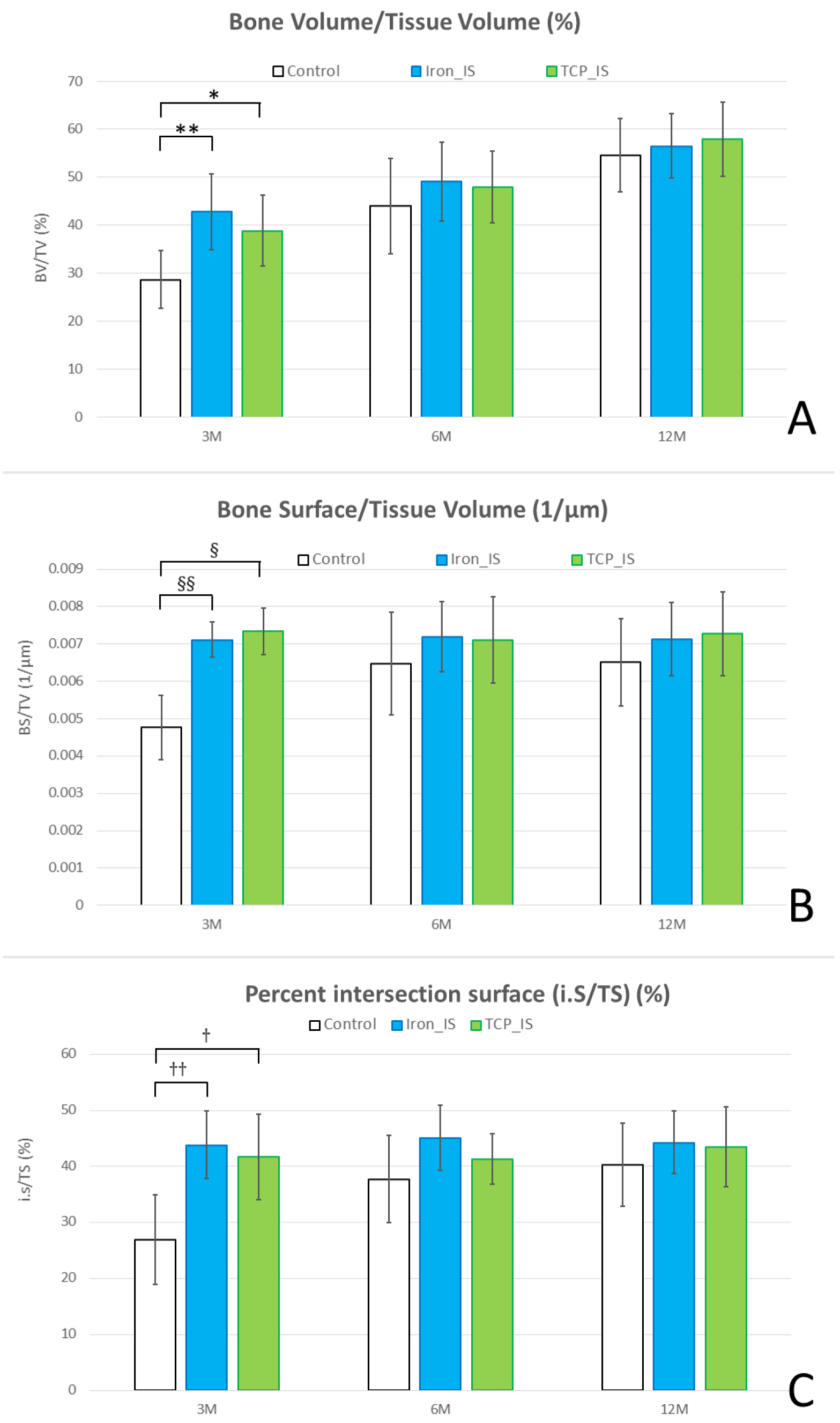
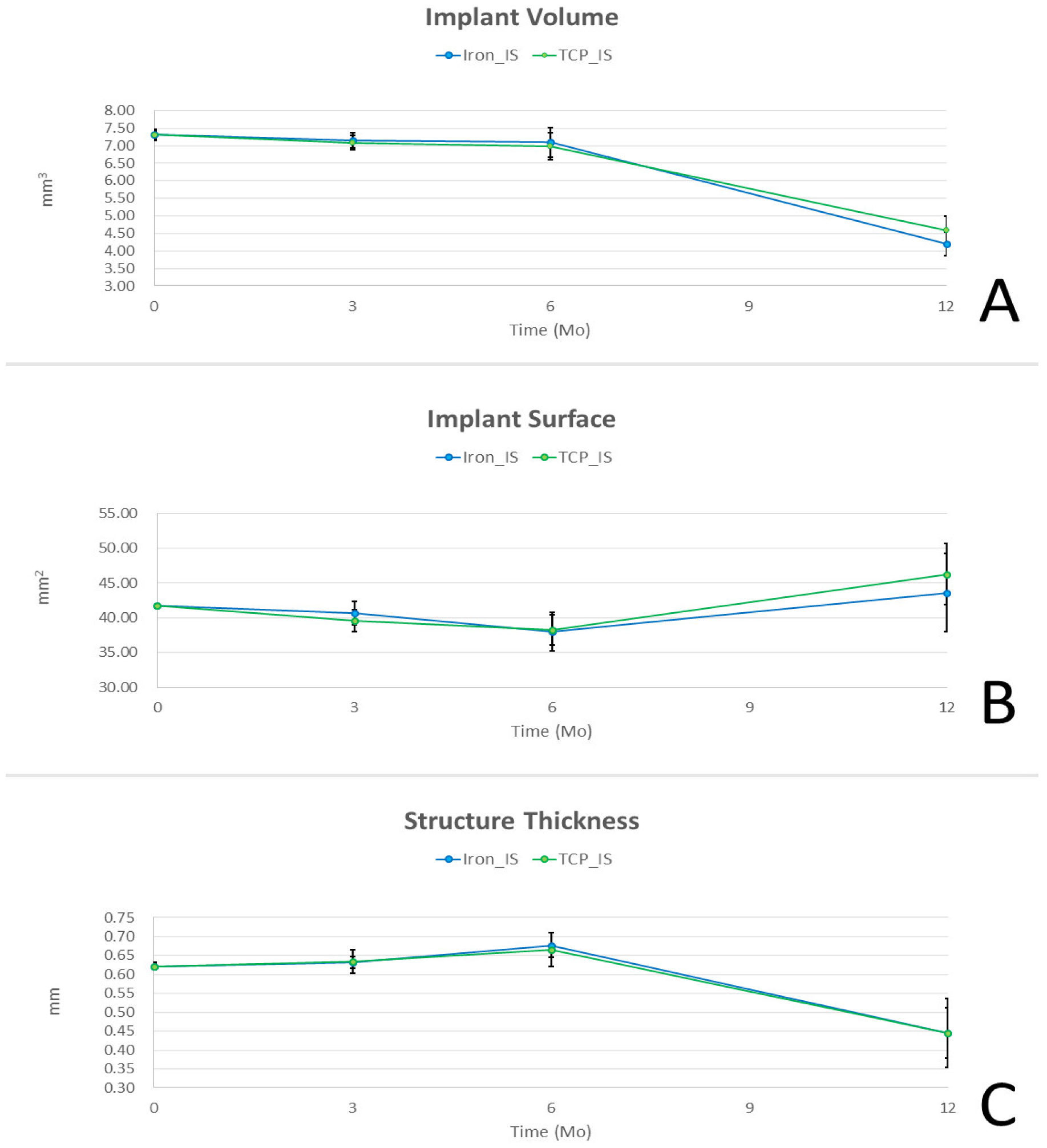
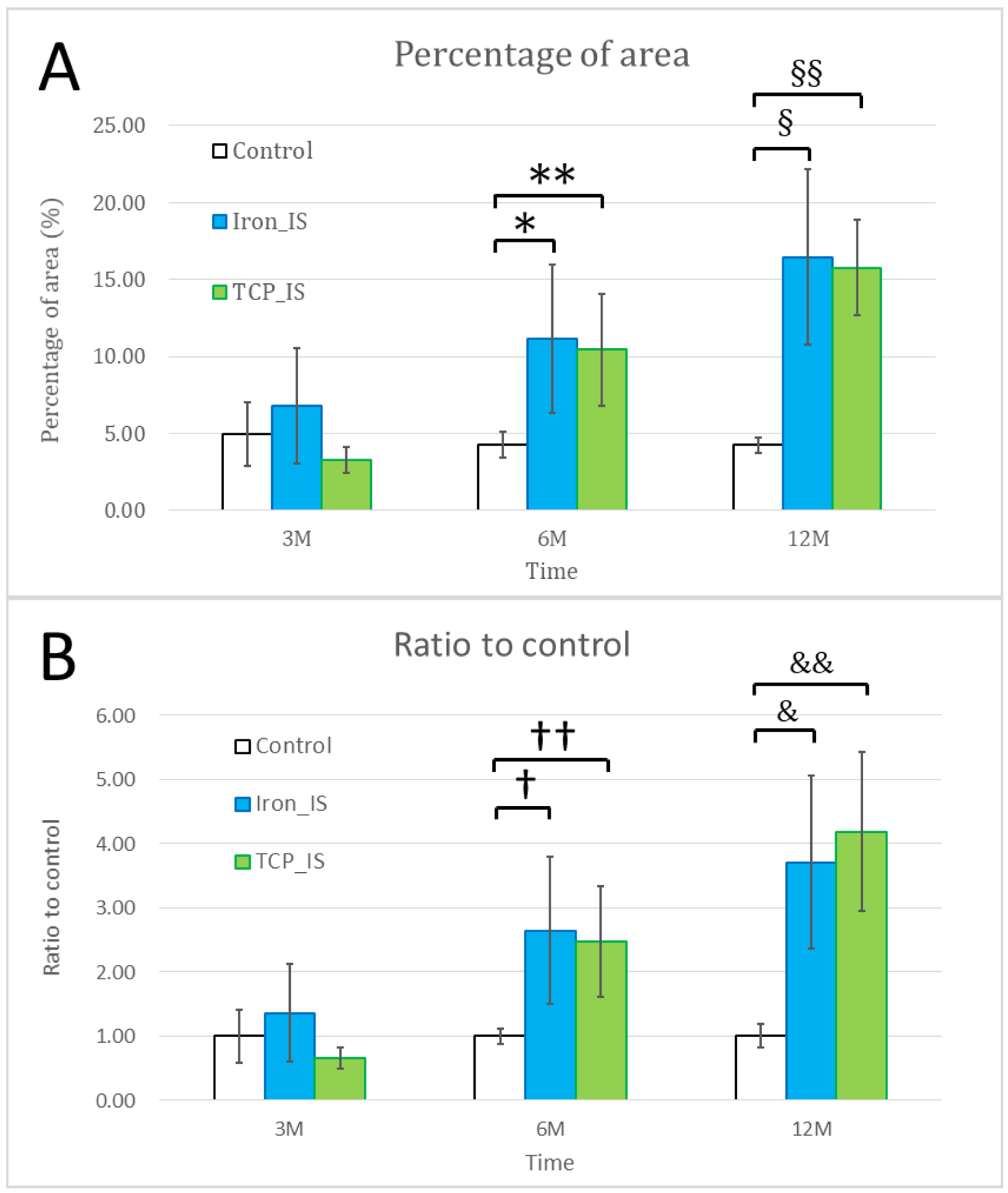
2.3. Micro-CT Analysis
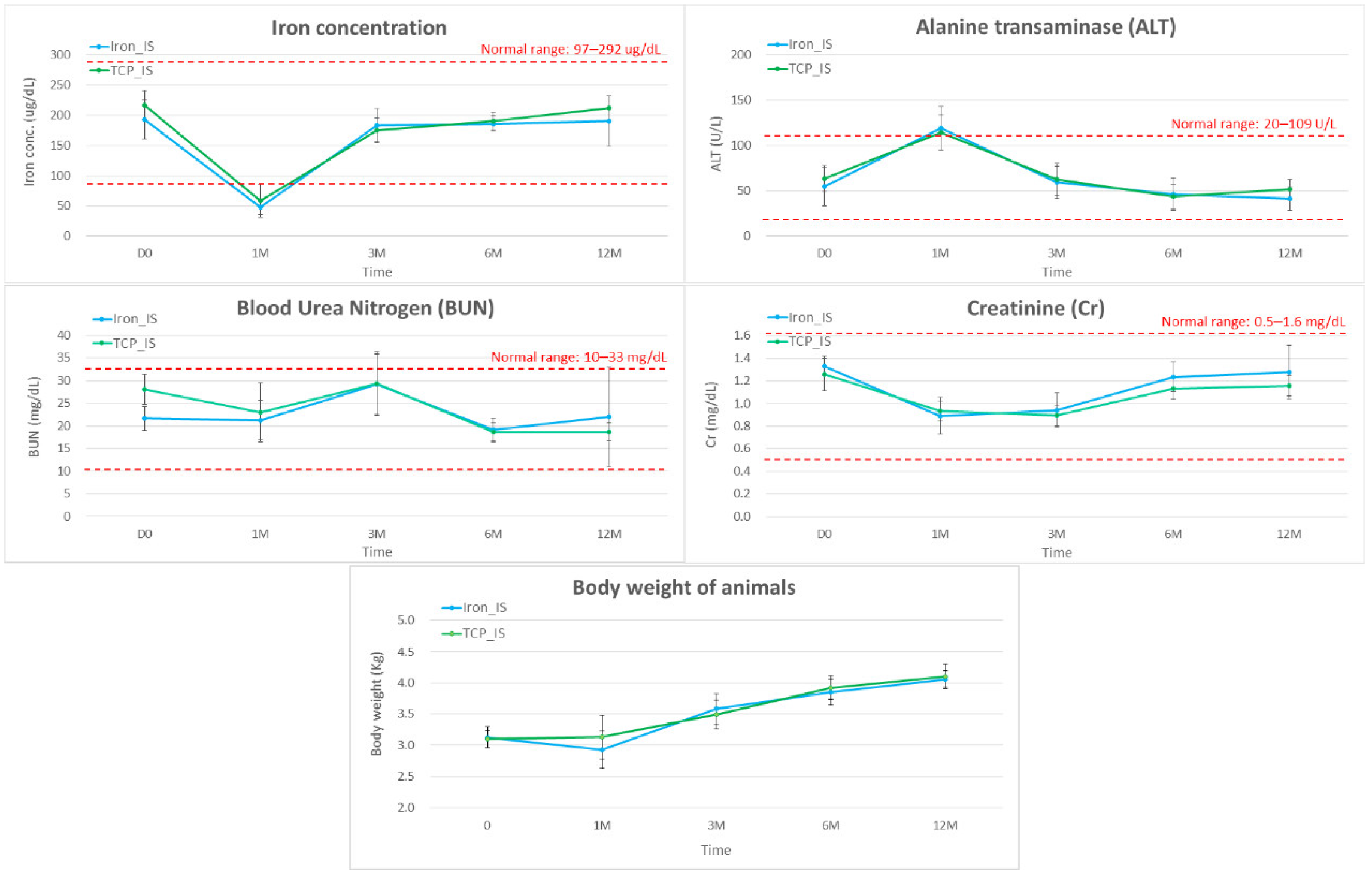
2.4. Biochemical Analysis
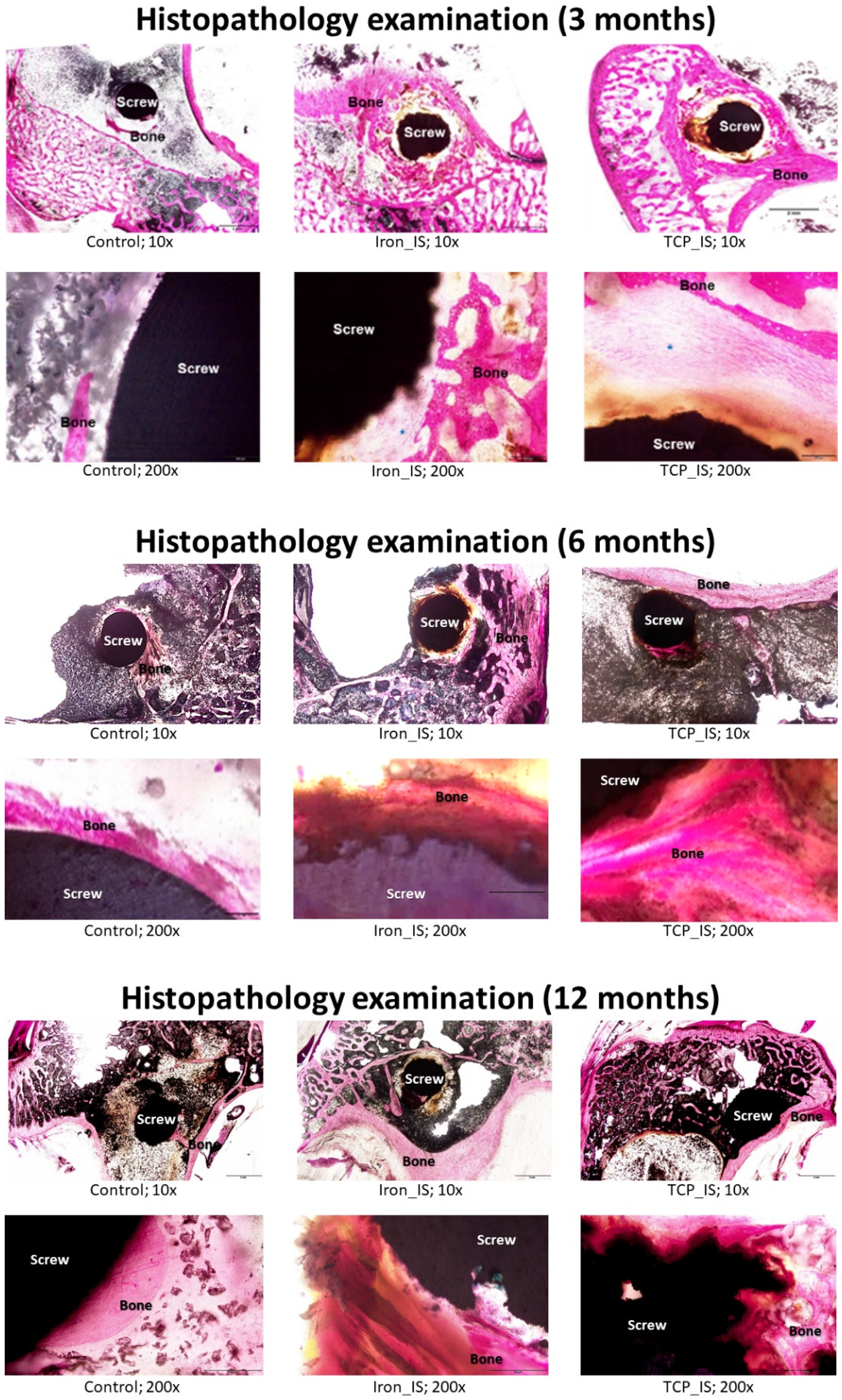
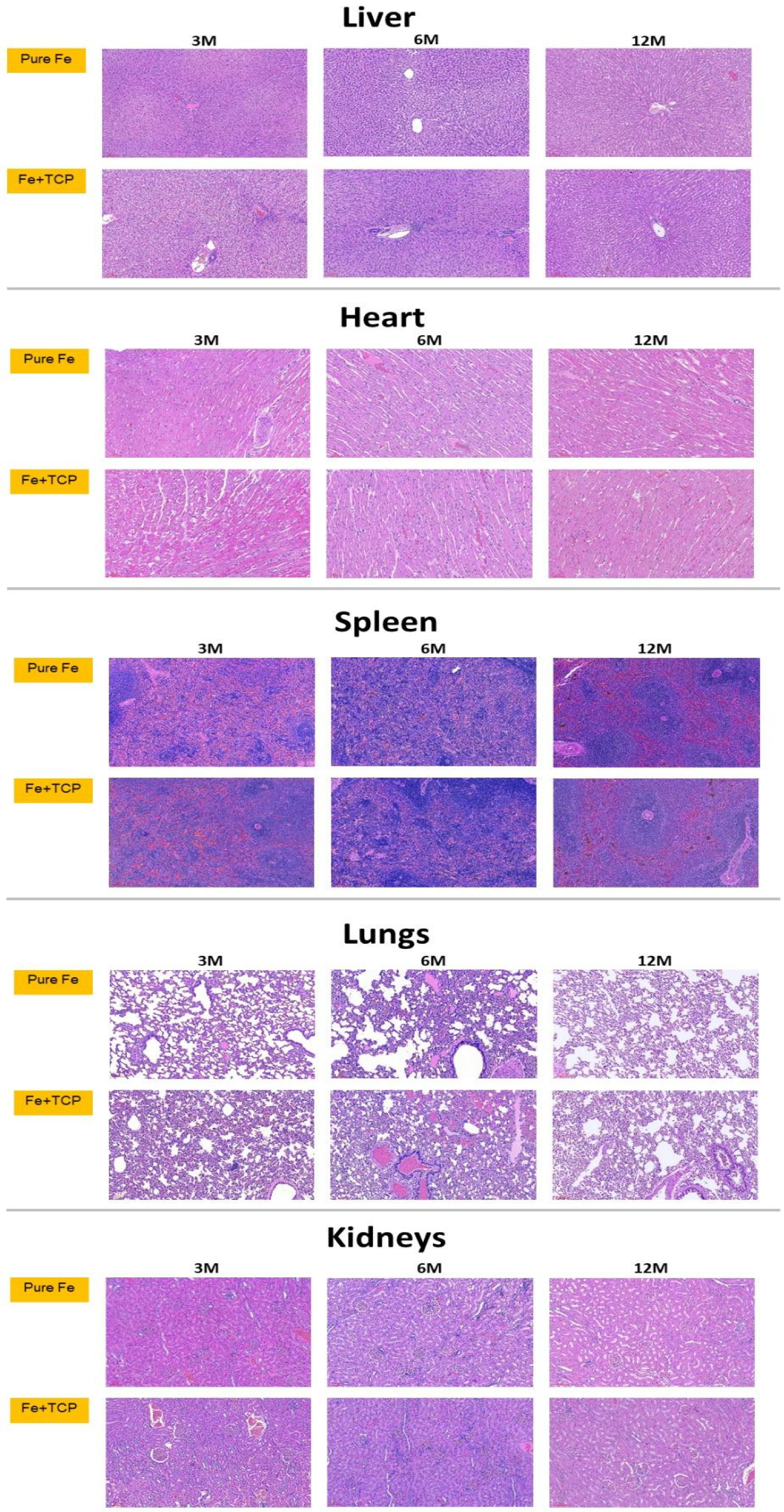
2.5. Histological and Histopathological Analyses
3. Methods
3.1. IS Development Using AM
3.2. In Vitro IS Immersion Test
3.3. In Vivo Animal Study Design
3.4. Surgical Methods
3.5. Biomechanical Analysis
3.6. Micro-CT Analysis
3.7. Histological Analysis
3.8. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, C.; Alves, M.; Montemor, M.F.; Carmezim, M.J. Bioresorbable metallic implants: Surface functionalization with nanoparticles and nanostructures. Adv. Mater. Appl. Micro Nano Scale 2017, 219–242. [Google Scholar]
- Park, J.B.; Bronzino, J.D. Biomaterials: Principles and Applications; Taylor & Francis: Boca Raton, FL, USA, 2002. [Google Scholar] [CrossRef]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable Iron and Porous Iron: Mechanical Properties, Degradation Behaviour, Manufacturing Routes and Biomedical Applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.-C.; Seok, H.-K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Shuai, C.; Li, S.; Peng, S.; Feng, P.; Lai, Y.; Gao, C. Biodegradable metallic bone implants. Mater. Chem. Front. 2019, 3, 544–562. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Mayer, J.; Wintermantel, E.; Leong, K.W. Biomedical applications of polymer-composite materials: A review. Compos. Sci. Technol. 2001, 61, 1189–1224. [Google Scholar] [CrossRef]
- Ramsay, S.D.; Pilliar, R.M.; Santerre, J.P. Fabrication of a biodegradable calcium polyphosphate/polyvinyl-urethane carbonate composite for high load bearing osteosynthesis applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 94, 178–186. [Google Scholar] [CrossRef]
- Figueiredo, L.; Fonseca, R.; Pinto, L.F.; Ferreira, F.C.; Almeida, A.; Rodrigues, A. Strategy to improve the mechanical properties of bioabsorbable materials based on chitosan for orthopedic fixation applications. J. Mech. Behav. Biomed. Mater. 2020, 103, 103572. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Yusop, A.H.; Daud, N.M.; Nur, H.; Kadir, M.R.A.; Hermawan, H. Controlling the degradation kinetics of porous iron by poly(lactic-co-glycolic acid) infiltration for use as temporary medical implants. Sci. Rep. 2015, 5, 11194. [Google Scholar] [CrossRef]
- Yusop, A.H.M.; Ulum, M.F.; Al Sakkaf, A.; Hartanto, D.; Nur, H. Insight into the bioabsorption of Fe-based materials and their current developments in bone applications. Biotechnol. J. 2021, 16, 2100255. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, D.; Dai, Y.; Lin, J.; Li, Y.; Wen, C. Microstructure, mechanical properties, degradation behavior, and biocompatibility of porous Fe-Mn alloys fabricated by sponge impregnation and sintering techniques. Acta Biomater. 2020, 114, 485–496. [Google Scholar] [CrossRef]
- Wegener, B.; Sichler, A.; Milz, S.; Sprecher, C.; Pieper, K.; Hermanns, W.; Jansson, V.; Nies, B.; Kieback, B.; Müller, P.E.; et al. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. 2020, 10, 9141. [Google Scholar] [CrossRef]
- Gorejová, R.; Oriňaková, R.; Králová, Z.O.; Baláž, M.; Kupková, M.; Hrubovčáková, M.; Haverová, L.; Džupon, M.; Oriňak, A.; Kaľavský, F.; et al. In Vitro Corrosion Behavior of Biodegradable Iron Foams with Polymeric Coating. Materials 2020, 13, 184. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef]
- Hong, D.; Chou, D.-T.; Velikokhatnyi, O.I.; Roy, A.; Lee, B.; Swink, I.; Issaev, I.; Kuhn, H.A.; Kumta, P.N. Binder-jetting 3D printing and alloy development of new biodegradable Fe-Mn-Ca/Mg alloys. Acta Biomater. 2016, 45, 375–386. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitze, P.J. Degradation performance of biodegradable FeMnC (Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, T.; Zheng, Y. Microstructure, mechanical property, biodegradation behavior, and biocompatibility of biodegradable Fe–Fe2O3 composites. J. Biomed. Mater. Res. Part A 2014, 102, 2277–2287. [Google Scholar] [CrossRef]
- Tsai, P.-I.; Chen, C.-Y.; Huang, S.-W.; Yang, K.-Y.; Lin, T.-H.; Chen, S.-Y.; Sun, J.-S. Improvement of bone-tendon fixation by porous titanium interference screw: A rabbit animal model. J. Orthop. Res. 2018, 36, 2633–2640. [Google Scholar] [CrossRef]
- Putra, N.; Leeflang, M.; Minneboo, M.; Taheri, P.; Fratila-Apachitei, L.; Mol, J.; Zhou, J.; Zadpoor, A. Extrusion-based 3D printed biodegradable porous iron. Acta Biomater. 2020, 121, 741–756. [Google Scholar] [CrossRef]
- Zadpoor, A.A. Bone tissue regeneration: The role of scaffold geometry. Biomater. Sci. 2015, 3, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Ulum, M.; Arafat, A.; Noviana, D.; Yusop, A.; Nasution, A.; Kadir, M.A.; Hermawan, H. In vitro and in vivo degradation evaluation of novel iron-bioceramic composites for bone implant applications. Mater. Sci. Eng. C 2014, 36, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Cheng, J.; Zheng, Y.F. In vitro degradation and biocompatibility of Fe–Pd and Fe–Pt composites fabricated by spark plasma sintering. Mater. Sci. Eng. C 2014, 35, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef]
- Tai, C.-C.; Lo, H.-L.; Liaw, C.-K.; Huang, Y.-M.; Huang, Y.-H.; Yang, K.-Y.; Huang, C.-C.; Huang, S.-I.; Shen, H.-H.; Lin, T.-H.; et al. Biocompatibility and Biological Performance Evaluation of Additive-Manufactured Bioabsorbable Iron-Based Porous Suture Anchor in a Rabbit Model. Int. J. Mol. Sci. 2021, 22, 7368. [Google Scholar] [CrossRef]
- Milewski, M.D.; Diduch, D.R.; Hart, J.M.; Tompkins, M.; Ma, S.-Y.; Gaskin, C.M. Bone Replacement of Fast-Absorbing Biocomposite Anchors in Arthroscopic Shoulder Labral Repairs. Am. J. Sports Med. 2012, 40, 1392–1401. [Google Scholar] [CrossRef]
- Prabhu, B.; Karau, A.; Wood, A.; Dadsetan, M.; Liedtke, H.; DeWitt, T. Bioresorbable Materials for Orthopedic Applications (Lactide and Glycolide Based). In Orthopedic Biomaterials: Progress in Biology, Manufacturing, and Industry Perspectives; Springer: Cham, Switzerland, 2018; pp. 287–344. [Google Scholar] [CrossRef]
- Huang, C.-C.; Lam, T.-N.; Amalia, L.; Chen, K.-H.; Yang, K.-Y.; Muslih, M.R.; Singh, S.S.; Tsai, P.-I.; Lee, Y.-T.; Jain, J.; et al. Tailoring grain sizes of the biodegradable iron-based alloys by pre-additive manufacturing microalloying. Sci. Rep. 2021, 11, 9610. [Google Scholar] [CrossRef]
- Yamakado, K.; Kitaoka, K.; Yamada, H.; Hashiba, K.; Nakamura, R.; Tomita, K. The influence of mechanical stress on graft healing in a bone tunnel. Arthrosc. J. Arthrosc. Relat. Surg. 2002, 18, 82–90. [Google Scholar] [CrossRef]
- Pyka, G.; Kerckhofs, G.; Schrooten, J.; Wevers, M. The effect of spatial micro-CT image resolution and surface complexity on the morphological 3D analysis of open porous structures. Mater. Charact. 2014, 87, 104–115. [Google Scholar] [CrossRef]
- Chiu, Y.-R.; Hsu, Y.-T.; Wu, C.-Y.; Lin, T.-H.; Yang, Y.-Z.; Chen, H.-Y. Fabrication of Asymmetrical and Gradient Hierarchy Structures of Poly-p-xylylenes on Multiscale Regimes Based on a Vapor-Phase Sublimation and Deposition Process. Chem. Mater. 2020, 32, 1120–1130. [Google Scholar] [CrossRef]
- Van Dalen, G.; Koster, M.W. 2D & 3D Particle Size Analysis of Micro-CT Images; Unilever Research and Development Netherlands: Vlaardingen, The Netherlands, 2012. [Google Scholar]
- Zheng, J.-F.; Xi, Z.-W.; Li, Y.; Li, J.-N.; Qiu, H.; Hu, X.-Y.; Luo, T.; Wu, C.; Wang, X.; Song, L.-F.; et al. Long-term safety and absorption assessment of a novel bioresorbable nitrided iron scaffold in porcine coronary artery. Bioact. Mater. 2022, 17, 496–505. [Google Scholar] [CrossRef]
- Peuster, M.; Wohlsein, P.; Brügmann, M.; Ehlerding, M.; Seidler, K.; Fink, C.; Brauer, H.; Fischer, A.; Hausdorf, G. A novel approach to temporary stenting: Degradable cardiovascular stents produced from corrodible metal—Results 6–18 months after implantation into New Zealand white rabbits. Heart 2001, 86, 563–569. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef]
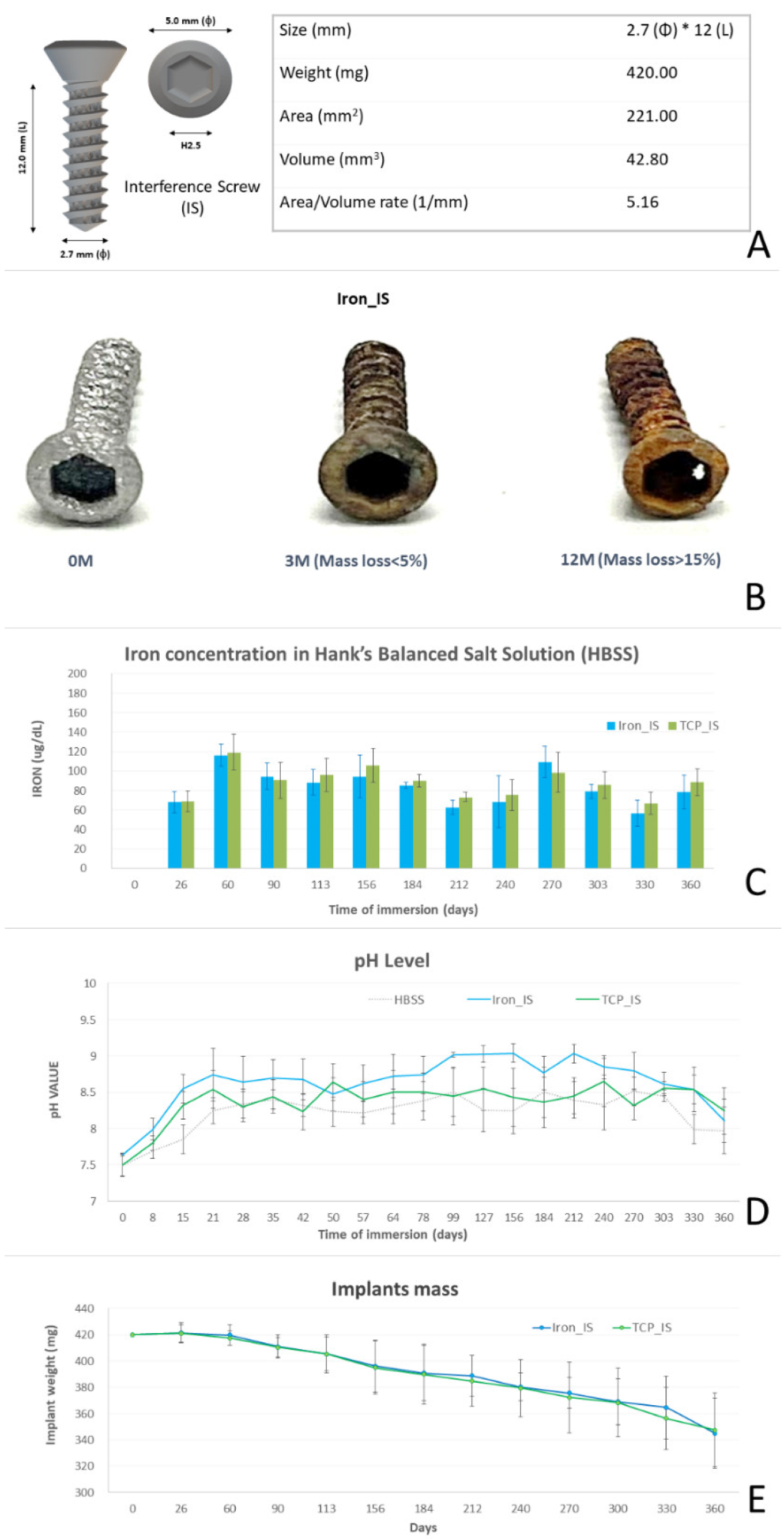

| Day (s) | 0 | 26 | 60 | 90 | 113 | 156 | 184 | 212 | 240 | 270 | 303 | 330 | 360 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Implant weight (mg) | Iron_IS | 420.00 ± 0.00 | 421.33 ± 7.64 | 419.67 ± 7.77 | 410.83 ± 8.86 | 405.13 ± 14.50 | 396.00 ± 19.95 | 390.63 ± 21.17 | 388.57 ± 15.71 | 380.07 ± 10.79 | 375.53 ± 11.67 | 368.80 ± 17.60 | 364.50 ± 23.95 | 344.67 ± 26.63 |
| TCP_IS | 420.00 ± 0.00 | 420.90 ± 6.50 | 417.30 ± 5.70 | 410.20 ± 7.40 | 405.20 ± 12.90 | 394.90 ± 20.50 | 389.80 ± 23.00 | 384.70 ± 19.50 | 379.30 ± 21.90 | 372.10 ± 27.10 | 368.20 ± 26.10 | 356.20 ± 23.70 | 347.30 ± 28.10 | |
| Weight loss (mg) | Iron_IS | NA | −1.33 | 1.67 | 8.83 | 5.70 | 9.13 | 5.37 | 2.07 | 8.50 | 4.53 | 6.73 | 4.30 | 19.83 |
| TCP_IS | NA | −0.90 | 3.60 | 7.10 | 5.00 | 10.30 | 5.10 | 5.10 | 5.40 | 7.20 | 3.90 | 12.00 | 8.90 | |
| Average weight loss per month (mg/m) | IRON_IS | 6.28 ± 4.95 | ||||||||||||
| TCP_IS | 6.06 ± 2.71 | |||||||||||||
| Time (Months) | |||
|---|---|---|---|
| Group | 3 | 6 | 12 |
| Control | 25.90 ± 6.96 | 39.68 ± 6.63 | 47.53 ± 12.36 |
| Iron_IS | 37.61 ± 5.14 | 44.88 ± 6.70 | 51.76 ± 13.53 |
| TCP_IS | 39.51 ± 6.46 | 47.20 ± 8.60 | 54.62 ± 11.63 |
| p value between groups | |||
| Control vs. Iron_IS | 0.0025 | 0.1560 | 0.5400 |
| Iron_IS vs. TCP_IS | 0.5374 | 0.5671 | 0.6702 |
| Control vs. TCP_IS | 0.0026 | 0.0919 | 0.2903 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tai, C.-C.; Huang, Y.-M.; Liaw, C.-K.; Yang, K.-Y.; Ma, C.-H.; Huang, S.-I.; Huang, C.-C.; Tsai, P.-I.; Shen, H.-H.; Sun, J.-S.; et al. Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study. Int. J. Mol. Sci. 2022, 23, 14626. https://doi.org/10.3390/ijms232314626
Tai C-C, Huang Y-M, Liaw C-K, Yang K-Y, Ma C-H, Huang S-I, Huang C-C, Tsai P-I, Shen H-H, Sun J-S, et al. Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study. International Journal of Molecular Sciences. 2022; 23(23):14626. https://doi.org/10.3390/ijms232314626
Chicago/Turabian StyleTai, Chien-Cheng, Yu-Min Huang, Chen-Kun Liaw, Kuo-Yi Yang, Chun-Hsien Ma, Shin-I Huang, Chih-Chieh Huang, Pei-I Tsai, Hsin-Hsin Shen, Jui-Sheng Sun, and et al. 2022. "Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study" International Journal of Molecular Sciences 23, no. 23: 14626. https://doi.org/10.3390/ijms232314626
APA StyleTai, C.-C., Huang, Y.-M., Liaw, C.-K., Yang, K.-Y., Ma, C.-H., Huang, S.-I., Huang, C.-C., Tsai, P.-I., Shen, H.-H., Sun, J.-S., & Chen, C.-Y. (2022). Biocompatibility and Biological Performance of Additive-Manufactured Bioabsorbable Iron-Based Porous Interference Screws in a Rabbit Model: A 1-Year Observational Study. International Journal of Molecular Sciences, 23(23), 14626. https://doi.org/10.3390/ijms232314626







