Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Etichal Approval
2.2. Animal Management
2.3. Dietary Treatments
2.4. Feeding Intake, Sampling, and Analysis
2.5. Blood and Colostrum Sample Collection and Analyses
2.6. Data Analysis
3. Results
3.1. DM and Fatty Acids Intake
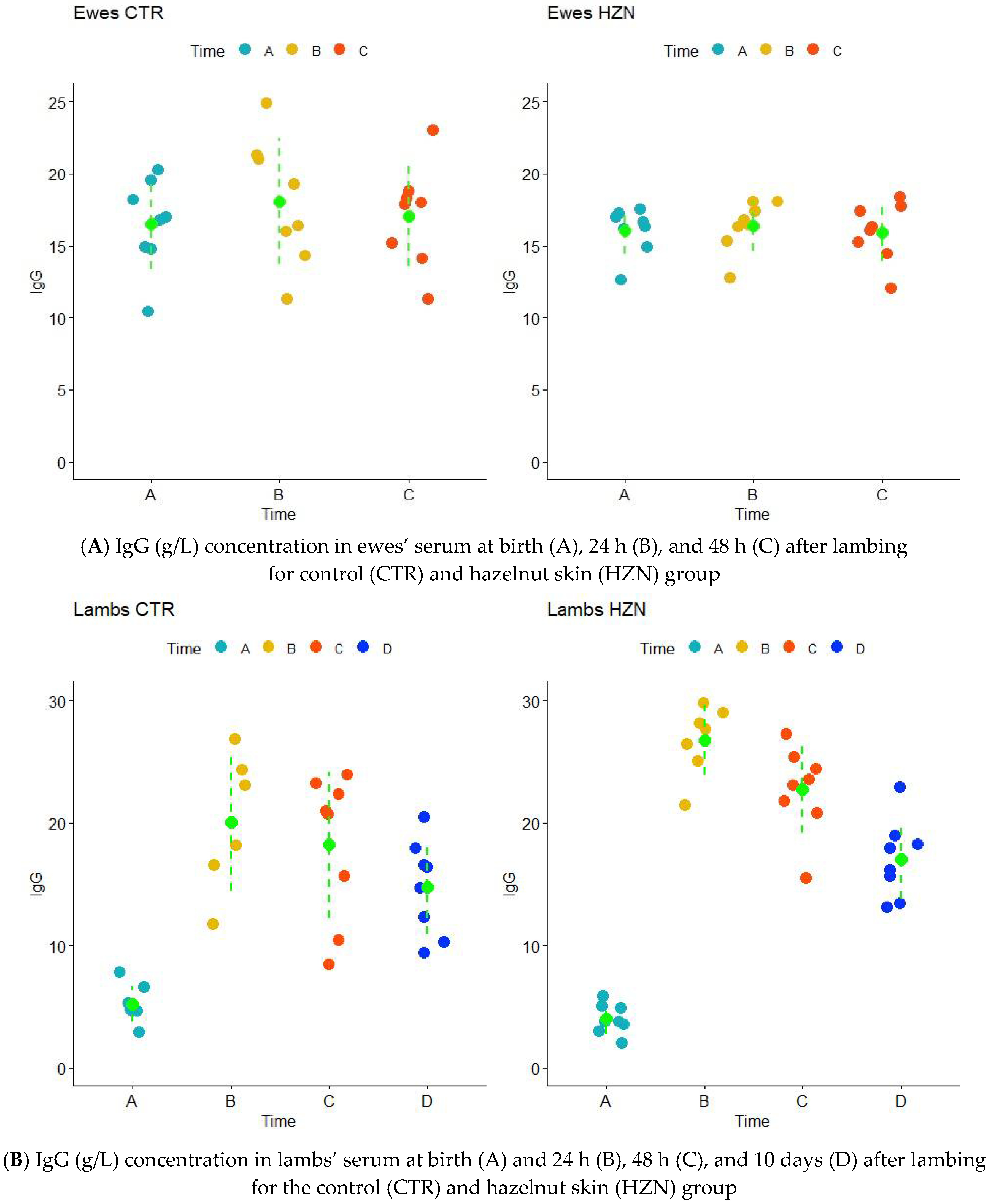
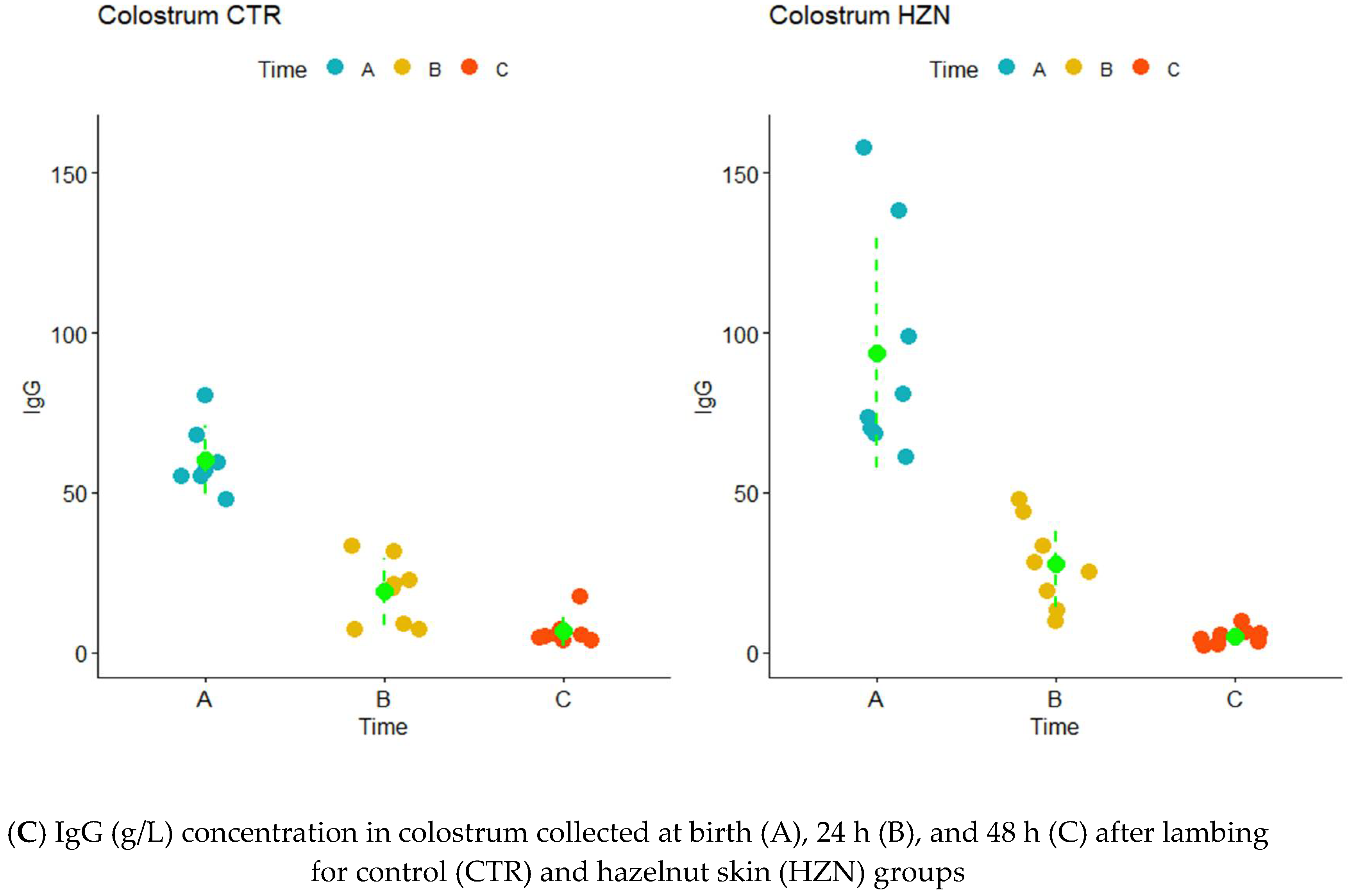
3.2. IgG Concentration
3.3. GGT and LDH Activity Levels IgG Concentration
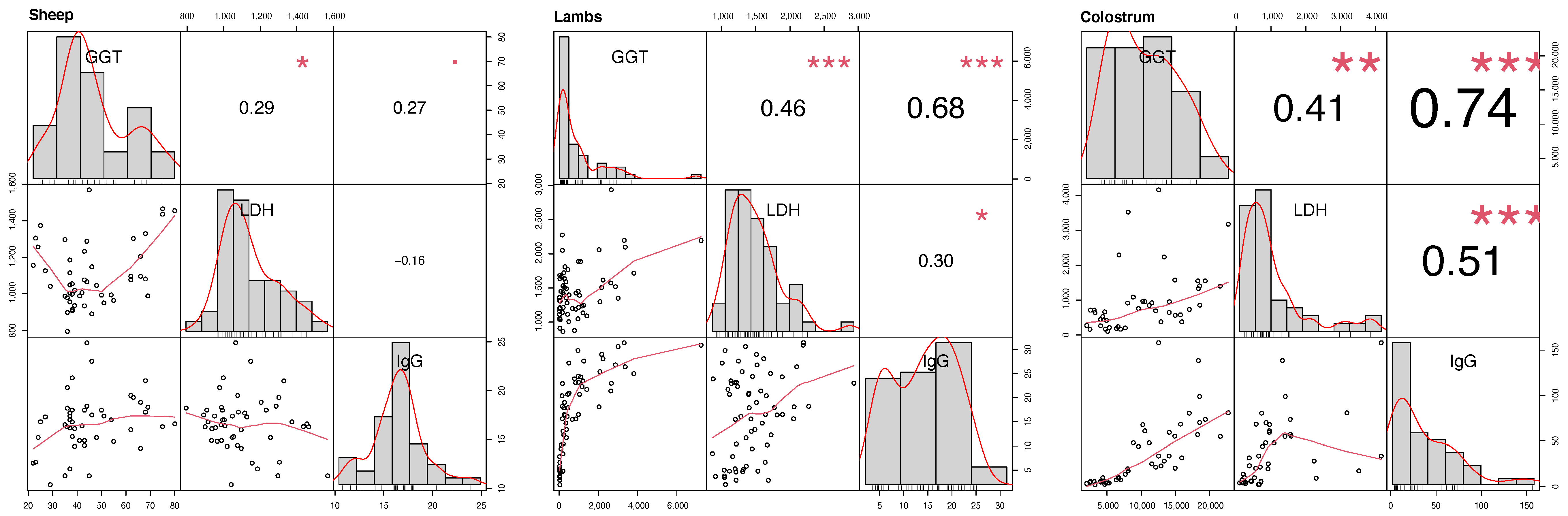
3.4. IgG, GGT, and LDH Enzyme Activity Levels Correlation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanza, M.; Bella, M.; Priolo, A.; Barbagallo, D.; Galofaro, V.; Landi, C.; Pennisi, P. Lamb meat quality as affected by a natural or artificial milk feeding regime. Meat Sci. 2006, 73, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Castellano, L.E.; Suárez-Trujillo, A.; Martell-Jaizme, D.; Cugno, G.; Argüello, A.; Castro, N. The effect of colostrum period management on BW and immune system in lambs: From birth to weaning. Animal 2015, 9, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Cabral, R.G.; Chapman, C.E.; Aragona, K.M.; Clark, E.; Lunak, M.; Erickson, P.S. Predicting colostrum quality from performance in the previous lactation and environmental changes. J. Dairy Sci. 2016, 99, 4048–4055. [Google Scholar] [CrossRef] [PubMed]
- Loste, A.; Ramos, J.J.; Fernández, A.; Ferrer, L.M.; Lacasta, D.; Verde, M.T.; Marca, M.C.; Ortín, A. Effect of colostrum treated by heat on immunological parameters in newborn lambs. Livest. Sci. 2008, 117, 176–183. [Google Scholar] [CrossRef]
- Weaver, D.M.; Tyler, J.W.; VanMetre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive Transfer of Colostral Immunoglobulins in Calves. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef]
- Ahmad, R.; Khan, A.; Javed, M.T.; Hussain, I. The level of immunoglobulins in relation to neonatal lamb mortality in Pak-Karakul sheep. Vet. Arh. 2000, 70, 129–139. [Google Scholar]
- Hernández-Castellano, L.; Wall, S.K.; Stephan, R.; Corti, S.; Bruckmaier, R. Milk somatic cell count, lactate dehydrogenase activity, and immunoglobulin G concentration associated with mastitis caused by different pathogens: A field study. Schweiz. Arch. Tierheilkd. 2017, 159, 283–290. [Google Scholar] [CrossRef][Green Version]
- Braun, J.P.; Trumel, C.; Bézille, P. Clinical biochemistry in sheep: A selected review. Small Rumin. Res. 2010, 92, 10–18. [Google Scholar] [CrossRef]
- Conneely, M.; Berry, D.P.; Sayers, R.; Murphy, J.P.; Lorenz, I.; Doherty, M.L.; Kennedy, E. Factors associated with the concentration of immunoglobulin G in the colostrum of dairy cows. Animal 2013, 7, 1824–1832. [Google Scholar] [CrossRef]
- Ahmadi, M.; Boldura, O.; Milovanov, C.; Dronca, D.; Mircu, C.; Hutu, I.; Popescu, S.; Padeanu, I.; Tulcan, C. Colostrum from Different Animal Species–A Product for Health Status Enhancement. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca 2016, 73, 1–7. [Google Scholar] [CrossRef]
- Torres-Rovira, L.; Pesantez-Pacheco, J.-L.; Hernandez, F.; Elvira-Partida, L.; Perez-Solana, M.-L.; Gonzalez-Martin, J.-V.; Gonzalez-Bulnes, A.; Astiz, S. Identification of factors affecting colostrum quality of dairy Lacaune ewes assessed with the Brix refractometer. J. Dairy Res. 2017, 84, 440–443. [Google Scholar] [CrossRef] [PubMed]
- Zarei, S.; Ghorbani, G.R.; Khorvash, M.; Martin, O.; Mahdavi, A.H.; Riasi, A. The Impact of Season, Parity, and Volume of Colostrum on Holstein Dairy Cows Colostrum Composition. Agric. Sci. 2017, 8, 572–581. [Google Scholar] [CrossRef]
- Massimini, G.; Peli, A.; Boari, A.; Britti, D. Evaluation of assay procedures for prediction of passive transfer status in lambs. Am. J. Vet. Res. 2006, 67, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Tarquino, C.F.; Flaiban, K.M.C.; Lisboa, J.A.N. Transferência de imunidade passiva em cordeiros de corte manejados extensivamente em clima tropical. Braz. J. Vet. Res. 2011, 31, 199–205. [Google Scholar] [CrossRef]
- Alves, A.C.; Alves, N.G.; Ascari, I.J.; Junquinera, F.B.; Coutinho, A.S.; Lima, R.R.; Pérez, J.R.O.; De Paula, S.O.; Furusho-Garcia, I.F.; Abreu, L.R. Colostrum composition of Santa Inês sheep and passive transfer of immunity to lambs. J. Dairy Sci. 2015, 98, 3706–3716. [Google Scholar] [CrossRef]
- Highland, M.A.; Berglund, A.K.; Knowles, D.P. Passive transfer in domestic and bighorn lambs total IgG in ewe sera and colostrum and serum IgG kinetics in lambs following colostrum ingestion are similar in domestic sheep and bighorn sheep (Ovis aries and Ovis canadensis). Sheep Goat Res. J. 2017, 32, 36–42. [Google Scholar]
- Kessler, E.C.; Bruckmaier, R.M.; Gross, J.J. Immunoglobulin G content and colostrum composition of different goat and sheep breeds in Switzerland and Germany. J. Dairy Sci. 2019, 102, 5542–5549. [Google Scholar] [CrossRef]
- Maden, M.; Birdane, F.M.; Altunok, V.; Dere, S. Serum and colostrum/milk alkaline phosphatase activities in the determination of passive transfer status in healthy lambs. Rev. Med. Vet. 2004, 155, 565–569. [Google Scholar]
- Britti, D.; Massimini, G.; Peli, A.; Luciani, A.; Boari, A. Evaluation of serum enzyme activities as predictors of passive transfer status in lambs. J. Am. Vet. Med. Assoc. 2005, 226, 951–955. [Google Scholar] [CrossRef]
- Hogan, I.; Doherty, M.; Fagan, J.; Kennedy, E.; Conneely, M.; Brady, P.; Ryan, C.; Lorenz, I. Comparison of rapid laboratory tests for failure of passive transfer in the bovine. Ir. Vet. J. 2015, 68, 1–10. [Google Scholar] [CrossRef]
- Gökçe, E.; Atakişi, O. Interrelationships of Serum and Colostral IgG (Passive Immunity) with Total Protein Concentrations and Health Status in Lambs. Kafkas Univ. Vet. Fak. Derg. 2018, 25, 387–396. [Google Scholar] [CrossRef]
- Tessman, R.K.; Tyler, J.W.; Parish, S.M.; Johnson, D.L.; Gant, R.G.; Grasseshi, H.A. Use of age and serum gamma-glutamyl-transferase activity to assess passive transfer status in lambs. J. Am. Vet. Med. Assoc. 1997, 211, 1163–1164. [Google Scholar] [PubMed]
- Tate, S.S.; Meister, A. γ-Glutamyl transpeptidase: Catalytic, structural and functional aspects. In The Biological Effects of Glutamic Acid and Its Derivatives; Najjar, V.A., Ed.; Developments in Molecular and Cellular Biochemistry Volume 1; Springer: Berlin/Heidelberg, Germany, 1981; pp. 357–368. [Google Scholar]
- Hu, G.; Tuomilehto, J.; Pukkala, E.; Hakulinen, T.; Antikainen, R.; Vartiainen, E.; Jousilahti, P. Joint effects of coffee consumption and serum gamma-glutamyltransferase on the risk of liver cancer. Hepatology 2008, 48, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.F.; Longui, C.A.; Miorin, L.A.; Sens, Y.A. Gamma-Glutamyltransferase Activity in Chronic Dialysis Patients and Renal Transplant Recipients With Hepatitis C Virus Infection. Transplant. Proc. 2008, 40, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.R.; Garcia, C.; Barber, T. Regulation of amino acid metabolism during lactation. Recent Res. Dev. Nutr. 2001, 4, 101–111. [Google Scholar]
- Pero, M.E.; Mirabella, N.; Lombardi, P.; Squillacioti, C.; De Luca, A.; Avallone, L. Gammaglutamyltransferase activity in buffalo mammary tissue during lactation. Anim. Sci. 2006, 82, 351–354. [Google Scholar] [CrossRef]
- Lehmann, M.; Wellnitz, O.; Bruckmaier, R.M. Concomitant lipopolysaccharide-induced transfer of blood-derived components including immunoglobulins into milk. J. Dairy Sci. 2013, 96, 889–896. [Google Scholar] [CrossRef]
- Lombardi, P.; Avallone, L.; Pagnini, U.; D’Angelo, D.; Bogin, E. Evaluation of Buffalo Colostrum Quality by Estimation of Enzyme Activity Levels. J. Food Prot. 2001, 64, 1265–1267. [Google Scholar] [CrossRef]
- Maden, M.; Altunok, V.; Birdane, F.M.; Aslan, V.; Nizamlioglu, M. Blood and Colostrum/Milk Serum γ-Glutamyltransferase Activity as a Predictor of Passive Transfer Status in Lambs. J. Vet. Med. Ser. B 2003, 50, 128–131. [Google Scholar] [CrossRef]
- Zarrilli, A.; Micera, E.; Lacarpia, N.; Lombardi, P.; Pero, M.E.; Pelagalli, A.; D’Angelo, D.; Mattia, M.; Avallone, L. Evaluation of goat colostrum quality by determining enzyme activity levels. Livest. Prod. Sci. 2003, 83, 317–320. [Google Scholar] [CrossRef]
- Zarrilli, A.; Micera, E.; Lacarpia, N.; Lombardi, P.; Pero, M.E.; Pelagalli, A.; D’Angelo, D.; Mattia, M.; Avallone, L. Evaluation of ewe colostrum quality by estimation of enzyme activity levels. Rev. Med. Vet. 2003, 154, 521–523. [Google Scholar]
- Aydogdu, U.; Guzelbektes, H. Effect of colostrum composition on passive calf immunity in primiparous and multiparous dairy cows. Vet. Med. 2019, 63, 1–11. [Google Scholar] [CrossRef]
- Belkasmi, F.; Madani, T.; Mouffok, C.; Semara, L. Enzymatic quality of colostrum in Ouled Djellal ewes, Algeria. Biol. Rhythm Res. 2019, 53, 1–9. [Google Scholar] [CrossRef]
- Pecka-Kiełb, E.; Zachwieja, A.; Wojtas, E.; Zawadzki, W. Influence of nutrition on the quality of colostrum and milk of ruminants. Mljekarstvo 2018, 68, 169–181. [Google Scholar] [CrossRef]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Serra, A.; Mele, M.; Bernabucci, U. Conjugated linoleic acid isomers strongly improve the redox status of bovine mammary epithelial cells (BME-UV1). J. Dairy Sci. 2015, 98, 7071–7082. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. Redox Biology in Transition Periods of Dairy Cattle: Role in the Health of Periparturient and Neonatal Animals. Antioxidants 2019, 8, 20. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary Polyphenol Supplementation in Food Producing Animals: Effects on the Quality of Derived Products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Mitlianga, P. Fruit and Vegetable Co-Products as Functional Feed Ingredients in Farm Animal Nutrition for Improved Product Quality. Agriculture 2015, 5, 1020–1034. [Google Scholar] [CrossRef]
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef]
- Schieber, A.; Stintzing, F.C.; Carle, R. By-products of plant food processing as a source of functional compounds—recent developments. Trends Food Sci. Technol. 2001, 12, 401–413. [Google Scholar] [CrossRef]
- Liso, G.; Palmieri, A.; Pirazzoli, C.; Moriello, M.S. Prospettive e opportunità in Italia per un’efficiente filiera corilicola. Suppl. Terra Vita 2017, 5, 6–15. (In Italian) [Google Scholar]
- Caccamo, M.; Valenti, B.; Luciano, G.; Priolo, A.; Rapisarda, T.; Belvedere, G.; Marino, V.M.; Esposto, S.; Taticchi, A.; Servili, M.; et al. Hazelnut as Ingredient in Dairy Sheep Diet: Effect on Sensory and Volatile Profile of Cheese. Front. Nutr. 2019, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Campione, A.; Natalello, A.; Valenti, B.; Luciano, G.; Rufino-Moya, P.J.; Avondo, M.; Morbidini, L.; Pomente, C.; Krol, B.; Wilk, M.; et al. Effect of Feeding Hazelnut Skin on Animal Performance, Milk Quality, and Rumen Fatty Acids in Lactating Ewes. Animals 2020, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Renna, M.; Lussiana, C.; Malfatto, V.; Gerbelle, M.; Turille, G.; Medana, C.; Ghirardello, D.; Mimosi, A.; Cornale, P. Evaluating the Suitability of Hazelnut Skin as a Feed Ingredient in the Diet of Dairy Cows. Animals 2020, 10, 1653. [Google Scholar] [CrossRef]
- Pereira Neves, A. Desempenho de Ovelhas Pantaneiras Submetidas à Sincronização de Estro e Suplementação Nutricional de Curto Prazo Antes da Estação Reprodutiva. Master’s Thesis, Federal University of Mato Grosso do Sul, Aquidauana, Brasil, 2016. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/156734/1/CNPC-2016-UPA-4.pdf?msclkid=85e646ddc7a411ecb3d8595e46127059 (accessed on 2 November 2021). (In Portuguese).
- Spezzigu, A.; Sale, S.; Bua, S. Controllo ormonale della dinamica follicolare nella pecora: Protocolli di sincronizzazione dei calori. Summa 2017, 63–73. (In Italian) [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2013. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Licitra, G.; Hernandez, T.M.; Van Soest, P.J. Standardization of procedures for nitrogen fractionation of ruminant feeds. Anim. Feed Sci. Technol. 1996, 57, 347–358. [Google Scholar] [CrossRef]
- Nutrient Research Council (NRC); Board on Agriculture and Natural Resources; Subcommittee on Dairy Cattle Nutrition; Committee on Animal Nutrition. Nutrient Requirements of Dairy Cattle: Seventh Revised Edition, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- Alves, S.P.; Cabrita, A.R.J.; Fonseca, A.J.M.; Bessa, R.J.B. Improved method for fatty acid analysis in herbage based on direct transesterification followed by solid-phase extraction. J. Chromatogr. A 2008, 1209, 212–219. [Google Scholar] [CrossRef]
- Cornale, P.; Renna, M.; Lussiana, C.; Bigi, D.; Chessa, S.; Mimosi, A. The Grey Goat of Lanzo Valleys (Fiurinà): Breed characteristics, genetic diversity, and quantitative-qualitative milk traits. Small Rumin. Res. 2014, 116, 1–13. [Google Scholar] [CrossRef]
- Iussig, G.; Renna, M.; Gorlier, A.; Lonati, M.; Lussiana, C.; Battaglini, L.M.; Lombardi, G. Browsing ratio, species intake, and milk fatty acid composition of goats foraging on alpine open grassland and grazable forestland. Small Rumin. Res. 2015, 132, 12–24. [Google Scholar] [CrossRef]
- Enri, S.R.; Probo, M.; Renna, M.; Caro, E.; Lussiana, C.; Battaglini, L.M.; Lombardi, G.; Lonati, M. Temporal variations in leaf traits, chemical composition and in vitro true digestibility of four temperate fodder tree species. Anim. Prod. Sci. 2020, 60, 643. [Google Scholar] [CrossRef]
- Hunter, A.G.; Reneau, J.K.; Williams, J.B. Factors Affecting IgG Concentration in Day-Old Lambs. J. Anim. Sci. 1977, 45, 1146–1151. [Google Scholar] [CrossRef]
- Amadori, M.; Archetti, I.L. La valutazione del benessere nella specie bovina. In Fondazione Iniziative Zooprofilattiche e Zootecniche; Yumpu: Brescia, Italy, 2002. (In Italian) [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In 2nd International Symposium on Information Theory, Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, USSR, 2–8 September 1971; Petrov, B.N., Csaki, F., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Sarkar, D. Lattice: Multivariate Data Visualisation with R; Springer: New York, NY, USA, 2008. [Google Scholar]
- Weiss, W.P.; Spears, J.W. Vitamin and trace mineral effects on immune function of ruminants. In Ruminant Physiology; Wageningen Academic Publishers: Utrecht, The Netherlands, 2006; pp. 473–496. [Google Scholar]
- Hatfield, P.G.; Daniels, J.T.; Kott, R.W.; Burgess, D.E.; Evans, T.J. Role of supplemental vitamin E in lamb survival and production: A review. J. Anim. Sci. 2000, 77, 1–9. [Google Scholar] [CrossRef]
- Bondo, T.; Jensen, S.K. Administration of RRR-α-tocopherol to pregnant mares stimulates maternal IgG and IgM production in colostrum and enhances vitamin E and IgM status in foals. J. Anim. Physiol. Anim. Nutr. 2010, 95, 214–222. [Google Scholar] [CrossRef]
- Wang, L.; Xu, X.; Su, G.; Shi, B.; Shan, A. High concentration of vitamin E supplementation in sow diet during the last week of gestation and lactation affects the immunological variables and antioxidative parameters in piglets. J. Dairy Res. 2016, 84, 8–13. [Google Scholar] [CrossRef]
- Żarczyńska, K.; Samardžija, M.; Sobiech, P. Influence of selenium administration to dry cows on selected biochemical and immune parameters of their offspring. Reprod. Domest. Anim. 2019, 54, 1284–1290. [Google Scholar] [CrossRef]
- Jensen, S.K.; Lauridsen, C. α-Tocopherol Stereoisomers. Vitam. Horm. 2007, 76, 281–308. [Google Scholar] [CrossRef]
- Sterndale, S.; Broomfield, S.; Currie, A.; Hancock, S.; Kearney, G.A.; Lei, J.; Liu, S.; Lockwood, A.; Scanlan, V.; Smith, G.; et al. Supplementation of Merino ewes with vitamin E plus selenium increases α-tocopherol and selenium concentrations in plasma of the lamb but does not improve their immune function. Animal 2018, 12, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Mazur-Kuśnirek, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in Monogastric Nutrition—A Review. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited review: Plant polyphenols and rumen microbiota responsible for fatty acid biohydrogenation, fiber digestion, and methane emission: Experimental evidence and methodological approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, W.K. Dietary Application of Tannins as a Potential Mitigation Strategy for Current Challenges in Poultry Production: A Review. Animals 2020, 10, 2389. [Google Scholar] [CrossRef] [PubMed]
- Caprarulo, V.; Giromini, C.; Rossi, L. Review: Chestnut and quebracho tannins in pig nutrition: The effects on performance and intestinal health. Animal 2020, 15, 100064. [Google Scholar] [CrossRef] [PubMed]
- Prodanović, R.; Nedić, S.; Simeunović, P.; Borozan, S.; Nedić, S.; Bojkovski, J.; Kirovski, D.; Vujanac, I. Effects of chestnut tannins supplementation of prepartum moderate yielding dairy cows on metabolic health, antioxidant and colostrum indices. Ann. Anim. Sci. 2021, 21, 609–621. [Google Scholar] [CrossRef]
- Vela, C.M. Avaliação da Absorção Colostral em Neonatos Ovinos da raça Bergamácia. Master’s Thesis, Paulista University, São Paulo, Brazil, 2011. Available online: https://repositorio.unesp.br/handle/11449/89253 (accessed on 23 December 2021). (In Portuguese).
- Pugh, D.G.; Braid, A.N. Reference intervals and conversions. In Sheep and Goat Medicine, 2nd ed.; Elsevier Saundars: Maryland Heights, MO, USA, 2018; pp. 596–597. [Google Scholar]
- Zhu, H.; Zhao, X.; Chen, S.; Tan, W.; Han, R.; Qi, Y.; Huang, D.; Yang, Y. Evaluation of colostrum bioactive protein transfer and blood metabolic traits in neonatal lambs in the first 24 hours of life. J. Dairy Sci. 2021, 104, 1164–1174. [Google Scholar] [CrossRef]
- Vatankhan, M. Relationship between immunoglobulin concentrations in the ewe’s serum and colostrum, and lamb’s serum in Lori-Bakhtiari Sheep. Iran. J. Appl. Anim. Sci. 2013, 3, 539–544. [Google Scholar]
- Bórnez, R.; Linares, M.B.; Vergara, H. Haematological, hormonal and biochemical blood parameters in lamb: Effect of age and blood sampling time. Livest. Sci. 2009, 121, 200–206. [Google Scholar] [CrossRef]
- Cestaro, A. Meccanismi di Trasferimento Della Componente Proteica Colostrale Nella Specie Bufalina. Ph.D. Thesis, University of Naples Federico II, Naples, Italy, 2008. (In Italian). [Google Scholar]
- Pauli, J.V. Colostral transfer of gamma glutamyl transferase in lambs. N. Z. Vet. J. 1983, 31, 150–151. [Google Scholar] [CrossRef]
- Chagunda, M.G.; Larsen, T.; Bjerring, M.; Ingvartsen, K.L. L-lactate dehydrogenase and N-acetyl-β-D-glucosaminidase activities in bovine milk as indicators of non-specific mastitis. J. Dairy Res. 2006, 73, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Experimental Zooprofilactic Institute of Lombardy and Emilia Romagna Bruno Ubertini (IZSLERBU). Valutazione del Benessere Animale nell’Allevamento degli Ovini e dei Caprini: Manuale Esplicativo Controllo Ufficiale; Centro di Referenza Nazionale per il Benessere Animale: Brescia, Italy, 2021. (In Italian) [Google Scholar]
| Gestation | Lactation | ||||
|---|---|---|---|---|---|
| CTR | HZN | CTR | HZN | ||
| Ingredients (g/kg DM) | |||||
| Hay | 664 | 562 | 719 | 637 | |
| Experimental concentrates 1 | |||||
| Barley | 110 | 111 | 89 | 90 | |
| Maize | 109 | 111 | 89 | 90 | |
| Soybean meal (44% CP) | 56 | 57 | 45 | 46 | |
| Rumen-protected fat | 18 | - | 15 | - | |
| Hazelnut skin | - | 116 | - | 94 | |
| Proximate composition (g/kg DM, unless otherwise stated) | |||||
| DM (g/kg) | 889 | 891 | 889 | 890 | |
| Ash | 61 | 53 | 71 | 65 | |
| CP | 116 | 121 | 111 | 115 | |
| RDP (% CP) | 66.5 | 60.0 | 66.6 | 61.0 | |
| EE | 35 | 56 | 31 | 49 | |
| NDF | 533 | 519 | 564 | 554 | |
| ADF | 306 | 314 | 326 | 323 | |
| ADL | 51 | 74 | 53 | 72 | |
| NSC 2 | 248 | 243 | 222 | 218 | |
| NEL (MJ/kg DM) | 6.1 | 6.3 | 5.9 | 6.1 | |
| Gestation | Effects | Lactation | Effects | |||||
|---|---|---|---|---|---|---|---|---|
| CTR | HZN | DT | SD | CTR | HZN | DT | SD | |
| DMI (kg/d) | 1.54 | 1.51 | ns | ns | 1.89 | 1.86 | ns | ns |
| C16:0 | 12.95 | 6.55 | *** | ns | 13.57 | 7.20 | *** | ns |
| C18:0 | 1.41 | 1.39 | *** | ns | 1.51 | 1.49 | *** | ns |
| C18:1c9 (OA) | 10.17 | 37.50 | *** | ns | 10.33 | 37.66 | *** | ns |
| C18:2 c6c9c12 (LA) | 10.93 | 13.54 | *** | ns | 11.43 | 14.04 | *** | ns |
| C18:3 c9c12c15 (ALA) | 2.62 | 2.28 | *** | ns | 3.30 | 2.96 | *** | ns |
| ∑SFA | 15.96 | 9.12 | *** | ns | 17.00 | 10.16 | *** | ns |
| ∑MUFA | 10.70 | 38.67 | *** | ns | 10.93 | 38.90 | *** | ns |
| ∑PUFA | 13.69 | 15.93 | *** | ns | 14.90 | 17.15 | *** | ns |
| Sheep 1 (pseudoR2 = 0.04) | Lambs 2 (pseudoR2 = 0.77) | Colostrum 1 (pseudoR2 = 0.75) | ||||
|---|---|---|---|---|---|---|
| IgG (g/L) | Intercept | HZN | Intercept | HZN | Intercept | HZN |
| Estimate | 17.2042 | −1.0833 | 15.228 | 2.503 | 29.078 | 12.947 |
| Standard Error | 0.5671 | 0.8021 | 3.833 | 1.036 | 17.898 | 5.286 |
| t-value | 30.335 | −1.351 | 3.973 | 2.415 | 3.139 | 43.999 |
| p-value | <2 × 10−16 | 0.183 | 0.0154 | 0.019 | 1.625 | 0.0184 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, I.; Tizzani, P.; Perona, G.; Lussiana, C.; Mimosi, A.; Ponzio, P.; Cornale, P. Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs. Animals 2022, 12, 3220. https://doi.org/10.3390/ani12223220
Viola I, Tizzani P, Perona G, Lussiana C, Mimosi A, Ponzio P, Cornale P. Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs. Animals. 2022; 12(22):3220. https://doi.org/10.3390/ani12223220
Chicago/Turabian StyleViola, Irene, Paolo Tizzani, Giovanni Perona, Carola Lussiana, Antonio Mimosi, Patrizia Ponzio, and Paolo Cornale. 2022. "Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs" Animals 12, no. 22: 3220. https://doi.org/10.3390/ani12223220
APA StyleViola, I., Tizzani, P., Perona, G., Lussiana, C., Mimosi, A., Ponzio, P., & Cornale, P. (2022). Hazelnut Skin in Ewes’ Diet: Effects on Colostrum Immunoglobulin G and Passive Transfer of Immunity to the Lambs. Animals, 12(22), 3220. https://doi.org/10.3390/ani12223220





