Synthesis of Fully Deacetylated Quaternized Chitosan with Enhanced Antimicrobial Activity and Low Cytotoxicity
Abstract
1. Introduction
2. Results and Discussion
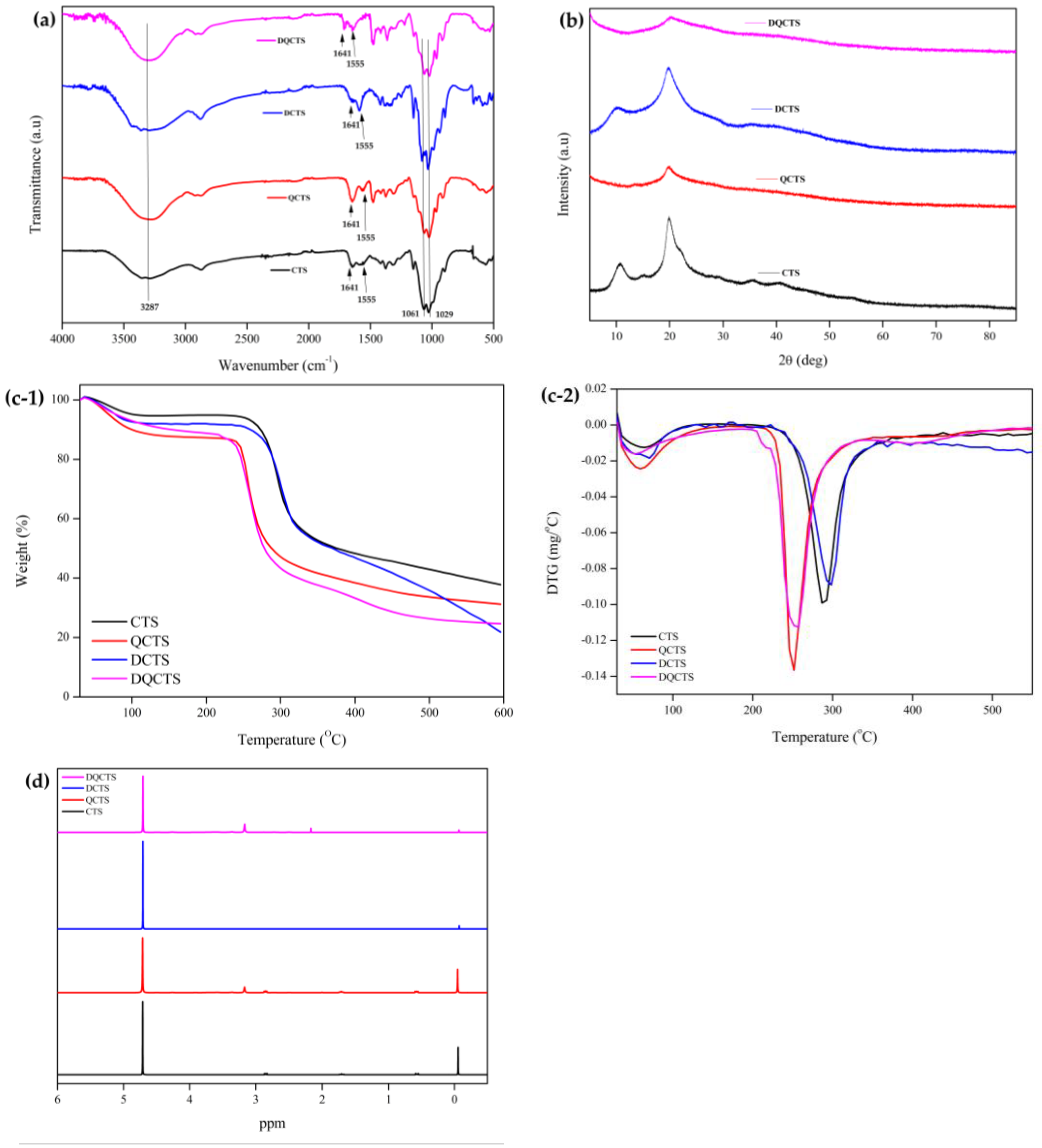
2.1. Characterization of Chitosan Compounds
2.2. Antimicrobial Activity
2.2.1. Minimum Inhibitory Concentrations (MIC), Minimum Bactericidal Concentrations (MBC), and Minimum Fungicidal Concentrations (MFC)
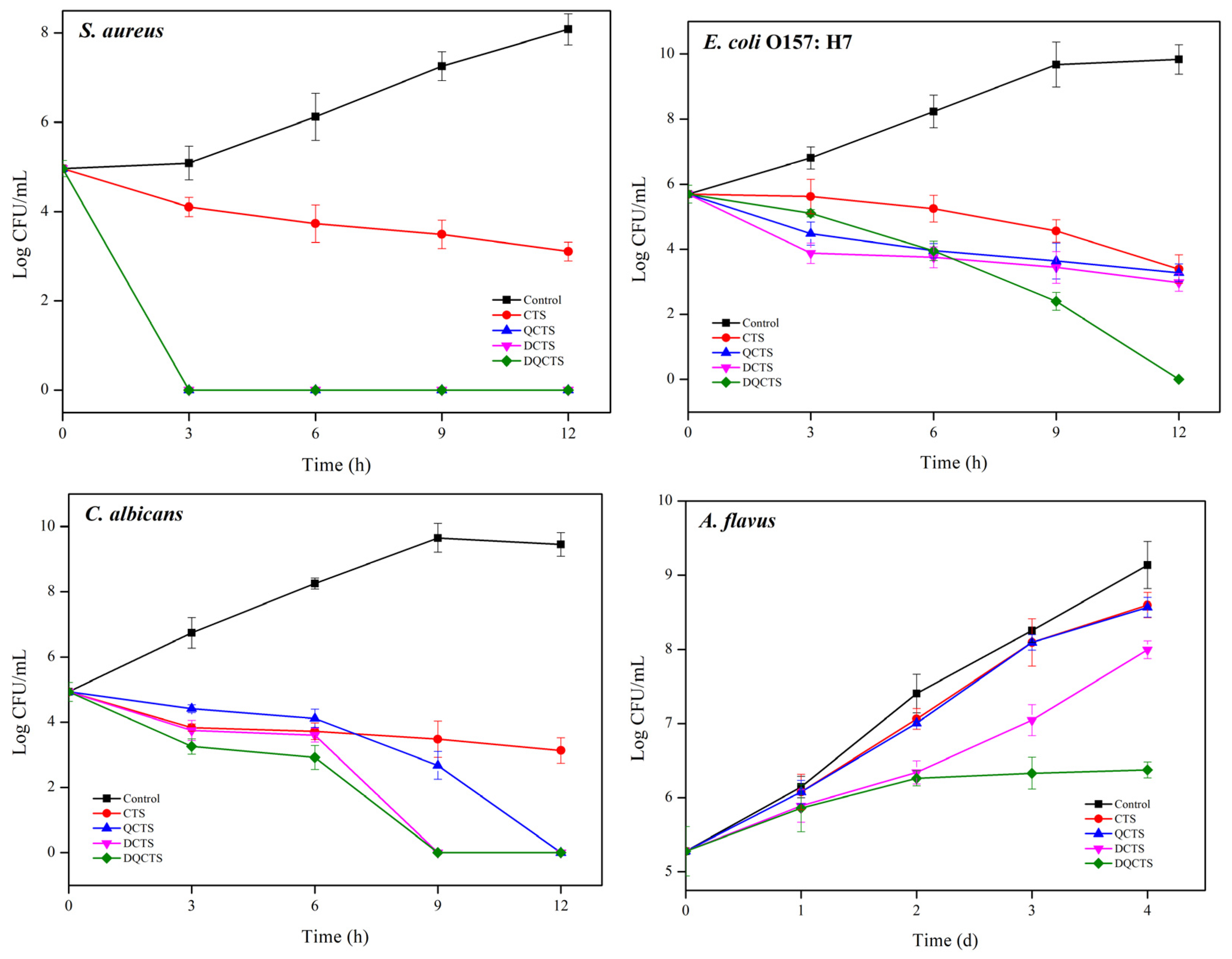
2.2.2. Time Kill Assay
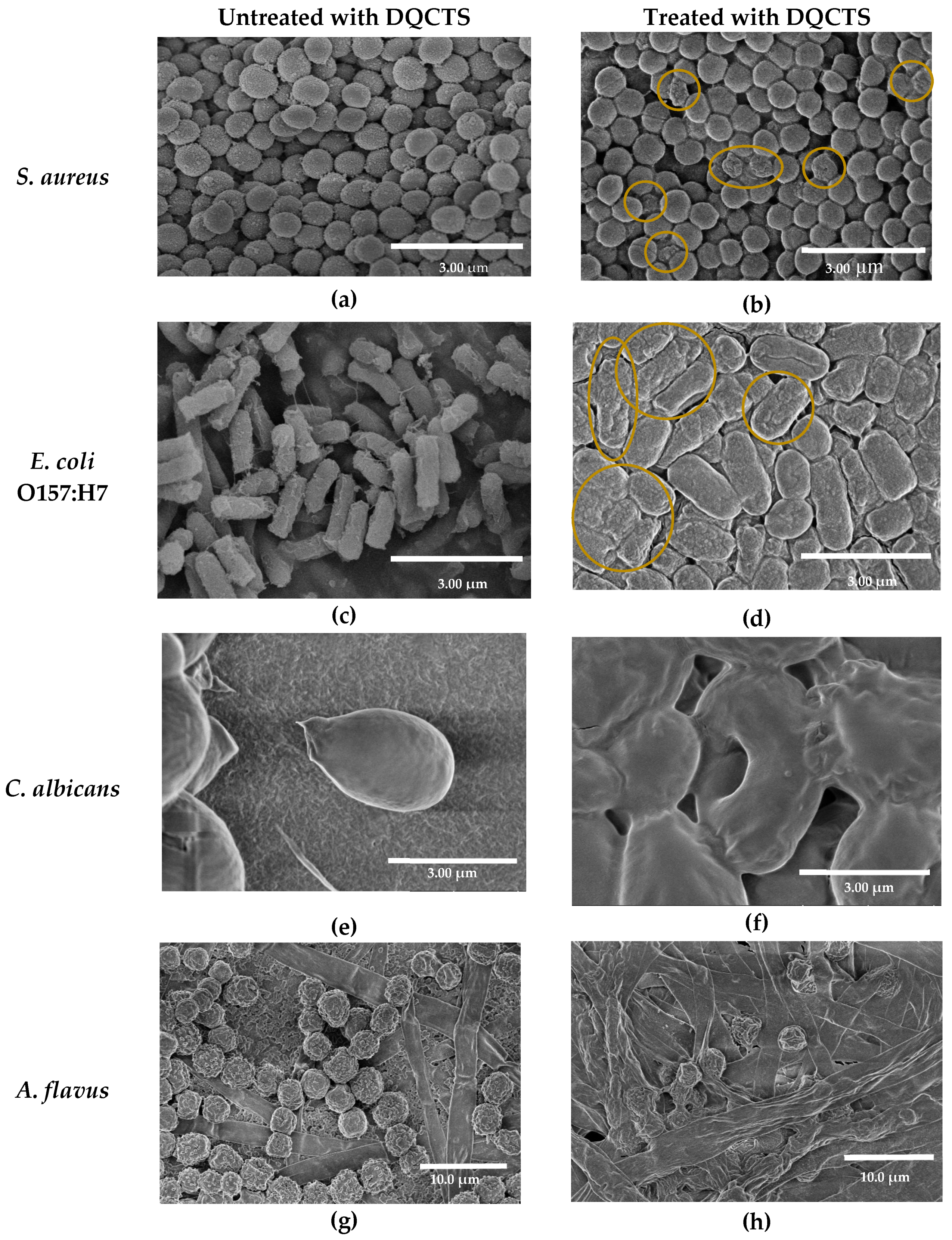
2.2.3. Effect of Fully Deacetylated Quaternized Chitosan (DQCTS) on the Morphology of Microorganisms
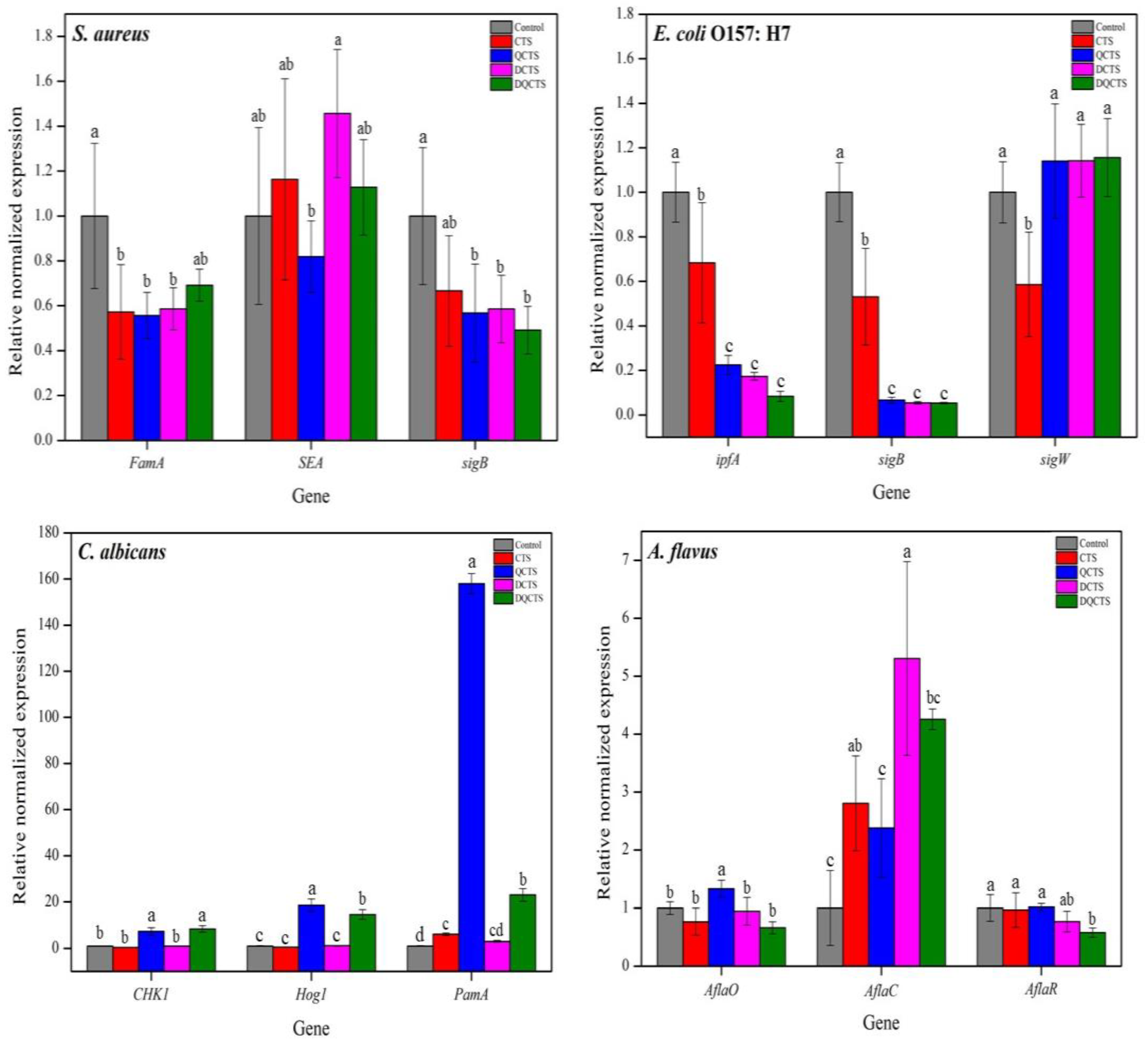
2.2.4. Relative Expression Levels of Catalase, Virulence Factor, and Stress Response Sigma Factors
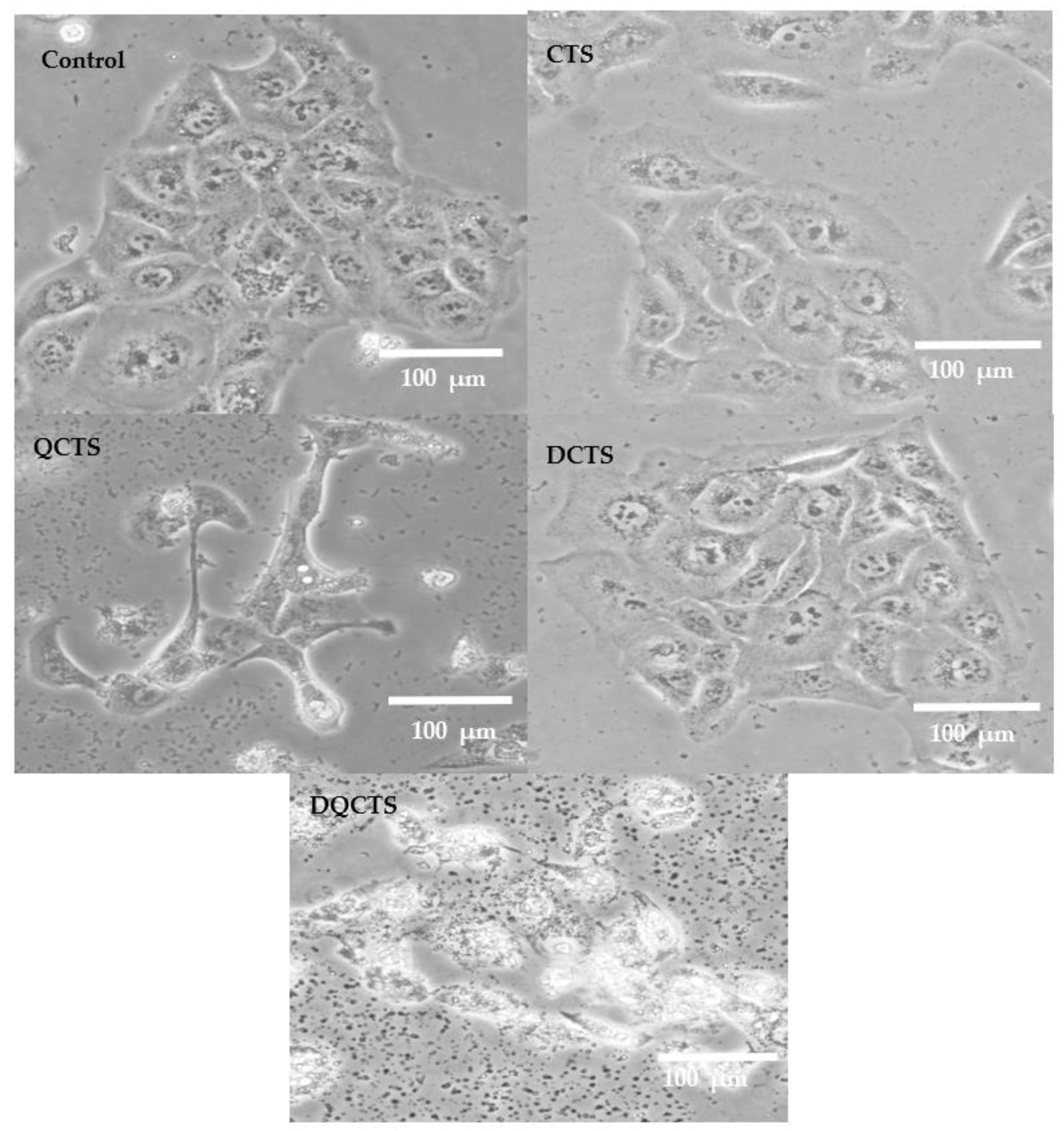
2.3. Cytotoxicity Test
3. Materials and Methods
3.1. Materials
3.2. Preparation of Fully Deacetylated Chitosan
3.3. Preparation of Quaternized Chitosan
3.4. Characterization of Functionalized Chitosan
3.5. Preparation of Microbial Cultures and Assay Media
3.6. Antimicrobial Activity
3.6.1. Minimum inhibitory Concentrations (MIC), Minimum Bactericidal Concentrations (MBC), and Minimum Fungicidal Concentrations (MFC)
3.6.2. Time Kill Assay
3.6.3. Effect of Chitosan Compounds on the Morphology of Microbial Cells
3.6.4. Quantitative Real-Time PCR (qRT-PCR)
3.7. Cytotoxicity Test
3.7.1. Cell Culture
3.7.2. Cell Viability
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butchosa, N.; Brown, C.; Larsson, P.T.; Berglund, L.A.; Bulone, V.; Zhou, Q. Nanocomposites of bacterial cellulose nanofibers and chitin nanocrystals: Fabrication, characterization and bactericidal activity. Green Chem. 2013, 15, 3404–3413. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Morley, K.L.; Chauve, G.; Kazlauskas, R.; Dupont, C.; Shareck, F.; Marchessault, R.H. Acetyl xylan esterase-catalyzed deacetylation of chitin and chitosan. Carbohydr. Polym. 2006, 63, 310–315. [Google Scholar] [CrossRef]
- Al Sagheer, F.; Al-Sughayer, M.; Muslim, S.; Elsabee, M. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Tolaimate, A.; Desbrieres, J.; Rhazi, M.; Alagui, A. Contribution to the preparation of chitins and chitosans with controlled physico-chemical properties. Polymer 2003, 44, 7939–7952. [Google Scholar] [CrossRef]
- He, X.; Li, K.; Xing, R.; Liu, S.; Hu, L.; Li, P. The production of fully deacetylated chitosan by compression method. Egypt J. Aquat. Res. 2016, 42, 75–81. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.H.; Yoon, K.S.; Shankar, S.; Rhim, J.-W. Comparative antibacterial and antifungal activities of sulfur nanoparticles capped with chitosan. Microb. Pathog. 2020, 144, 104178. [Google Scholar] [CrossRef]
- Niamlang, P.; Tongrain, T.; Ekabutr, P.; Chuysinuan, P.; Supaphol, P. Preparation, characterization, and biocompatibility of poly(vinyl alcohol) films containing tetracycline hydrochloride-loaded quaternized chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P.; Tantayanon, S. Antibacterial activity of quaternary ammonium chitosan containing mono or disaccharide moieties: Preparation and characterization. Int. J. Biol. Macromol. 2009, 44, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical applications of quaternized chitosan. Polymers. 2021, 13, 2514. [Google Scholar] [CrossRef] [PubMed]
- Terayama, H.; Terayama, E. High molecular antibacterial substances derived from chitin. About the manufacture of Macramin. J. Antibiot. 1948, 2, 44–45. [Google Scholar]
- Polnok, A.; Borchard, G.; Verhoef, J.; Sarisuta, N.; Junginger, H. Influence of methylation process on the degree of quaternization of N-trimethyl chitosan chloride. Eur. J. Pharm. Biopharm. 2004, 57, 77–83. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, L.; Du, L.; Guo, S.; Wang, X.; Tang, T. Adjustment of the antibacterial activity and biocompatibility of hydroxypropyltrimethyl ammonium chloride chitosan by varying the degree of substitution of quaternary ammonium. Carbohydr. Polym. 2010, 81, 275–283. [Google Scholar] [CrossRef]
- Jaworska, M.; Sakurai, K.; Gaudon, P.; Guibal, E. Influence of chitosan characteristics on polymer properties. I: Crystallographic properties. Polym. Int. 2003, 52, 198–205. [Google Scholar] [CrossRef]
- Suh, J.F.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [PubMed]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Tharun, J.; Hwang, Y.; Roshan, R.; Ahn, S.; Kathalikkattil, A.C.; Park, D. A novel approach of utilizing quaternized chitosan as a catalyst for the eco-friendly cycloaddition of epoxides with CO2. Catal. Sci. Technol. 2012, 2, 1674–1680. [Google Scholar] [CrossRef]
- Thien, D.; An, N.; Hoa, N. Preparation of fully deacetylated chitosan for adsorption of Hg (ii) ion from aqueous solution. Chem. Sci. J. 2015, 6, 1. [Google Scholar]
- Xie, H.; Zhang, S.; Li, S. Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2. Green Chem. 2006, 8, 630–633. [Google Scholar] [CrossRef]
- Gonil, P.; Sajomsang, W.; Ruktanonchai, U.R.; Pimpha, N.; Sramala, I.; Nuchuchua, O.; Saesoo, S.; Chaleawlert-Umpon, S.; Puttipipatkhachorn, S. Novel quaternized chitosan containing β-cyclodextrin moiety: Synthesis, characterization and antimicrobial activity. Carbohydr. Polym. 2011, 83, 905–913. [Google Scholar] [CrossRef]
- Franklin, T.J.; Snow, G.A. Biochemistry of Antimicrobial Action; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Jones, L.; O'Shea, P. The electrostatic nature of the cell surface of Candida albicans: A role in adhesion. Exp. Mycol. 1994, 18, 111–120. [Google Scholar] [CrossRef]
- Sagoo, S.; Board, R.; Roller, S. Chitosan inhibits growth of spoilage micro-organisms in chilled pork products. Food Microbiol. 2002, 19, 175–182. [Google Scholar] [CrossRef]
- Kotzé, A.R.; Lueβen, H.L.; de Leeuw, B.J.; Verhoef, J.C.; Junginger, H.E. N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: In vitro evaluation in intestinal epithelial cells (Caco-2). Pharm. Res. 1997, 14, 1197–1202. [Google Scholar] [CrossRef]
- Thanou, M.; Kotze, A.; Scharringhausen, T.; Luessen, H.; De Boer, A.; Verhoef, J.; Junginger, H. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Controlled Release 2000, 64, 15–25. [Google Scholar] [CrossRef]
- Van Schaik, W.; Abee, T. The role of σB in the stress response of Gram-positive bacteria–targets for food preservation and safety. Curr. Opin. Biotechnol. 2005, 16, 218–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, A.; Tang, J.; Tang, C.; Chen, J. Comparative effects of food preservatives on the production of staphylococcal enterotoxin I from Staphylococcus aureus isolate. J. Food Qual. 2017, 2017, 9495314. [Google Scholar] [CrossRef]
- Rubini, D.; Varthan, P.V.; Jayasankari, S.; Vedahari, B.N.; Nithyanand, P. Suppressing the phenotypic virulence factors of Uropathogenic Escherichia coli using marine polysaccharide. Microb. Pathog. 2020, 141, 103973. [Google Scholar] [CrossRef] [PubMed]
- San Jose, C.; Monge, R.A.; Perez-Diaz, R.; Pla, J.; Nombela, C. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 1996, 178, 5850–5852. [Google Scholar] [CrossRef]
- Kruppa, M.; Goins, T.; Cutler, J.E.; Lowman, D.; Williams, D.; Chauhan, N.; Menon, V.; Singh, P.; Li, D.; Calderone, R. The role of the Candida albicans histidine kinase (CHK1) gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res. 2003, 3, 289–299. [Google Scholar] [PubMed]
- Regularly Limit of Total Aflatoxin. Available online: http://www.foodsafetykorea.go.kr/portal/safefoodlife/food/foodRvlv/foodRvlv.do (accessed on 1 October 2022).
- Regularly Limit of Total Aflatoxin. Available online: https://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm119194.htm (accessed on 1 October 2022).
- Li, Z.; Lin, S.; An, S.; Liu, L.; Hu, Y.; Wan, L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019, 131, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, W.; Sun, J.; Hou, G.; Chen, Q.; Cong, W.; Zhao, F. Non-toxic O-quaternized chitosan materials with better water solubility and antimicrobial function. Int. J. Biol. Macromol. 2016, 84, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Sun, G.; Zhang, X.; Dai, H.; Liu, Y.; He, C.; Leong, K.W. PEI-g-chitosan, a novel gene delivery system with transfection efficiency comparable to polyethylenimine in vitro and after liver administration in vivo. Bioconjug. Chem. 2006, 17, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Rhim, J. Preparation and characterization of agar/lignin/silver nanoparticles composite films with ultraviolet light barrier and antibacterial properties. Food Hydrocoll. 2017, 71, 76–84. [Google Scholar] [CrossRef]
- Gruškienė, R.; Deveikytė, R.; Makuška, R. Quaternization of chitosan and partial destruction of the quaternized derivatives making them suitable for electrospinning. Chemija 2013, 24, 325–334. [Google Scholar]
- Kim, Y.H.; Lim, J.Y.; Yoon, K.S. Effect of water activity, Yuza (Citrus junos Sieb. ex Tanaka) powder and the mixture of sodium lactate and sodium acetate on quality and safety in beef jerky. Food Nutr. Sci. 2019, 10, 588. [Google Scholar] [CrossRef]
- Shankar, S.; Rhim, J. Amino acid mediated synthesis of silver nanoparticles and preparation of antimicrobial agar/silver nanoparticles composite films. Carbohydr. Polym. 2015, 130, 353–363. [Google Scholar] [CrossRef]
- Ontong, J.C.; Paosen, S.; Shankar, S.; Voravuthikunchai, S.P. Eco-friendly synthesis of silver nanoparticles using Senna alata bark extract and its antimicrobial mechanism through enhancement of bacterial membrane degradation. J. Microbiol. Methods 2019, 165, 105692. [Google Scholar] [CrossRef]
- Choo, K.; Kim, M.; Nansa, S.A.; Bae, M.K.; Lee, C.; Lee, S. Redox potential change by the cystine importer affected on enzymatic antioxidant protection in Deinococcus geothermalis. Antonie Van Leeuwenhoek 2020, 113, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nailis, H.; Coenye, T.; Van Nieuwerburgh, F.; Deforce, D.; Nelis, H.J. Development and evaluation of different normalization strategies for gene expression studies in Candida albicans biofilms by real-time PCR. BMC Mol. Biol. 2006, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Moyne, A.; Marco, M.L. RNA-based detection does not accurately enumerate living Escherichia coli O157: H7 cells on plants. Front. Microbiol. 2016, 7, 223. [Google Scholar] [CrossRef][Green Version]
- Vázquez-Sánchez, D.; Galvão, J.A.; Oetterer, M. Contamination sources, biofilm-forming ability and biocide resistance of Staphylococcus aureus in tilapia-processing facilities. Food Sci. Technol. Int. 2018, 24, 209–222. [Google Scholar] [CrossRef]
- Kim, H.; Kwon, H.; Kim, K.; Lee, S. Antifungal and Antiaflatoxigenic Activities of 1, 8-Cineole and t-Cinnamaldehyde on Aspergillus flavus. Appl. Sci. 2018, 8, 1655. [Google Scholar] [CrossRef]
- Babić, M.; Pajić, M.; Radinović, M.; Boboš, S.; Bulajić, S.; Nikolić, A.; Velebit, B. Effects of Temperature Abuse on the Growth and Staphylococcal Enterotoxin A Gene (sea) Expression of Staphylococcus aureus in Milk. Foodborne Pathog. Dis. 2019, 16, 282–289. [Google Scholar] [CrossRef]
- Cheetham, J.; MacCallum, D.M.; Doris, K.S.; da Silva Dantas, A.; Scorfield, S.; Odds, F.; Smith, D.A.; Quinn, J. MAPKKK-independent regulation of the Hog1 stress-activated protein kinase in Candida albicans. J. Biol. Chem. 2011, 286, 42002–42016. [Google Scholar] [CrossRef]
- Lackmann, J.; Schneider, S.; Edengeiser, E.; Jarzina, F.; Brinckmann, S.; Steinborn, E.; Havenith, M.; Benedikt, J.; Bandow, J.E. Photons and particles emitted from cold atmospheric-pressure plasma inactivate bacteria and biomolecules independently and synergistically. J. R. Soc. Interface 2013, 10, 20130591. [Google Scholar] [CrossRef]
- Brown, A.J.; Budge, S.; Kaloriti, D.; Tillmann, A.; Jacobsen, M.D.; Yin, Z.; Ene, I.V.; Bohovych, I.; Sandai, D.; Kastora, S. Stress adaptation in a pathogenic fungus. J. Exp. Biol. 2014, 217, 144–155. [Google Scholar] [CrossRef]
- Park, E.; Park, S.; Kim, S.; Lee, K.; Chang, J. Lung fibroblasts may play an important role in clearing apoptotic bodies of bronchial epithelial cells generated by exposure to PHMG-P-containing solution. Toxicol. Lett. 2018, 286, 108–119. [Google Scholar] [CrossRef] [PubMed]

| Microorganism | MIC/MB(F)C (μg/mL) | |||
|---|---|---|---|---|
| CTS | QCTS | DCTS | DQCTS | |
| S. aureus | 750 1/1000 2 | 250/250 | 750/750 | 250/250 |
| E. coli O157:H7 | 1000/>1000 | 250/500 | 750/>1000 | 250/250 |
| A. flavus | >1000/>1000 3 | >1000/>1000 | >1000/>1000 | >1000/>1000 |
| C. albicans | 1000/>1000 | 750/1000 | 62.5/125 | 15.6/31.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.H.; Yoon, K.S.; Lee, S.-J.; Park, E.-J.; Rhim, J.-W. Synthesis of Fully Deacetylated Quaternized Chitosan with Enhanced Antimicrobial Activity and Low Cytotoxicity. Antibiotics 2022, 11, 1644. https://doi.org/10.3390/antibiotics11111644
Kim YH, Yoon KS, Lee S-J, Park E-J, Rhim J-W. Synthesis of Fully Deacetylated Quaternized Chitosan with Enhanced Antimicrobial Activity and Low Cytotoxicity. Antibiotics. 2022; 11(11):1644. https://doi.org/10.3390/antibiotics11111644
Chicago/Turabian StyleKim, Yeon Ho, Ki Sun Yoon, Sung-Jae Lee, Eun-Jung Park, and Jong-Whan Rhim. 2022. "Synthesis of Fully Deacetylated Quaternized Chitosan with Enhanced Antimicrobial Activity and Low Cytotoxicity" Antibiotics 11, no. 11: 1644. https://doi.org/10.3390/antibiotics11111644
APA StyleKim, Y. H., Yoon, K. S., Lee, S.-J., Park, E.-J., & Rhim, J.-W. (2022). Synthesis of Fully Deacetylated Quaternized Chitosan with Enhanced Antimicrobial Activity and Low Cytotoxicity. Antibiotics, 11(11), 1644. https://doi.org/10.3390/antibiotics11111644










